Abstract
Mcl-1 is a member of the Bcl-2 family that is expressed in early monocyte differentiation and that can promote viability on transfection into immature myeloid cells. However, the effects of Mcl-1 are generally short lived compared with those of Bcl-2 and are not obvious in some transfectants. To further explore the effects of this gene, mice were produced that expressed Mcl-1 as a transgene in hematolymphoid tissues. The Mcl-1 transgene was found to cause moderate viability enhancement in a wide range of hematopoietic cell types, including lymphoid (B and T) as well as myeloid cells at both immature and mature stages of differentiation. However, enhanced hematopoietic capacity in transgenic bone marrow and spleen was not reflected in any change in pool sizes in the peripheral blood. In addition, among transgenic cells, mature T cells remained long lived compared with B cells and macrophages could live longer than either of these. Interestingly, when hematopoietic cells were maintained in tissue culture in the presence of interleukin-3, Mcl-1 enhanced the probability of outgrowth of continuously proliferating myeloid cell lines. Thus, Mcl-1 transgenic cells remained subject to normal in vivo homeostatic mechanisms controlling viable cell number, but these constraints could be overridden under specific conditions in vitro. Within the organism, Bcl-2 family members may act at “viability gates” along the differentiation continuum, functioning as part of a system for controlled hematopoietic cell amplification. Enforced expression of even a moderate viability-promoting member of this family such as Mcl-1, within a conducive intra- and extracellular environment in isolation from normal homeostatic constraints, can substantially increase the probability of cell immortalization.
© 1998 by The American Society of Hematology.
THE BCL-2 FAMILY contains a growing number of members that promote cell viability, including Bcl-x, Mcl-1, and A1. Mcl-1 was originally identified based on its rapid upregulation in ML-1 human myeloblastic leukemia cells initiating monocytic differentiation.1 Mcl-1 enhances viability on transfection into murine myeloid progenitor (FDC-P1) and Chinese hamster ovary cells.2,3 This enhancement is generally moderate in in vitro systems, with viability being maintained for a shorter period of time with Mcl-1 than with Bcl-23 and no enhancement being observed in some cell lines.4 The effects of introduction of Mcl-1 into an intact animal system have not yet been described.
The Bcl-2–related genes exhibit different tissue- and differentiation stage–specific patterns of expression, which may be one reason for the existence of a variety of family members with apparently similar functions. In the case of Mcl-1, the pattern of expression in myeloid cells in vivo is similar to that observed in the ML-1 cell line; that is, expression is abundant at immature stages of differentiation and is subsequently downregulated.5,6 Expression in lymphoid cells likewise occurs at specific differentiation stages, with Mcl-1 being abundant in lymph node germinal center cells that are undergoing affinity maturation but scant in mature resting lymphocytes in the mantle zone.6 This is the reciprocal of the pattern observed with Bcl-2.6,7 Because of this differentiation stage specificity of expression, Mcl-1 is generally only detectable at low levels in whole tissue extracts.6 Bcl-x and A18 also display distinct patterns of expression during differentiation. For example, Bcl-2 is predominant over Bcl-x at early stages of lymphoid development, Bcl-x is predominant at intermediate stages (eg, in double-positive thymocytes), and Bcl-2 is re-expressed and Bcl-x downregulated in mature cells (ie, in single-positive thymocytes9). Overall, various Bcl-2 family members are differentially expressed in cells of different lineages at different stages of development.
The effects of Bcl-2 family members have been studied in transgenic and null (knock-out) mice, as well as in transfected cell lines. With Bcl-2 and Bcl-x, enhanced cell viability is observed in transgenic mice whereas enhanced cell death occurs in null animals.9-16 The null animals show differences between the different family members, because Bcl-x–null mice exhibit embryonic lethality with apoptosis in the hematolymphoid and nervous systems,17 whereas the Bcl-2–null mice are normal at birth but subsequently undergo fulminent lymphocyte apoptosis.18 Another difference is that Bcl-x contributes to the survival of thymocytes at an intermediate (double-positive) stage of differentiation, whereas Bcl-2 has effects in more mature (single-positive) cells, mirroring the above-described pattern of expression.16 Thus, in the intact animal, Bcl-2 family members exhibit developmental stage specificity of function as well as of expression.
Several studies have suggested that Bcl-2 family members can promote cell survival without necessarily affecting cell differentiation. This was first observed when Bcl-2 was transfected into a hematopoietic cell line that can undergo differentiation (FDC-PMix)19: When transfectants were placed under apoptosis-inducing conditions, Bcl-2 promoted the survival of cells capable of differentiating normally. Similar results were recently obtained when Bcl-2 was expressed as a transgene in mice having a defect in the development of either the lymphoid or the myeloid lineage. Mice lacking the interleukin-7 (IL-7) receptor exhibit a decrease in T-cell precursors,20 which is probably related to the fact that IL-7 normally promotes expression of Bcl-2. The introduction of a Bcl-2 transgene allowed these cells to survive and differentiate, resulting in a normalization of the T lymphocyte pool.21,22 Analogously, Bcl-2 promoted the survival and differentiation of monocytes in mice lacking monocyte colony-stimulating factor.23 Finally, A1 has been reported to promote differentiation in addition to viability.24Taken togther, these findings suggest a model in which Bcl-2 family members control viability at critical gates along the differentiation continuum, by either promoting cell viability and allowing continued differentiation, or, alternatively, allowing cell death.
Although Bcl-2 has been shown to enhance cell viability for several days to weeks in a wide variety of cell lines, there have been only isolated reports relating to cell immortalization. Here, whereas Bcl-2 alone has not been observed to allow immortalization, a combination of EμBcl-2 plus Myc was found effective, allowing the outgrowth of pre–B-cell lines from mouse bone marrow.25 Analogously, mice transgenic for Bcl-2 alone initially exhibit lymphoproliferation,10 whereas mice transgenic for Bcl-2 plus Myc exhibit rapid tumorigenesis.26 Furthermore, a fraction of mice transgenic for Bcl-2 alone develop tumors after a long latency period, and these frequently exhibit alterations in the endogenous c-myc gene.27 Finally, tumor cells expressing both Bcl-2 and Myc die rapidly on explantation into tissue culture, but can be propagated as immortal bipotential (lymphoid/myeloid) progenitor cell lines on a specific stromal layer.28 Thus, cell immortalization in the presence of a combination of Bcl-2 plus Myc has been observed under specific conditions.25 28
Because of the insights gained from studies of mice transgenic for Bcl-2, we applied a transgenic approach to further our understanding of Mcl-1, particularly of its effects in hematopoietic cells. We prepared mice that prominently expressed the Mcl-1 transgene in hematolymphoid tissues, using a mini-gene construct consisting of the Mcl-1 human genomic locus and presumptive regulatory elements. The Mcl-1 transgene caused viability enhancement in hematopoietic cells of various lineages and at various stages of differentiation. As in transfected lines, this effect was moderate and Mcl-1 seemed to act within the context of endogenous homeostatic determinants of viability. Quite unexpectedly, the transgene had a striking effect when myeloid cells were explanted into tissue culture in the presence of IL-3. Here, we observed the outgrowth of continuously proliferating cell lines (consisting of mast cells or monocytes) each time transgenic cells were maintained in bulk culture and from greater than 5% of clones that formed in semisolid medium. In contrast, nontransgenic cells could be maintained in culture for a period of months, but were never observed to form a continuous cell line (immortalization frequency < 2.5 × 10-8), in agreement with the results of previous investigators.29-34 Taken together, our findings indicate that enforced expression of even a moderate viability-enhancing member of the Bcl-2 family such as Mcl-1 can contribute to a dramatic alteration in cell fate: viability promotion by the Mcl-1 transgene, in the context of a conducive intra- and extracellular milieu and in isolation from homeostatic constraints, could substantially increase the probability of escape from the normal limitation on cell lifespan to allow for immortalization.
MATERIALS AND METHODS
Preparation of the Mcl-1 transgene construct.
Cloned fragments of human genomic DNA were used to prepare a transgene construct containing all exons and introns of Mcl-1, the 3′-untranslated region, and approximately 10.5 kb of the genomic 5′-flanking region and 1.7 kb of the 3′-flanking region (Fig 1A). This was accomplished by ligation of three genomic fragments from the two overlapping lambda phage clones (clones B3 and B5). First, a 3.7-kb XbaI-XbaI fragment (Fragment 1, Fig 1A) from clone B5 was ligated to a 2.8-kbNotI-XbaI fragment (Fragment 2) from clone B3, and then into the NotI-XbaI–digested pBluescriptSK+vector. The resulting plasmid was then digested with NotI andSalI, releasing a fragment containing the above 6.5 kb of Mcl-1 along with, at its 3′-end, 57 bp of vector multiple cloning site sequence terminating in a SalI site. This fragment was then ligated to an 11-kb SalI-NotI fragment (Fragment 3) from clone B3, and into SalI-digested pBluescript. A plasmid containing the resultant 17.5-kb SalI-SalI insert oriented correctly, pSS17.5, was purified on a cesium chloride gradient,35 and the insert was purified by gel electrophoresis/electroelution36 and Qiagen column chromatography (Qiagen, Chatsworth, CA). The integrity of the Mcl-1 insert was confirmed by restriction enzyme mapping and DNA sequencing of the ligation joints.
