Abstract
Results to date indicate that high-dose therapy (HDT) with autologous stem cell support improves survival of patients with symptomatic multiple myeloma (MM). We performed a multicenter, sequential, randomized trial designed to assess the optimal timing of HDT and autotransplantation. Among 202 enrolled patients who were up to 56 years old, 185 were randomly assigned to receive HDT and peripheral blood stem cell (PBSC) autotransplantation (early HDT group, n = 91) or a conventional-dose chemotherapy (CCT) regimen (late HDT group, n = 94). In the late HDT group, HDT and transplantation were performed as rescue treament, in case of primary resistance to CCT or at relapse in responders. PBSC were collected before randomization, after mobilization by chemotherapy, and, in the two groups, HDT was preceded by three or four treatments with vincristine, doxorubicin, and methylprednisolone. Data were analyzed on an intent-to-treat basis using a sequential design. Within a median follow-up of 58 months, estimated median overall survival (OS) was 64.6 months in the early HDT group and 64 months in the late group. Survival curves were not different (P = .92, log-rank test). Median event-free survival (EFS) was 39 months in the early HDT group whereas median time between randomization and CCT failure was 13 months in the late group. Average time without symptoms, treatment, and treatment toxicity (TWiSTT) were 27.8 months (95% confidence interval [CI]; range, 23.8 to 31.8) and 22.3 months (range, 16.0 to 28.6) in the two groups, respectively. HDT with PBSC transplantation obtained a median OS exceeding 5 years in young patients with symptomatic MM, whether performed early, as first-line therapy, or late, as rescue treatment. Early HDT may be preferred because it is associated with a shorter period of chemotherapy.
© 1998 by The American Society of Hematology.
FOR THE LAST 30 YEARS, the mainstay of treatment in multiple myeloma (MM) has been melphalan and prednisone.1 With this treatment or other standard-dose drug combinations, the median survival is less than 3 years and may reach 5 years only in selected responder patients.2Complete remission (CR) is rare and less than 5% of patients live longer than 10 years.
Evidence of a dose-response effect for alkylating agents3has prompted studies of high-dose therapy (HDT) regimens followed by transplantation of syngeneic, allogeneic, or autologous bone marrow (BM) or peripheral blood stem cell (PBSC).4-8Transplantation from an allogeneic donor may have the advantage over autotransplantation of a potential “graft versus myeloma” effect, but the procedure still has a high level of related mortality.9
The phase II studies that have assessed HDT and autotransplantation in MM patients reported high response rates with 20% to 50% of apparent CR, and suggested a survival benefit over conventional-dose chemotherapy (CCT).10-13 This was recently confirmed by Attal et al,14 who randomly compared HDT plus autologous BM transplantation to CCT.
Since 1986, we have performed HDT and transplantation in myeloma patients using autologous PBSC rather than BM stem cells.15In 1990, considering that most young patients with symptomatic MM should receive HDT, either at diagnosis or when the disease would be refractory to a first-line CCT regimen, we initiated a multicenter sequential randomized clinical trial designed to assess the optimal timing of HDT and PBSC autotransplantation. We report the results of this trial in 202 previously untreated patients.
MATERIALS AND METHODS
Patients.
Eligibility criteria included age less than 56 years and symptomatic MM. Patients were excluded if they had the following: (1) stable stage I MM (Durie and Salmon classification); (2) previous cytotoxic chemotherapy (other than one course of steroids and/or alkylating agents) or radiotherapy (other than a local irradiation not contraindicating further total body irradiation [TBI]); (3) severe abnormalities of cardiac, pulmonary, and hepatic functions; (4) serum creatinine level >300 μmol/L. All patients gave informed consent and the study was approved by an institutional ethics committee.
PBSC collection.
After enrollment, PBSC were collected by cytapheresis during recovery from short-term aplasia induced by a reinforced CHOP regimen (cyclophosphamide [CY] 1,500 mg/m2, doxorubicin 90 mg/m2, vincristine 1.4 mg/m2, and steroids).15 Evaluation of apheresis products was performed using an in vitro assay for granulocyte-macrophage colony-forming units (CFU-GM). Quantification of CD34+hematopoietic progenitor cells was not performed routinely. A second CHOP course, combined with granulocyte colony-stimulating factor (G-CSF, 5 μg/kg) since 1993, was administered eventually to obtain a sufficient number of PBSC (at least 10 × 104CFU-GM/kg of body weight).
Randomization.
After PBSC collection patients were randomly assigned to one of the two HDT groups, provided that (1) sufficient quantities of PBSC had been collected, and (2) kidney or other organ compromise did not occur during PBSC collection. Randomization was stratified according to the center and was carried out by telephone.
Early HDT arm.