The protein product of the Mcl-1 mini-transgene is prominently expressed in hematolymphoid tissues. (A) The Mcl-1 mini-gene construct used in the generation of transgenic mice (pSS17.5 plasmid) contains all exons (▪, including the 3′-untranslated region [▧] and introns (□) of human Mcl-1. It also contains Mcl-1 5′- and 3′-genomic flanking regions (▨). Fragments 1, 2, and 3 are genomic subclones used in making the transgene construct. Restriction sites are indicated as follows: S, SalI; X,XhoI; B, BamHI; and Xb, XbaI. The BS3.0 genomic subclone shown was used as probe to identify transgenic mice by Southern blotting. (B) Expression of the Mcl-1 transgene was assayed in a variety of tissues by Western blotting. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 50 μg of protein, except lane 13 which represents 100 μg of protein. (C) Expression of the human Mcl-1 transgene in the spleen and lymph node (L.N.) of a transgenic mouse (Tr.) was assayed in parallel with human lymph node. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 100 μg of protein, except lane 4 which represents 50 μg of protein. (D) Expression of the Mcl-1 transgene was assayed in enriched T- and B-cell populations from transgenic mouse spleen. Unfractionated spleen from a transgenic (Tr.) and a nontransgenic (Non-Tr.) mouse, as well as ML-1 cells incubated in the presence or absence of 5 × 10-10 mol/L TPA for 3 hours served as controls. Each lane represents 50 μg of protein. (E) Expression of the Mcl-1 transgene was assayed in cell lines derived from cells from the spleen or bone marrow of transgenic mice. Lanes 1 through 3 (mast cell lines) represent 1 × 106 cells and lane 4 represents 5 × 105 cells. Lanes 5 and 6 (monocytic cell lines) and lane 7 represent 50 μg protein.
The protein product of the Mcl-1 mini-transgene is prominently expressed in hematolymphoid tissues. (A) The Mcl-1 mini-gene construct used in the generation of transgenic mice (pSS17.5 plasmid) contains all exons (▪, including the 3′-untranslated region [▧] and introns (□) of human Mcl-1. It also contains Mcl-1 5′- and 3′-genomic flanking regions (▨). Fragments 1, 2, and 3 are genomic subclones used in making the transgene construct. Restriction sites are indicated as follows: S, SalI; X,XhoI; B, BamHI; and Xb, XbaI. The BS3.0 genomic subclone shown was used as probe to identify transgenic mice by Southern blotting. (B) Expression of the Mcl-1 transgene was assayed in a variety of tissues by Western blotting. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 50 μg of protein, except lane 13 which represents 100 μg of protein. (C) Expression of the human Mcl-1 transgene in the spleen and lymph node (L.N.) of a transgenic mouse (Tr.) was assayed in parallel with human lymph node. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 100 μg of protein, except lane 4 which represents 50 μg of protein. (D) Expression of the Mcl-1 transgene was assayed in enriched T- and B-cell populations from transgenic mouse spleen. Unfractionated spleen from a transgenic (Tr.) and a nontransgenic (Non-Tr.) mouse, as well as ML-1 cells incubated in the presence or absence of 5 × 10-10 mol/L TPA for 3 hours served as controls. Each lane represents 50 μg of protein. (E) Expression of the Mcl-1 transgene was assayed in cell lines derived from cells from the spleen or bone marrow of transgenic mice. Lanes 1 through 3 (mast cell lines) represent 1 × 106 cells and lane 4 represents 5 × 105 cells. Lanes 5 and 6 (monocytic cell lines) and lane 7 represent 50 μg protein.
Preparation of Mcl-1 transgenic mice.
Microinjection of insert DNA (4 ng/mL in 10 mmol/L Tris-Cl, pH 7.5, containing 0.25 mmol/L EDTA) was performed by DNX Corporation (Princeton, NJ). The founder mice (C57B6/SJL-F1) obtained were mated with normal C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). The presence or absence of the Mcl-1 transgene was determined by Southern blotting,35 using as a probe a subclone of human genomic DNA (clone BS3.0 [in the Bluescript vector]) that had been labeled by the random primer method.37 This probe detects a 16-kbXhoI band in genomic DNA from mice made transgenic for human Mcl-1 not in nontransgenic mice.
Expression of the Mcl-1 transgene.
Expression of the protein product of the human Mcl-1 transgene was assayed by Western blotting using a rabbit polyclonal anti–Mcl-1 antibody that specifically detects human Mcl-1 and does not detect cross-reacting bands in mouse cells.5 Tissues were excised, minced rapidly, placed into tubes containing ice-cold tissue lysis buffer (50 mmol/L HEPES, pH 7.2; 150 mmol/L NaCl; 0.2% NP-40; 2 mmol/L EGTA; 15 mmol/L EDTA; and 1% sodium dodecyl sulfate), homogenized with a Tissue Tearor (Biospec Products, Inc, Racine, WI), boiled for 10 minutes, and centrifuged at 16,000g for 5 minutes (room temperature). Protein concentration in the resultant supernatants was assayed using the DC Protein Assay Kit (Bio-Rad, Hercules, CA). Immunoblotting was performed using previously described methods.2
T- and B-cell–enriched populations were separated as follows. Enrichment for B cells was performed by complement-mediated lysis of T cells.38 Briefly, spleen cells were obtained and erythrocytes removed using ACK lysis buffer (0.15 mol/L NH4Cl; 1.0 mmol/L KHCO3; 0.1 mmol/L EDTA, pH 7.2). Cells were then incubated on ice for 45 minutes with an anti–Thy-1 monoclonal antibody in Hank’s balanced salt solution (HBSS; BioWhittaker, Walkersville, MD) containing 5% fetal calf serum (FCS). After collection by centrifugation, cells were incubated at 37°C for 30 minutes in HBSS containing 5% FCS (HBSS/FCS) and 5% rabbit serum as a source of complement. After three washes with cold HBSS/FCS, the T-cell–depleted population (generally 95% B cells) was assayed for cell viability and protein concentration.
Enrichment for T cells was performed by depletion of B cells, which was accomplished by panning on dishes coated with goat anti-mouse Ig antibody. Briefly, after lysis of erythrocytes as above, spleen cells were incubated at 4°C (with intermittent swirling) in plastic dishes precoated with goat anti-mouse Ig, using a buffer of phosphate-buffered saline ([PBS] 137 mmol/L NaCl; 2.7 mmol/L KCl; 10 mmol/L Na2HPO4; 1.8 mmol/L KH2PO4, pH 7.4) containing 5% FCS. After 70 minutes, cells remaining in suspension were obtained, the panning process repeated, cells in suspension again harvested, and the B-cell–depleted population (generally approximately 90% T cells) washed three times with HBSS/FCS and assayed for protein concentration.
Isolation and short-term in vitro culture of cells from spleen and bone marrow.
Suspensions of spleen cells were prepared by dispersing freshly removed spleen tissue in Delbecco’s Modified Eagle medium containing 10% fetal bovine serum (FBS), 50 U/mL penicillin, and 50 μg/mL streptomycin (standard medium), and rinsing the splenocytes through a 210-μm Spectra/Mesh Polypropylene filter (Spectrum, Houston, TX). After centrifugation (5 minutes at 200g), cells were resuspended in standard medium, counted to determine the total number of splenocytes recovered, and incubated at 4 × 106cells/mL in tissue culture. Cell viability was monitored on subsequent days of culture by trypan blue dye exclusion.39 In some experiments, the tissue culture medium was additionally supplemented with 100 to 200 pmol/L IL-3 or IL-2 (R & D Systems, Minneapolis, MN).
Suspensions of bone marrow cells were obtained by flushing the tibia with standard medium using a 10-mL syringe equipped with a 22-gauge needle.
The fraction of cells of various lineages surviving on in vitro culture, as compared with the number present on day 0, was calculated as follows: (the number of viable T, B, or myeloid cells remaining on day 1 or 3)/(the number of viable T, B, or myeloid cells initially present on day 0). The numerator and denominator of this ratio were calculated from the viable cell number (determined using trypan blue) and the percentage of viable cells expressing lineage markers (determined by flow cytometry as described below). Similarly, the fraction of colony-forming cells surviving on in vitro culture was calculated as the following ratio: (the number present on day 1)/(the number present on day 0).
Flow cytometry.
The primary antibodies used were monoclonal rat anti-mouse antibodies (fluorescein isothiocyanate [FITC]- or phycoerythrin [PE]-conjugated, or biotinylated forms) directed against the following markers: CD3, CD5, and CD45R (B220) (Sigma Chemical Company, St Louis, MO); CD4, CD8, IgM, CD34, CD13, Thy1.2, and Sca-1 (Pharmingen, San Diego, CA); CD11b, F4/80, c-kit, and Gran-1 (Caltag, South San Francisco, CA); and MP12 and MP20 (Bachem, King of Prussia, PA). PE- or FITC-conjugated rat IgG was used as a negative control. Flow cytometry was performed using a FACSCAN (Becton Dickinson, San Jose, CA) and a total of 10,000 cells (viable plus dead) was assayed for each sample. When dead cells were present, the data were analyzed by gating on the viable cell population with a reference set by the propidium iodide staining. Where two-color staining was performed, single-color staining was carried out in parallel to set the electronic compensation.
Assay for IgE receptors was performed as follows: cells were incubated for 45 minutes on ice with purified mouse IgE (Pharmingen; 1 μg/106 cells in 50 μL of staining buffer), washed with staining buffer, and then incubated for 30 minutes on ice with an FITC-conjugated rat anti-mouse IgE antibody (Pharmingen; 1 μg/106 cells in 50 μL of staining buffer). Cells were washed and analyzed using a FACSCAN as above.
For each antibody used, the percentage positive cells was calculated as follows: a threshold was set such that the fluorescence of at least 95% of the cells stained with the negative control antibody fell below the threshold. Cells that exhibited fluorescence above this threshold with the test antibody were considered positive.
Assay of colony formation in semisolid medium.
Cells obtained from the bone marrow were assayed for the ability to form various types of colonies in methylcellulose medium. The medium used for assay of myeloid/erythroid colonies was Methocult M3534 (Stemcell Technologies, Inc, Vancouver, Canada), which contains IL-3, IL-6, stem cell factor, FBS, bovine serum albumin, 2-mercaptoethanol, insulin, and transferrin; this medium was supplemented with erythropoietin (3 U/mL; R & D Systems). The medium used for assay of B lymphoid colonies was Methocult M3630 (Stemcell Technologies), which contains IL-7, FBS, and 2-mercaptoethanol. Bone marrow cells were plated at 1.5 × 104 cells/35-mm dish for assay of myeloid/erythroid colonies and at 4 × 104 cells/35-mm dish for assay of B lymphoid colonies. Colonies were counted on day 7 using a Zeiss Axioskop inverted microscope (10× lens). In initial tests, spleen cells were similarly tested for formation of myeloid colonies, using the Methocult M3534 medium without erythropoeitin supplementation. Spleen cells were plated at 1.5 × 105 cells/35-mm dish and the colonies that formed were counted by eye on day 9.
Long-term in vitro culture for the derivation of cell lines from spleen and bone marrow cells.
Cells from the spleen were incubated overnight in standard medium (5 × 105 cells/mL) and then transferred (at the same cell density) into long-term culture medium which consisted of X-VIVO 15 medium (BioWhittaker) supplemented with 10% FBS, 200 pmol/L IL-3 (R & D systems), and 20% WEHI-3B– conditioned medium prepared as described previously.40 Cells from the bone marrow were treated identically except that they were incubated in standard medium for 4 days before transfer to long-term culture medium. Nonadherent and adherent cells were maintained separately as follows. At weekly intervals, nonadherent cells were collected by centrifugation and recultured in fresh medium (5 × 105 cells/mL) in a new flask. Remaining adherent cells were discarded for the first two passages when fibroblasts were abundant. Thereafter, loosely adherent monocytic cells were also observed. These cells were passaged by reculturing loosely adherent cells tht were found in suspension at confluence. Continued passage in this fashion resulted in decreasing numbers of fibroblasts and the emergence of monocytic cell lines.
Tissue histology.
For light microscopy studies, fixation in 10% neutral buffered formalin (Curtis-Matheson Scientific, Houston, TX); paraffin embedding; sectioning and staining with hematoxylin and eosin; as well as histochemical staining for myeloperoxidase, alpha naphthyl butyrate esterase, and chloroacetate esterase were performed in the Dartmouth-Hitchcock Hospital Pathology Department facility (Lebanon, NH).
For transmission electron microscopy studies, samples were prepared as follows: cells were rinsed in 0.1 mol/L phosphate buffer (pH 7.4) containing 4% sucrose. They were then fixed in 2% glutaraldehyde/1% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 15 minutes (room temperature), followed by replacement with fresh fixative and fixation for an additional 1 hour (4°C). After fixation, cells were rinsed in 0.1 mol/L phosphate buffer (pH 7.4) and postfixed in 1% OsO4 in 0.1 mol/L phosphate buffer (pH 7.4). Cells were dehydrated through a graded series of ethanols and propylene oxide and embedded in epon (LX112). Thin sections were stained with 2% uranyl acetate in methanol for 20 minutes, followed by 5 minutes in Reynold’s lead citrate. Micrographs were taken at 80 kV on a JEOL 100CX electron microscope (JOEL USA, Inc, Peabody, MA).
RESULTS
The Mcl-1 transgene is prominently expressed in hematolymphoid tissues.
Because Mcl-1 exerts effects in hematopoietic cells on transfection,2 we wished to test for effects in hematopoietic tissues in transgenic mice. To this end, we constructed a minigene containing the Mcl-1 human genomic locus complete with 5′- and 3′-flanking regions (Fig 1A). We used this construct, instead of lineage-specific expression constructs, in the hopes of promoting transgene expression in a variety of hematolymphoid tissues. This hope was based on the fact that a similar approach had been used with other genes,41-44 where it was found to result in elevated expression in tissues that normally express the gene product. Thus, although data comparing the regulatory regions of human and mouse Mcl-1 are not yet available, the approach of using human genomic constructs for the preparation of transgenic mice has been applied successfully for a variety of other genes. In addition, the highly conserved carboxyl portion of the Bcl-2 that is important for function is greater than 90% identical in the human and murine Mcl-1 gene products. Finally, the human gene product is known to have viability-promoting effects in a murine hematopoietic cell line.2 Using this approach, we obtained two transgenic founder animals (female). These were mated with nontransgenic mice, and offspring that were transgenic (heterozygous) for human Mcl-1 were compared with nontransgenic littermates of the same sex, or, if not available, nontransgenic animals of the same age and sex from concurrent litters. The experiments described below were performed using offspring from one of the founders, with similar effects being observed with the other founder.
The mice obtained indeed exhibited prominent transgene expression in a variety of hematopoietic and lymphoid tissues, establishing the use of the approach involving a human genomic construct in transgenic mice. Using an antibody that detects human (but not mouse) Mcl-1, the protein product of the human transgene was found to be abundant in bone marrow, lymph node, thymus, and spleen, and to be expressed at lower levels in some other tissues (kidney, small intestine, uterus, lung, and liver; Fig 1B). In the spleen, expression was prominent in both B- and T-cell–enriched populations (Fig 1D). The level of Mcl-1 transgene expression was considerably higher than the level of Mcl-1 expression in human lymph node assayed in parallel (Fig 1C), and approached that observed in ML-1 cells treated with 12-O-tetradecanoylphorbol 13-acetate (TPA) (Fig 1C and D). These levels are well within the range known to produce viability-enhancing effects in transgenic cell lines.2
The Mcl-1 transgene promotes survival in mature lymphoid and myeloid cells but does not completely override endogenous determinants of cell death.
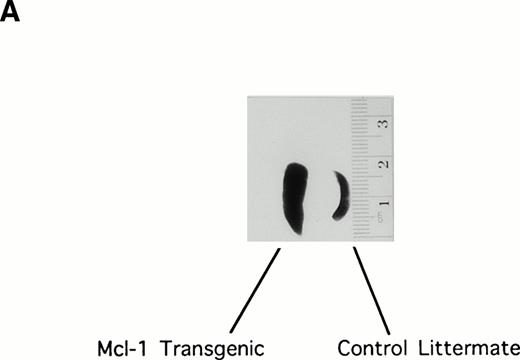
Initial examination of the Mcl-1 transgenic mice showed enlargement of the spleen, which was moderate but was observed in the majority of animals (Fig 2A). Spleens were therefore explanted from a series of 25 transgenic mice (6 weeks to 1 year in age) to assay total splenocyte number. Twenty-three of these animals showed an increase in splenocyte number as compared with matched controls, the average increase being 1.6-fold (SE = 0.08; P < .01 Student’s one-tailed t-test).
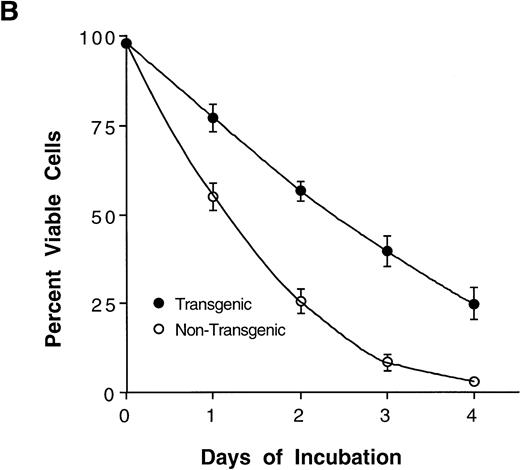
The Mcl-1 transgene causes splenic enlargement and enhances spleen cell survival in vitro. (A) Shown are spleens from two 12-week-old male littermates, one transgenic for Mcl-1 (left) and one nontransgenic (right). (B) Spleens from transgenic and nontransgenic mice were explanted, and the splenocytes were placed in suspension in standard medium, incubated in tissue culture, and assayed daily for cell viability by trypan blue dye exclusion. The points shown are the mean ± SE for cells from 18 transgenic mice and 12 matched nontransgenic controls. The significance of the difference between nontransgenic and transgenic cells, as assessed by analysis of variance with the Scheffé test, indicated a P value of <.01 for days 1, 2, 3, and 4.
The Mcl-1 transgene causes splenic enlargement and enhances spleen cell survival in vitro. (A) Shown are spleens from two 12-week-old male littermates, one transgenic for Mcl-1 (left) and one nontransgenic (right). (B) Spleens from transgenic and nontransgenic mice were explanted, and the splenocytes were placed in suspension in standard medium, incubated in tissue culture, and assayed daily for cell viability by trypan blue dye exclusion. The points shown are the mean ± SE for cells from 18 transgenic mice and 12 matched nontransgenic controls. The significance of the difference between nontransgenic and transgenic cells, as assessed by analysis of variance with the Scheffé test, indicated a P value of <.01 for days 1, 2, 3, and 4.