After PBSC collection, patients included in the early HDT arm received three or four monthly courses of VAMP, combining on days 1 to 4 continuous 24-hour infusion of vincristine (0.4 mg/d) and doxorubicin (9 mg/m2/d) and intravenous (IV) methylprednisolone (0.4 g/d). HDT and transplantation followed VAMP courses regardless of the disease response. Pretransplant cytoreduction combined a multidrug regimen (lomustine orally 120 mg/m2 day −8, VP16 250 mg/m2 day −8 to −6, CY 60 mg/kg day −5, and melphalan 140 mg/m2 day −4) with TBI (1,200 cGy [800 cGy to the lung] in six fractions on days −3 to −1 or 1,000 cGy during a 6-hour period on day −2). Previously harvested PBSC were reinfused at day 0. HDT was performed in protected units.
Late HDT arm.
After PBSC collection, patients included in the late HDT arm received monthly courses of the VMCP regimen that combined vincristine (1.4 mg/m2 IV on day 1), melphalan (6 mg/m2 orally on days 1 to 4), CY (600 mg/m2 IV on day 1), and prednisone (80 mg/m2 orally on days 1 to 4). In patients who achieved at least partial response, VMCP courses were pursued until a stable plateau phase was reached (defined below).
Monthly courses of VAMP followed by HDT and transplantation, according to the same protocol as used in the early HDT arm, were performed as rescue treatment, in case of disease progression on VMCP, in case of disease resistance after 6 courses of VMCP, or in case of relapse in responders.
Interferon (IFN).
Treatment with recombinant IFN-α (3 × 106U subcutaneously thrice weekly) was proposed in both arms to all patients in CCT or HDT-induced remission. IFN was discontinued, at the physician’s discretion, when it induced persisting side effects.
Response criteria.
Disease response was defined as follows: (1) CR: 5% or fewer plasma cells of normal morphology on BM smears and absence of monoclonal Ig (MIg) by immunochemical analysis (including immunofixation) of serum and of 100-fold concentrated urine samples. (2) Minimal residual disease (MRD): 5% or fewer plasma cells on BM smears associated with a decrease in the MIg level of at least 90%. (3) Partial response (PR): greater than 50% decrease in serum MIg and/or greater than 75% decrease in urinary Bence Jones (BJ) protein levels. (4) Resistant disease: absence of a decrease in serum MIg and urinary BJ protein levels of at least 50% and 75%, respectively, and absence of signs of disease progression. (5) Progressive disease: greater than 25% increase in MIg level or occurrence of hypercalcemia or of a plasma cell tumor.
A stable plateau phase was considered to exist in patients who achieved at least PR, when three consecutive measurements of MIg concentration 2 months apart varied by less than 20%. Relapse was defined by the reappearance of MIg, a greater than 25% increase in its level, or by other unequivocal signs of disease progression such as hypercalcemia or plasma cell tumor.
Statistical analysis.
The main endpoint was overall survival (OS) from randomization, regardless of the cause of death. Secondary endpoints were event-free survival (EFS) and time without symptoms, treatment, and treatment toxicity (TWiSTT) adapted from Cole et al.16
The study was conducted as a sequential trial using a triangular test design, performed as previously described,17 with accumulated data examined after 10 deaths. The study was designed to have a type I error of 5% with a power of 80% to detect a 20% reduction in the mortality rate among early HDT compared with late HDT patients.
All comparisons were on an intention-to-treat basis. Survival curves were estimated by the Kaplan-Meier method and compared with the use of the log-rank test, using September 1, 1997 as the reference date. The SAS (SAS Institute, Cary, NC) software package was used.
RESULTS
The trial began in January 1990. In April 1995, at the time of the fourth sequential analysis, 179 patients were randomized, 88 and 91 in the early and late HDT groups, respectively. Forty (21 and 19) deaths were reported. In the triangle test, the lower triangular boundary was crossed, indicating that there was less than a 20% benefit in survival with early as compared to late HDT (P < .05). Accordingly, accrual was stopped in June 1995.
Patient characteristics.
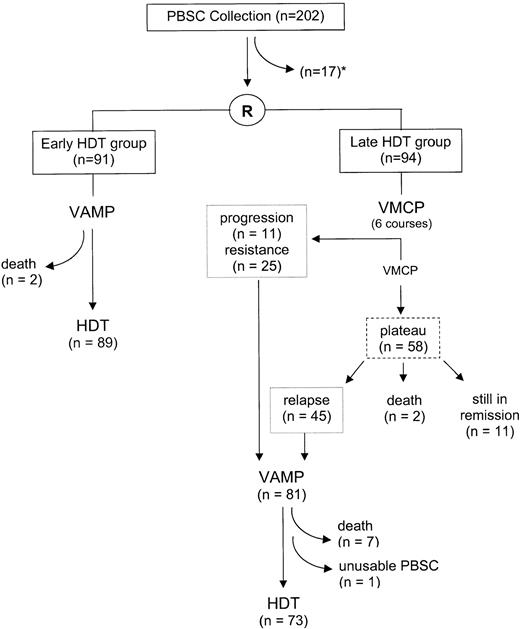
In June 1995, 202 patients were included in 14 centers. Seventeen enrolled patients did not proceed to randomization because of death (n = 4), severe infectious complications (n = 2) during the CHOP-induced aplasia, renal failure (n = 2), insufficient PBSC quantities (n = 7, including 6 who did not receive G-CSF), protocol violation, or patient’s decision (n = 2) (Fig 1). Baseline characteristics of the early (n = 91) and late (n = 94) HDT groups were close, except for myeloma isotype distribution (Table 1). Median durations between PBSC mobilization and randomization were 35 days (range, 25 to 107) and 38 days (range, 23 to 157), respectively.