In Bcl-2 transgenic mice, splenic enlargement is accompanied by enhanced splenocyte survival on incubation in tissue culture. To assay for such an effect in the case of Mcl-1, we monitored splenocyte viability over 4 days of incubation in vitro. At every time point assayed, viability was significantly higher in cultures from transgenic animals than in parallel cultures from nontransgenic controls (Fig 2B). Thus, the average percentage of viable cells declined to approximately 50% within 1 day in nontransgenic cultures, whereas an equivalent decline required about twice as much time in transgenic cultures. The enhancement of viability was particularly noticeable on day 4, when nontransgenic cells had nearly all died whereas about 25% of transgenic cells remained viable. In sum, Mcl-1 enhanced spleen cell survival, a result that paralleled very closely previous findings in transfected cell lines.2 3
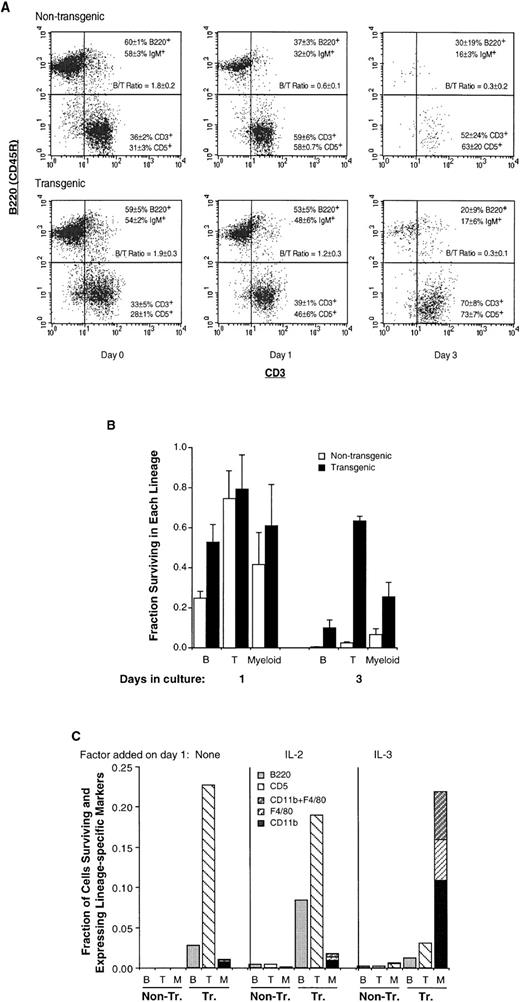
We next monitored B- and T-cell markers in transgenic splenocytes, to determine which lineage(s) were affected by Mcl-1. We found that the increase in transgenic splenocyte number in vivo (Fig 2A) represented an increase in both B and T cells, the relative proportions of these two being unchanged (Fig 3A, day 0). Likewise, the increase in transgenic splenocyte viability in vitro (Fig2B) represented the survival of cells of both lineages (Fig 3A, day 3 where both B and T cells remain present). Overall, Mcl-1 promoted the survival of cells of both lymphoid lineages, both in vivo and in vitro (Figs 2 and 3A).
The Mcl-1 transgene enhances the survival of mature lymphoid (B and T) as well as myeloid cells but does not override endogenous determinants of viability. (A) Spleen cells from transgenic and nontransgenic mice were assayed by two-color flow cytometry for the presence of B and T cell cell markers, either immediately on explantation (day 0) or after 1 or 3 days of of incubation, as in Fig2B. The dot plots shown were obtained by gating on the viable cell population and the percentages of B220+ and CD3+ cells, and cell-surface IgM+ and CD5+ cells, represent the mean ± SD of two to three animals. Results from parallel assays are shown in Table 1. The B/T cell ratio indicated was calculated from both sets of markers. The number of cells recovered from the spleen in these experiments averaged 2.9 ± 0.6 (SD) × 108 for nontransgenic and 4.6 ± 1.3 × 108 for transgenic mice. The percentage of viable cells on day 0 was greater than 95% and the average percentage of viable cells on days 1, 2, and 3, respectively, was 51% ± 6% (SD), 19% ± 4%, and 2.4% ± 0.7% for nontransgenic mice and 75% ± 8%, 56% ± 5%, and 40% ± 8% for transgenic mice. (B) The fractions of T lymphoid, B lymphoid, and myeloid cells surviving on days 1 and 3 (compared with the initial numbers present on day 0) were calculated from the experiment in (A). The values for the T-cell markers CD3+ and CD5+ cells were averaged, as were those for the B-cell markers B220+ and IgM+; CD11b was assayed in duplicate as a myeloid marker. Bars represent the SE of two to three animals. On day 0, the total number of viable nontransgenic T, B, and myeloid cells averaged 9.7 ± 0.1 × 107, 1.7 ± 0.2 × 108, and 1.5 ± 0.4 × 107 (SE of three animals), and the total number of viable transgenic T, B, and myeloid cells averaged 1.4 ± 0.1 × 108, 2.6 ± 0.3 × 108, and 2.8 ± 0.5 × 107. (C) Spleen cells from Mcl-1 transgenic and nontransgenic mice were incubated in vitro as in (A), with IL-2 or IL-3 (100 pmol/L) being added on day 1 as indicated. On day 9, the viable cell population was assayed by flow cytometry for CD11b and F4/80, CD11b and CD5, or B220. At this time, the percentage of viable cells in transgenic cultures was 26% to 28%, as determined by propidium iodide staining. Essentially no viable cells remained in nontransgenic cultures to which no factor had been added on day 1, and only small numbers of viable cells (≤1%) could be detected in nontransgenic cultures to which growth factors had been added. B, B220+ cells; T, CD5+ cells; M, CD11b+ and/or F4/80+ cells. The CD11b+ cells shown were CD5−, and the CD5+ cells shown were CD11b−, with ≤3% of cells being CD11b+CD5+.
The Mcl-1 transgene enhances the survival of mature lymphoid (B and T) as well as myeloid cells but does not override endogenous determinants of viability. (A) Spleen cells from transgenic and nontransgenic mice were assayed by two-color flow cytometry for the presence of B and T cell cell markers, either immediately on explantation (day 0) or after 1 or 3 days of of incubation, as in Fig2B. The dot plots shown were obtained by gating on the viable cell population and the percentages of B220+ and CD3+ cells, and cell-surface IgM+ and CD5+ cells, represent the mean ± SD of two to three animals. Results from parallel assays are shown in Table 1. The B/T cell ratio indicated was calculated from both sets of markers. The number of cells recovered from the spleen in these experiments averaged 2.9 ± 0.6 (SD) × 108 for nontransgenic and 4.6 ± 1.3 × 108 for transgenic mice. The percentage of viable cells on day 0 was greater than 95% and the average percentage of viable cells on days 1, 2, and 3, respectively, was 51% ± 6% (SD), 19% ± 4%, and 2.4% ± 0.7% for nontransgenic mice and 75% ± 8%, 56% ± 5%, and 40% ± 8% for transgenic mice. (B) The fractions of T lymphoid, B lymphoid, and myeloid cells surviving on days 1 and 3 (compared with the initial numbers present on day 0) were calculated from the experiment in (A). The values for the T-cell markers CD3+ and CD5+ cells were averaged, as were those for the B-cell markers B220+ and IgM+; CD11b was assayed in duplicate as a myeloid marker. Bars represent the SE of two to three animals. On day 0, the total number of viable nontransgenic T, B, and myeloid cells averaged 9.7 ± 0.1 × 107, 1.7 ± 0.2 × 108, and 1.5 ± 0.4 × 107 (SE of three animals), and the total number of viable transgenic T, B, and myeloid cells averaged 1.4 ± 0.1 × 108, 2.6 ± 0.3 × 108, and 2.8 ± 0.5 × 107. (C) Spleen cells from Mcl-1 transgenic and nontransgenic mice were incubated in vitro as in (A), with IL-2 or IL-3 (100 pmol/L) being added on day 1 as indicated. On day 9, the viable cell population was assayed by flow cytometry for CD11b and F4/80, CD11b and CD5, or B220. At this time, the percentage of viable cells in transgenic cultures was 26% to 28%, as determined by propidium iodide staining. Essentially no viable cells remained in nontransgenic cultures to which no factor had been added on day 1, and only small numbers of viable cells (≤1%) could be detected in nontransgenic cultures to which growth factors had been added. B, B220+ cells; T, CD5+ cells; M, CD11b+ and/or F4/80+ cells. The CD11b+ cells shown were CD5−, and the CD5+ cells shown were CD11b−, with ≤3% of cells being CD11b+CD5+.
Histological examination agreed with the above flow cytometric results in that the architecture of the spleen was generally preserved in transgenic mice: splenic enlargement was not associated with any dramatic follicular expansion, except in rare mice exhibiting expansion of the while pulp. However, extramedullary hematopoiesis was very active in the majority of transgenic animals. Occasional animals (two of nine tested) also exhibited an increase in thymocyte number, but no other consistent gross morphological alterations were noted.
Bcl-2 or Bcl-x transgenic mice made using lineage- or differentiation stage–specific constructs often exhibit a preferential expansion of one lymphoid lineage over the other and/or enhanced survival at specific stages of differentiation (eg, the follicular expansion observed in some strains of Bcl-2 transgenic mice).11,16 However, the above histological and flow cytometric observations did not suggest a preferential effect in the Mcl-1 transgenic mice, which probably related to the fact that Mcl-1 was expressed in both B and T cells (Fig 1C). To confirm a lack of preferential effect and to further characterize the differentiation phenotype of the transgenic splenocytes, we performed a more comprehensive flow cytometric characterization. Lymphoid cells from transgenic spleen were found to exhibit a normal mature cell phenotype, and, other than the increase in spleen cell number, no overt abnormality or preferentially expanded subpopulation was detected (Table 1). Thus, B cells expressed cell-surface IgM and IgD and T cells were predominantly either CD4 or CD8 single positive. In sum, Mcl-1 seemed to cause enhanced survival in various types of cells (Figs 2 and 3) without grossly altering the pattern of cell differentiation or causing preferential expansion of a particular cell type. The apparent contrast between these findings and previous reports of the effects of lineage-specific Bcl-2 constructs is probably more apparent than real, because Bcl-2 can promote survival in both lymphoid lineages and other cell types when appropriate promoters are used.
Cell-Surface Marker Expression in Hematopoietic Cells From Mcl-1 Transgenic Mice
| . | Percent Positive Cells . | |
|---|---|---|
| Nontransgenic Mice . | Transgenic Mice . | |
| Splenocytes | ||
| IgM | 64 ± 8 | 61 ± 9 |
| IgD | 55 ± 3 | 45 ± 7 |
| CD4 | 17 ± 2 | 19 ± 1 |
| CD8 | 12 ± 1 | 13 ± 1 |
| CD4+CD8+ | <1 | <1 |
| CD11b | 3 ± 2 | 5 ± 3 |
| c-kit | <1 | <1 |
| Bone marrow cells | ||
| IgM | 28 ± 8 | 25 ± 4 |
| Thy 1.2 | 2.4 ± 0.2 | 2.6 ± 0.6 |
| Gran-1 | 19 ± 2 | 25 ± 1 |
| c-kit | 1.1 ± 0.1 | 0.6 ± 0.3 |
| . | Percent Positive Cells . | |
|---|---|---|
| Nontransgenic Mice . | Transgenic Mice . | |
| Splenocytes | ||
| IgM | 64 ± 8 | 61 ± 9 |
| IgD | 55 ± 3 | 45 ± 7 |
| CD4 | 17 ± 2 | 19 ± 1 |
| CD8 | 12 ± 1 | 13 ± 1 |
| CD4+CD8+ | <1 | <1 |
| CD11b | 3 ± 2 | 5 ± 3 |
| c-kit | <1 | <1 |
| Bone marrow cells | ||
| IgM | 28 ± 8 | 25 ± 4 |
| Thy 1.2 | 2.4 ± 0.2 | 2.6 ± 0.6 |
| Gran-1 | 19 ± 2 | 25 ± 1 |
| c-kit | 1.1 ± 0.1 | 0.6 ± 0.3 |
Whereas the proportions of B versus T cells in the spleen were not altered in transgenic mice (Fig 3A, day 0), these proportions were altered as cells were maintained in culture. B cells were initially more abundant than T cells (B/T cell ratio of ≥1.8 on day 0, Fig 3A). However, on day 1, nontransgenic cultures showed a substantial decrease in the percentage of B cells and an increase in the percentage of T cells (B/T cell ratio of 0.6), suggesting that B cells were dying more rapidly than T cells. This decrease in the B/T cell ratio was delayed in transgenic cultures. Calculation of the fraction of viable B cells remaining on day 1 (as compared with day 0, Fig 3B) showed a value of approximately 0.25 in nontransgenic cultures and 0.5 in transgenic cultures; the fraction of viable T cells remaining at this time was ≥0.74 in both cases. In other words, the death of B cells occurred earlier than that of T cells and this rapid B-cell death was partially inhibited by Mcl-1. The slower process of T-cell death was also inhibited by Mcl-1, because almost no nontransgenic T cells remained present on day 3 but the fraction of remaining transgenic T cells was greater than 0.6. The observation that the death of B cells occurred more rapidly than that of T cells is in agreement with the fact that mature, resting B cells (with the exception of memory cells) are generally short lived compared with mature T cells45,46. In sum, Mcl-1 enhanced cell survival in both lymphoid lineages, and seemed to act within the context of other determinants of viability such that T cells remained long lived compared with B cells.
Myeloid cells, as represented by the CD11b marker, were present in low numbers in the above splenocyte cultures (≤5% on average; Table 1). These cells also exhibited extended survival in the presence of Mcl-1, because the fraction of CD11b+ cells surviving on day 3 (compared with the initial number on day 0) was 0.07 in nontransgenic and 0.25 in transgenic cultures (Fig 3B). By adding various growth factors to the medium, we found that the survival of these cells could be further extended by the addition of IL-3 (Fig 3C). Thus, nontransgenic cells could not remain viable for an extended period even in the presence of IL-2 or IL-3 (<1% viable on day 9), whereas a proportion of the transgenic cells remained viable (26% to 28%). In the absence of supplemental growth factors (or in the presence of IL-2), the surviving transgenic cells consisted primarily of T cells. However, in the presence of IL-3, the majority of surviving cells (80%) expressed the general myeloid marker CD11b and/or the monocyte/macrophage marker F4/80. In sum, like lymphoid cells, transgenic myeloid cells exhibited enhanced survival in vitro, and this was particularly pronounced in the presence of IL-3.
The Mcl-1 transgene enhances bone marrow hematopoietic capacity but does not alter peripheral blood pools.
To extend the above observation of an effect in myeloid cells, we cultured spleen cells in semisolid medium in the presence of factors that promote the formation of myeloid colonies from precursors (IL-3, IL-6, and stem cell factor). On average, 0.037% of transgenic spleen cells formed macroscopically detectable colonies (SE=0.01%; n = 5), which represented an approximate 3.2-fold increase (S.E. = 0.6) over parallel nontransgenic cultures and accorded with the very active extramedullary hematopoiesis noted on histological examination.
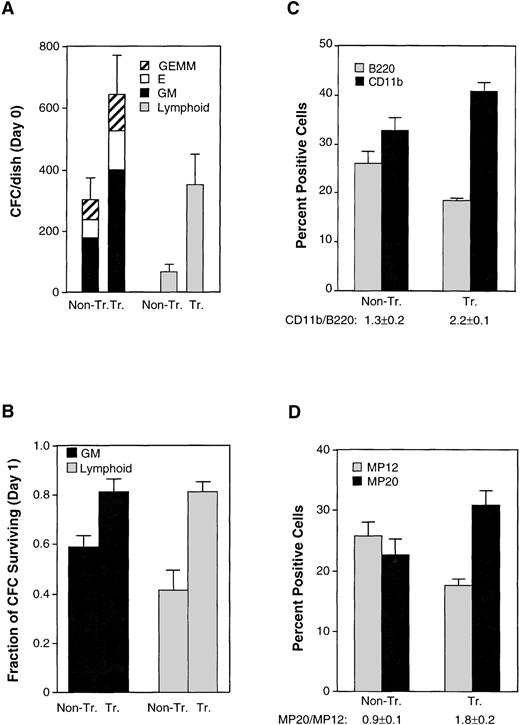
To assess more comprehensively the effects of Mcl-1 on hematopoietic precursors, we obtained cells from bone marrow and assayed for the formation of myeloid, erythroid, and lymphoid colonies in semisolid medium containing the appropriate growth factors. When assayed immediately on explantation of the spleen, transgenic animals showed a doubling of the yield of myeloid and erythroid colonies and an approximate fivefold increase in that of B lymphoid colonies (Fig 4A). In addition, the colonies derived from transgenic mice were larger in size than those from nontransgenic controls (data not shown). We also tested for the ability of colony-forming cells to survive in the absence of their specific growth factors.47 This was performed by maintaining bone marrow cells in liquid culture in standard medium before transfer to semisolid medium containing the growth factors. Both myeloid and erythroid colonies from transgenic mice showed enhanced survival (Fig 4B). In sum, transgenic mice exhibited an increase in the number of colony-forming cells that could be derived from transgenic bone marrow, as well as an increase in survival when these cells were deprived of specific growth factor requirements. These data showing enhanced survival in a variety of hematopoietic precursors provide a parallel to the enhancement of survival observed in mature cells of various lineages.