Main Baseline Characteristics of the 185 Randomized Patients, According to Treatment Group
| . | Early HDT Group (n = 91) . | Late HDT Group (n = 94) . |
|---|---|---|
| Age (yr) | 48 ± 5 | 47 ± 5 |
| Male gender | 39 (43) | 42 (45) |
| Durie-Salmon stage | ||
| I-150 | 3 (3) | 2 (2) |
| II | 9 (10) | 15 (16) |
| III | 79 (87) | 76 (82) |
| M component (MIg) | ||
| IgG | 50 (55) | 39 (41) |
| IgA-151 | 13 (14) | 34 (36) |
| IgM | 1 (1) | 0 |
| IgD | 3 (3) | 5 (5) |
| BJ protein only-151 | 21 (23) | 13 (14) |
| None | 3 (3) | 3 (3) |
| Hemoglobin (g/L) | 107 ± 22 | 107 ± 21 |
| ≤100 g/L | 30 (33) | 36 (38) |
| Serum calcium (mmol/L) | 2.5 ± 0.4 | 2.6 ± 0.7 |
| >2.6 mmol/L | 22 (24) | 20 (21) |
| Serum albumin (g/L) | 39.6 ± 6.6 | 39.0 ± 6.3 |
| Serum creatinine (μmol/L) | 112 ± 58 | 128 ± 117 |
| >150 μmol/L | 14 (15) | 15 (16) |
| BM plasmocytosis (%) | 36 ± 23 | 34 ± 24 |
| Serum β2microglobulin (mg/L) | 3.8 ± 2.9 | 3.7 ± 2.4 |
| ≥3 mg/L | 41 (46) | 46 (51) |
| CRP (mg/L)-152 | 7.0 ± 12.1 | 8.2 ± 14.1 |
| >6 mg/L | 11 (22) | 16 (27) |
| . | Early HDT Group (n = 91) . | Late HDT Group (n = 94) . |
|---|---|---|
| Age (yr) | 48 ± 5 | 47 ± 5 |
| Male gender | 39 (43) | 42 (45) |
| Durie-Salmon stage | ||
| I-150 | 3 (3) | 2 (2) |
| II | 9 (10) | 15 (16) |
| III | 79 (87) | 76 (82) |
| M component (MIg) | ||
| IgG | 50 (55) | 39 (41) |
| IgA-151 | 13 (14) | 34 (36) |
| IgM | 1 (1) | 0 |
| IgD | 3 (3) | 5 (5) |
| BJ protein only-151 | 21 (23) | 13 (14) |
| None | 3 (3) | 3 (3) |
| Hemoglobin (g/L) | 107 ± 22 | 107 ± 21 |
| ≤100 g/L | 30 (33) | 36 (38) |
| Serum calcium (mmol/L) | 2.5 ± 0.4 | 2.6 ± 0.7 |
| >2.6 mmol/L | 22 (24) | 20 (21) |
| Serum albumin (g/L) | 39.6 ± 6.6 | 39.0 ± 6.3 |
| Serum creatinine (μmol/L) | 112 ± 58 | 128 ± 117 |
| >150 μmol/L | 14 (15) | 15 (16) |
| BM plasmocytosis (%) | 36 ± 23 | 34 ± 24 |
| Serum β2microglobulin (mg/L) | 3.8 ± 2.9 | 3.7 ± 2.4 |
| ≥3 mg/L | 41 (46) | 46 (51) |
| CRP (mg/L)-152 | 7.0 ± 12.1 | 8.2 ± 14.1 |
| >6 mg/L | 11 (22) | 16 (27) |
Mean values (±SD) are given for continuous variables, while numbers of patients (percentages) are given otherwise.
All progressive, ie, >25% increase in MIg level confirmed by at least 3 consecutive measurements 2 months apart.
P value = .22 and .13 by the Fisher’s exact two-sided test, for MIgA and BJ, respectively.
Based on 108 assessed patients, 49 in the early HDT group and 59 in the late group.
Overall survival.
At the reference date of September 1, 1997, the median follow-up was 58 months (range, 25 to 91). Among the 17 eligible patients who could not be randomized, 14 died. The median OS of the 202 enrolled patients was 64 months (95% CI; range, 52 to 76) since PBSC collection.