The Mcl-1 transgene enhances hematopoietic capacity in the bone marrow although peripheral blood pools remain unaltered. (A) Bone marrow cells from nontransgenic (Non-Tr.) and transgenic (Tr.) mice were plated into methylcellulose medium containing factors supporting the growth of either myeloid and erythroid precursors (left set of bars) or B lymphoid precursors (right set of bars). The plating density was 1.5 × 104 cells/35-mm dish for assay of myeloid/erythroid colonies and 4 × 104 cells/35-mm dish for assay of B lymphoid colonies. Colonies were counted after 7 days. The values shown represent the mean ± SD of three independent experiments using four transgenic and three control mice (P < .05 by Student’s t-test). GM, colonies containing myeloid (granulocyte-macrophage) cells; E, colonies containing erythroid cells; GEMM, colonies containing both myeloid and erythroid cells. (B) The bone marrow cells assayed in (A) were cultured in standard medium (containing 10% FBS only) for 24 hours before plating into growth factor–containing methylcellulose medium. The fraction of colony-forming cells remaining on day 1 was calculated by comparison with the number of present on day 0. The significance between nontransgenic and transgenic animals, as determined by Student’st-test, was P < .05 for both GM and lymphoid colonies. (C) Bone marrow from transgenic and nontransgenic mice (10- to 12-weeks-old) was assayed by flow cytometry for the B220 and CD11b cell-surface markers. The total number of cells recovered from the bone marrow averaged 1.5 ± 0.3 (SD) × 107 cells for nontransgenic mice and 1.3 ± 0.1 × 107 cells for the transgenic mice. Bars represent the SE of three animals. The CD11b+/B220+ ratio is listed on the figure. The significance of the difference in this ratio in nontransgenic as compared with transgenic animals, as assessed by analysis of variance with the Scheffé test, which indicated a P value of <.01. (D) The bone marrow cells assayed in (C) were also assayed for the MP20 and MP12 markers. The percentage of MP20+MP12+ double-positive cells averaged 11% ± 0.5% (SD) in nontransgenic and 14% ± 2% in transgenic mice. The MP20+/MP12+ ratio is listed on the figure. The significance of the difference in this ratio in nontransgenic as compared with transgenic animals, as assessed by analysis of variance with the Scheffé test, which indicated aP value of <.05.
The Mcl-1 transgene enhances hematopoietic capacity in the bone marrow although peripheral blood pools remain unaltered. (A) Bone marrow cells from nontransgenic (Non-Tr.) and transgenic (Tr.) mice were plated into methylcellulose medium containing factors supporting the growth of either myeloid and erythroid precursors (left set of bars) or B lymphoid precursors (right set of bars). The plating density was 1.5 × 104 cells/35-mm dish for assay of myeloid/erythroid colonies and 4 × 104 cells/35-mm dish for assay of B lymphoid colonies. Colonies were counted after 7 days. The values shown represent the mean ± SD of three independent experiments using four transgenic and three control mice (P < .05 by Student’s t-test). GM, colonies containing myeloid (granulocyte-macrophage) cells; E, colonies containing erythroid cells; GEMM, colonies containing both myeloid and erythroid cells. (B) The bone marrow cells assayed in (A) were cultured in standard medium (containing 10% FBS only) for 24 hours before plating into growth factor–containing methylcellulose medium. The fraction of colony-forming cells remaining on day 1 was calculated by comparison with the number of present on day 0. The significance between nontransgenic and transgenic animals, as determined by Student’st-test, was P < .05 for both GM and lymphoid colonies. (C) Bone marrow from transgenic and nontransgenic mice (10- to 12-weeks-old) was assayed by flow cytometry for the B220 and CD11b cell-surface markers. The total number of cells recovered from the bone marrow averaged 1.5 ± 0.3 (SD) × 107 cells for nontransgenic mice and 1.3 ± 0.1 × 107 cells for the transgenic mice. Bars represent the SE of three animals. The CD11b+/B220+ ratio is listed on the figure. The significance of the difference in this ratio in nontransgenic as compared with transgenic animals, as assessed by analysis of variance with the Scheffé test, which indicated a P value of <.01. (D) The bone marrow cells assayed in (C) were also assayed for the MP20 and MP12 markers. The percentage of MP20+MP12+ double-positive cells averaged 11% ± 0.5% (SD) in nontransgenic and 14% ± 2% in transgenic mice. The MP20+/MP12+ ratio is listed on the figure. The significance of the difference in this ratio in nontransgenic as compared with transgenic animals, as assessed by analysis of variance with the Scheffé test, which indicated aP value of <.05.
In view of the above alterations in precursor number, we surveyed the cytometric marker profile of transgenic bone marrow. Transgenic mice were found to exhibit a subtle increase in the proportion of CD11b+ cells and decrease in the proportion of B220+ cells, resulting in a 1.7-fold increase in the CD11b+/B220+ ratio (Fig 4C). A small increase in Gran1+ cells was also observed (Table 1). Because of the subtle nature of these changes, we also used a second set of markers, MP20 and MP1248, which delineate bone marrow populations consisting primarily but not entirely of mature myelomonocytic (MP20 single-positive) and lymphoid (MP12 single-positive) cells where erythroid cells are negative for both these markers (MP12−MP20−). In the Mcl-1 transgenic mice, we observed a shift in the proportions of these markers that paralleled the shift in the CD11b+/B220+ ratio (Fig 4D). These markers do not represent populations identical to those marked by CD11b and B220.48-51 In particular, the MP20 single-positive fraction contains mature granulocytes and monocytes, whereas less mature cells of the myeloid lineage reside in the double-positive (MP20+MP12+) fraction, and (at even less mature stages) in the MP12 single-positive fraction.48 The proportion of MP20 single-positive cells was therefore smaller than the proportion of CD11b+ cells, because CD11b is present on some immature cells. Nonetheless, the results from these two different sets of markers, when taken together, suggested that the presence of Mcl-1 resulted in a shift in the proportion of myeloid relative to lymphoid cells in the bone marrow.
Despite the above shift in the bone marrow, no change was apparent in peripheral blood total or differential cell counts from transgenic mice (approximately 107 red blood cells/mL and 6,000 to 7,000 white blood cells/mL of which 88% to 89% were lymphocytes, 10% were segmented cells and 1% to 2% were monocytes). The above-described moderate expansion of myeloid pools in the bone marrow was reminiscent of the moderate expansion of lymphoid pools observed in the spleen (Fig2). The fact that expansion in hematopoietic organs was not reflected in the peripheral blood suggests that the endogenous homeostatic mechanisms that regulate the ultimate numbers of viable cells in the periphery are not affected by Mcl-1. We have recently obtained a monoclonal antibody to Mcl-1 and are developing a flow cytometric assay for the gene product. We plan to use this assay to determine whether the transgene is expressed in periferal lymphoid and myeloid cells and whether these cells exhibit enhanced survival. This will give an indication of whether endogenous homeostatic mechanisms operate by affecting transgene expression or alternatively by overriding the effects of the transgene product.
The Mcl-1 transgene increases the probability of myeloid cell immortalization on explantation to a conducive tissue culture environment.
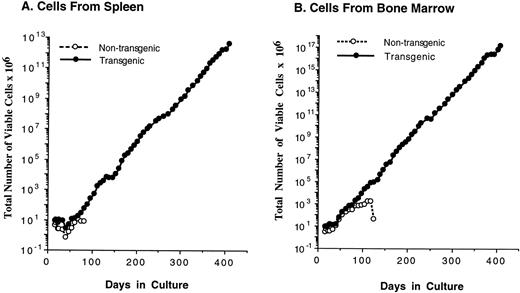
Because myeloid cells from transgenic mice displayed enhanced survival in the presence of IL-3 (Fig 3C), we attempted to maintain these cells in culture for an extended period of time. To our surprise, we found that continuously proliferating cell lines consistently grew out from bulk spleen-cell cultures from transgenic mice, a phenomenon that was never observed in parallel nontransgenic cultures (Fig 5A and Table 2). Cell lines also arose from bone marrow cultures from transgenic (but not nontransgenic) mice (Fig 5B). These cell lines consisted of immature mast cells as determined by morphology, ultrastructure, and other characteristics such as the presence of receptors for IgE (Fig 5D and E). Similar mast cells could be maintained for some time in nontransgenic cultures, but invariably eventually died (Fig 5 and Table 2) and never yielded a continuous cell line. These cells resembled very closely the previously described “P cells” which persist in cultures supplemented with IL-3 for up to 3 months, but eventually die without immortalization.29,32 33Because we likewise did not observe immortalization in control cultures, the frequency of immortalization of nontransgenic cells could only be estimated. An estimate would be that this frequency is less than 2.5 × 10-8 because no immortalized cell lines arose from a total of eight nontransgenic cultures initiated with 5 × 106 cells each (4 × 107 initial cells; Table 2). In contrast to the results with nontransgenic cultures, a cell line arose each of nine times that nonadherent cells from a transgenic mouse were maintained in the presence of IL-3 (Table2). These cell lines expressed the Mcl-1 transgene (Fig 1E), proliferated continuously provided IL-3 was present, and could be maintained in culture for an indefinite period (>1 year and >70 population doublings).
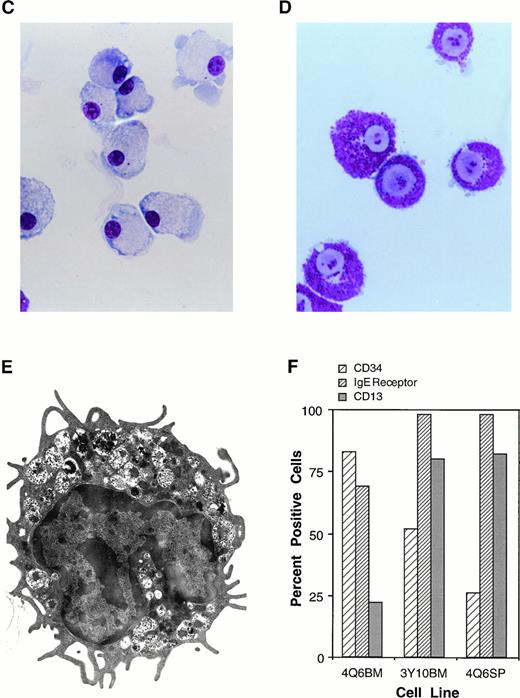
The Mcl-1 transgene promotes immortalization of monocytic and mast cell lines. (A and B) Establishment of cell lines from splenocytes and bone marrow cells from Mcl-1 transgenic mice. Cells from a transgenic and a nontransgenic mouse were cultured in long-term culture medium (containing IL-3 as described in Materials and Methods) and assayed at approximately weekly intervals for the total number of viable cells derived from the original culture, which contained a total of 5 × 106 cells. (C) Light microscopic view of a monocytic cell line (stained with Wright’s Giemsa, original magnification × 630). Histochemical staining showed this line to be strongly positive for alpha-naphthyl butyrate esterase activity, weakly positive for chloroacetate esterase activity, and negative for myeloperoxidase activity. (D) Light microscopic view of a mast cell line (stained with Wright’s Giemsa, original magnification × 800). (E) Electron microscopic view of a mast cell line (original magnification × 8,300) showing characteristics typical of immature mast cells, including large, often lobulated, nuclei and cytoplasmic granules containing a central dense core and a mixture of particles and vesicles.57 63 (F) Characterization of mast cell lines. Cell surface markers and histochemical staining properties were assayed for the indicated three cell lines. The percentage of c-kit+ cells was high in all cell lines (99%), as was the percentage of Sca1+ cells (89% for 4Q6BM, 90% for 3Y10BM, and 97% for 4Q6SP). Negligible percentages of cells expressed CD11b, Thy 1.2, CD3, CD5, or B220. Histochemical staining for chloroacetate esterase activity showed the following: 4Q6BM exhibited staining in the majority of cells (varying intensity in different cells); 3Y10BM contained a mixture of negative and weakly positive cells; and 4Q6SP contained mostly negative cells, with a minority of cells exhibiting weakly positive staining. The three cell lines were negative for myeloperoxidase and alpha-naphthyl butyrate esterase activities.
The Mcl-1 transgene promotes immortalization of monocytic and mast cell lines. (A and B) Establishment of cell lines from splenocytes and bone marrow cells from Mcl-1 transgenic mice. Cells from a transgenic and a nontransgenic mouse were cultured in long-term culture medium (containing IL-3 as described in Materials and Methods) and assayed at approximately weekly intervals for the total number of viable cells derived from the original culture, which contained a total of 5 × 106 cells. (C) Light microscopic view of a monocytic cell line (stained with Wright’s Giemsa, original magnification × 630). Histochemical staining showed this line to be strongly positive for alpha-naphthyl butyrate esterase activity, weakly positive for chloroacetate esterase activity, and negative for myeloperoxidase activity. (D) Light microscopic view of a mast cell line (stained with Wright’s Giemsa, original magnification × 800). (E) Electron microscopic view of a mast cell line (original magnification × 8,300) showing characteristics typical of immature mast cells, including large, often lobulated, nuclei and cytoplasmic granules containing a central dense core and a mixture of particles and vesicles.57 63 (F) Characterization of mast cell lines. Cell surface markers and histochemical staining properties were assayed for the indicated three cell lines. The percentage of c-kit+ cells was high in all cell lines (99%), as was the percentage of Sca1+ cells (89% for 4Q6BM, 90% for 3Y10BM, and 97% for 4Q6SP). Negligible percentages of cells expressed CD11b, Thy 1.2, CD3, CD5, or B220. Histochemical staining for chloroacetate esterase activity showed the following: 4Q6BM exhibited staining in the majority of cells (varying intensity in different cells); 3Y10BM contained a mixture of negative and weakly positive cells; and 4Q6SP contained mostly negative cells, with a minority of cells exhibiting weakly positive staining. The three cell lines were negative for myeloperoxidase and alpha-naphthyl butyrate esterase activities.
Cell Lines Established From Mcl-1 Transgenic Mice
| Cell Line* (tissue of origin) . | Months in Culture (months survival of nontransgenic control) . |
|---|---|
| Nonadherent mast cell lines | |
| UU3SP (spleen)1-153 | 5† (<1) |
| O4SP (spleen) | 6† (1.51-155) |
| I2SP (spleen) | 2† (1.51-155) |
| 4Q6SP (spleen) | 22† (3) |
| 4Q6BM (bone marrow)1-153 | 22† (4) |
| 3Y10BM (bone marrow) | 7† (<1) |
| 4W2BM (bone marrow) | 6† (3) |
| 5C2BM (bone marrow) | 13‡ (5) |
| 5B4BM (bone marrow) | 13‡ (6) |
| Adherent monocytic cell lines | |
| 4Q6BM-Adh (bone marrow)1-153 | 8‡ (4) |
| UU3SP-Adh (spleen)1-153 | 18‡ (<1) |
| E2SP-Adh (spleen) | 24‡ (ND) |
| Cell Line* (tissue of origin) . | Months in Culture (months survival of nontransgenic control) . |
|---|---|
| Nonadherent mast cell lines | |
| UU3SP (spleen)1-153 | 5† (<1) |
| O4SP (spleen) | 6† (1.51-155) |
| I2SP (spleen) | 2† (1.51-155) |
| 4Q6SP (spleen) | 22† (3) |
| 4Q6BM (bone marrow)1-153 | 22† (4) |
| 3Y10BM (bone marrow) | 7† (<1) |
| 4W2BM (bone marrow) | 6† (3) |
| 5C2BM (bone marrow) | 13‡ (5) |
| 5B4BM (bone marrow) | 13‡ (6) |
| Adherent monocytic cell lines | |
| 4Q6BM-Adh (bone marrow)1-153 | 8‡ (4) |
| UU3SP-Adh (spleen)1-153 | 18‡ (<1) |
| E2SP-Adh (spleen) | 24‡ (ND) |
Abbreviation: ND, not determined.
A cell inoculum containing a total of 5 × 106 cells (in 10 mL) was maintained in standard medium (10% FBS) for 4 days, and then continuously cultured in the presence of IL-3 as described in Materials and Methods, except for cell lines 5C2BM and 5B6BM which were placed directly into IL-3–containing medium.
Transgenic lines terminated voluntarily.
Transgenic lines still in culture.
The pair of clones UU3SP and UU3SP-Adh, as well as the pair 4Q6BM and 4Q6BM-Adh, were derived by separately culturing adherent versus nonadherent cells from a single mouse.
Cells were cultured simultaneously from two separate transgenic mice, with a single nontransgenic mouse serving as the control for both.
Whereas cell lines arose each time transgenic cells were maintained in culture, Mcl-1 did not seem to cause direct immortalization of all of the cells in the culture. Thus, during the initial 1 to 2 months in culture, little or no net increase in cell number occurred (Fig 5A and B); only after this “crisis”-like period did exponential growth become apparent. In principle, a cell line can arise out of a single immortalized cell. Therefore, to estimate the fraction of transgenic cells subject to immortalization in the presence of Mcl-1, we plated spleen cells in semisolid medium and picked 60 individual, macroscopically visible clones from two separate transgenic mice. On transfer to liquid culture, five of these clones were propagated continuously. Nontransgenic clones did not develop into cell lines, as expected from the above results with bulk cultures. The frequency of immortalization of transgenic cells was then estimated to be in the range of 3 × 10-5 (approximately 30 cells/106 transgenic spleen cells), based on the above-described finding that approximately 0.037% of transgenic spleen cells formed macroscopically visible colonies and that approximately 8% (5 of 60) of these were capable of immortalization. Given an immortalization frequency for nontransgenic cells of less than 2.5 × 10-8, immortalization in Mcl-1 transgenic cultures was estimated to be increased by a factor of greater than 1,000-fold.
In addition to the above mast cell lines obtained when nonadherent transgenic cells were maintained in IL-3 (and adherent cells were discarded), we observed monocytic cell lines to arise in three experiments in which adherent cells were maintained separately in bulk culture in the presence of IL-3 (nonadherent cells being discarded as described in Materials and Methods; Fig 5C and Table 2). In contrast, we did not observe cell lines to arise in the presence of IL-7. Thus, beyond enhancing the survival of myeloid cells, Mcl-1 allowed for the immortalization of mast and monocytic cells on incubation in the presence of IL-3.
DISCUSSION
In the work described here, we prepared mice that expressed Mcl-1 as a transgene in hematopoietic and lymphoid tissues. Initial observations of these mice showed moderate splenic enlargement, which was associated with increased numbers of B and T lymphocytes. The transgenic mice also exhibited expansion of the myeloid relative to the lymphoid compartment in the bone marrow. On explantation into tissue culture, mature B, T, and myeloid cells, as well as immature colony-forming cells, showed enhanced survival. The finding of lymphoid and myeloid expansion in the spleen and bone marrow, respectively, could reflect the fact that much lymphoid expansion normally occurs in the spleen whereas much myeloid expansion normally occurs in the bone marrow. In other words, the Mcl-1 transgene amplified existing hematopoietic processes. The increased yield of colony-forming cells, observed with both spleen and bone marrow, probably also reflects the ability of Mcl-1 to amplify hematopoiesis. Although viability enhancement by the Mcl-1 transgene was observed in a spectrum of cell types at various stages of differentiation, this effect was generally moderate (cell survival on the order of several days to a week), mirroring previous results in transfected cell lines.2,3 In addition, Mcl-1 did not interfere with the ability of these cells to undergo differentiation, paralleling similar observations with Bcl-2.19 52
The Mcl-1 transgene seemed to exert its effects within the context of other homeostatic regulators that influence cell viability. For example, whereas Mcl-1 enhanced the viability of a variety of hematopoietic cells, T cells lived longer than B cells and cells bearing macrophage markers could live longer than either of these. In a similar vein, the enhanced viability observed in cells from the spleen and bone marrow was not reflected in any change in peripheral blood pools. These findings suggested that the final outcome, in terms of viable cell number, depended on the combined action of Mcl-1 plus additional endogenous factors, where such homeostatic factors were capable of adjusting to the effects of the transgene. A similar phenomenon has been observed with Bcl-2, where expression of the transgene in T cells caused partial inhibition of negative selection in the thymus although self-reactive cells did not reach the periphery.12,53 Likewise, expression of the Bcl-2 transgene in myeloid cells enhanced survival in vitro but did not increase pool sizes in vivo.54 Overall, the effects of the Bcl-2 family members in vivo are subject to normal homeostatic influences even under conditions of enforced expression.
In addition to moderate survival-enhancing effects in a broad spectrum of cell types, Mcl-1 had effects capable of resulting in a striking alteration in cell fate when myeloid cells were cultured in the presence of IL-3. The presence of the transgene resulted in an increased probability of the outgrowth of continuously proliferating cell lines. We first observed this phenomenon serendipitously, when nonadherent spleen were maintained in the presence of IL-3. With bulk nonadherent cell cultures, an immature mast cell line arose from each transgenic culture, but not from the parallel nontransgenic control. By picking individual clones from semisolid medium, we found that a significant fraction of the transgenic cells that formed macroscopically visible colonies could grow continuously as cell lines (>5%). The overall frequency of immortalization was estimated to be in the range of 3 × 10-5, in contrast to a frequency of less than 2.5 × 10-8 in nontransgenic cultures. The immature mast cells we observed are very similar to the P cells initially characterized by Schrader et al.32,33 P cells are immature mast cells that persist in tissue culture in the presence of IL-3 (or IL-3 plus stem cell factor55-58), but survive for a maximum of only several weeks or months.30,32 Whereas cells persist for only a limited time when mouse hematopoietic cells are cultured directly into IL-3–containing medium, continuous cell lines do arise when cells are initially maintained in Dexter-type long-term culture, where the cell inoculum is incubated for several months in contact with a complex stromal layer.30 59 The Mcl-1 transgenic cell lines arose without the use of a Dexter-type long-term culture. Thus, Mcl-1 may have substituted for the effects of the long-term culture environment. It will be interesting to determine whether or not the Mcl-1 transgenic lines are tumorigenic, because immortalized cells from Dexter-type cultures generally are not. Overall, either the Mcl-1 transgene or the long-term culture environment may provide a survival advantage that results in an enhanced probability of cell immortalization.
In addition to the Mcl-1 transgene, several other factors may have contributed to immortalization. These include the internal cell environment, additional genetic changes, and the external cell milieu. The importance of the internal cell environment is seen in the observation that cell lines of myeloid but not lymphoid origin were obtained with Mcl-1, contrasting with the pre-B or bipotential progenitor lines previously observed with Bcl-2 plus Myc.25,28 It will be interesting to determine whether immortalization with Mcl-1 is enhanced by oncogenes such as Myc, as is the case with Bcl-2. The importance of additional genetic changes is seen in the fact that immortalization in the presence of Mcl-1 did not arise as an invariable response to the introduced gene. The importance of the external cell milieu is seen in the fact that Mcl-1 transgenic cell lines arose in the presence of IL-3 (but not IL-7) and in the absence of endogenous homeostatic mechanisms that normally modulate Bcl-2 family responses. The external environment (in this case, the stromal layer) was similarly found to be critical in some cases of immortalization with Bcl-2 plus Myc.28
Members of the Bcl-2 family normally act in specific cell types at specific stages of differentiation. Indeed, one model for hematopoietic cell amplification is that regulation may be exerted at a series of viability gates present at critical points along the differentiation continuum. These gates can be visualized as being under the control of growth and differentiation factors, many of which regulate the expression of Bcl-2 family members (eg, upregulation of Bcl-2 by IL-7,21 A1 by granulocyte-macrophage–CSF,60 Bcl-x by CD40,15 and Mcl-1 by lymphocyte-conditioned medium5). In other words, growth differentiation factor–induced expression of viability-enhancing genes is postulated to allow cells to pass through the gate and to continue to proliferate and differentiate. A network of such gates would allow for a fine level of control over cell amplification. This would also allow for rapid changes in response to external stimuli, such as an increased or decreased requirement for additional cells of a particular type. Finally, the existence of successive gates would allow errors or miscalculations from early gates to be corrected at subsequent gates. Seen in the context of this model, the Mcl-1 transgene studied here enhanced the ability of cells to pass through many of these viability gates, but did not eliminate the self-correcting feature of the system as a whole. However, when cells expressing the Mcl-1 transgene were removed from the endogenous constraints inherent in the system and placed in a conducive growth factor-rich environment, self correction was at least partially abrogated, allowing for eventual cell immortalization and paralleling results with other genes.61 62
In summary, Mcl-1 had moderate viability-enhancing effects in a spectrum of hematopoietic cells in transgenic animals, but acted within the context of the overall homeostatic regulation of viable cell number. In addition, in specific types of myeloid cells, the presence of Mcl-1 allowed for a high probability of immortalization when cells were placed in a conducive environment in isolation from homeostatic regulatory controls. This ability to have a long-term impact on cell fate, shown under specific intra- and extracellular conditions, may be more important in terms of diseases such as cancer than short-term viability enhancement, which is observed with Bcl-2 family members in a host of cell types and under a wide variety of conditions.
ACKNOWLEDGMENT
We thank Dr John Gearhart for expert advice in the early stages of this project. We thank Drs Christopher Lowrey, Paul Wallace, and Nancy Speck for their thoughtful reading of the manuscript. The transgenic founder mice were prepared by DNX corporation. Electron microscopy was by Louisa Howard and Charles Daghlian.
Supported by a grant from the National Cancer Institute (R01-CA57359). The cloning of the Mcl-1 gene used in preparing the transgenic mice was supported by R29-CA54385.
Address reprint request to Ruth W. Craig, PhD, Department of Pharmacology and Toxicology, HB 7650, Dartmouth Medical School, Hanover, NH 03755.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. The protein product of the Mcl-1 mini-transgene is prominently expressed in hematolymphoid tissues. (A) The Mcl-1 mini-gene construct used in the generation of transgenic mice (pSS17.5 plasmid) contains all exons (▪, including the 3′-untranslated region [▧] and introns (□) of human Mcl-1. It also contains Mcl-1 5′- and 3′-genomic flanking regions (▨). Fragments 1, 2, and 3 are genomic subclones used in making the transgene construct. Restriction sites are indicated as follows: S, SalI; X,XhoI; B, BamHI; and Xb, XbaI. The BS3.0 genomic subclone shown was used as probe to identify transgenic mice by Southern blotting. (B) Expression of the Mcl-1 transgene was assayed in a variety of tissues by Western blotting. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 50 μg of protein, except lane 13 which represents 100 μg of protein. (C) Expression of the human Mcl-1 transgene in the spleen and lymph node (L.N.) of a transgenic mouse (Tr.) was assayed in parallel with human lymph node. ML-1 cells incubated with 5 × 10-10 mol/L TPA for 3 hours served as a positive control. Each lane represents 100 μg of protein, except lane 4 which represents 50 μg of protein. (D) Expression of the Mcl-1 transgene was assayed in enriched T- and B-cell populations from transgenic mouse spleen. Unfractionated spleen from a transgenic (Tr.) and a nontransgenic (Non-Tr.) mouse, as well as ML-1 cells incubated in the presence or absence of 5 × 10-10 mol/L TPA for 3 hours served as controls. Each lane represents 50 μg of protein. (E) Expression of the Mcl-1 transgene was assayed in cell lines derived from cells from the spleen or bone marrow of transgenic mice. Lanes 1 through 3 (mast cell lines) represent 1 × 106 cells and lane 4 represents 5 × 105 cells. Lanes 5 and 6 (monocytic cell lines) and lane 7 represent 50 μg protein.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3226/5/m_blod42149001w.jpeg?Expires=1769234407&Signature=Gfv~hVk0q13a7tblT6tdLd8rSCXGON~e1k027wSudR16VhqK3Yoci1ZYBhsQyG2TSZoo7e9W6hLni1OzzCodfK1dzCtnneXbQyqtKy7w2O51saHOSzmukK6UPPbDU0jiJ3AnmYeBTAAL-4Gf7CXlIGfzPeed7nMhbdZAgA2DKVzo2x27isw2bTxPoS-G3iza55EK5Kr3UXts-n~x90zFy3kHYEb1qliIzjlduwWI6l4yW4mpaXP8uZcAFM-WMslpyJp5wB-JASaeB2LA9~dBrwXhXO0ZV11Tjfu4sHB79KptYKRVHB9-GFEi2KhbxTV5Z3Sm-qbpbG6493apEgD4CQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal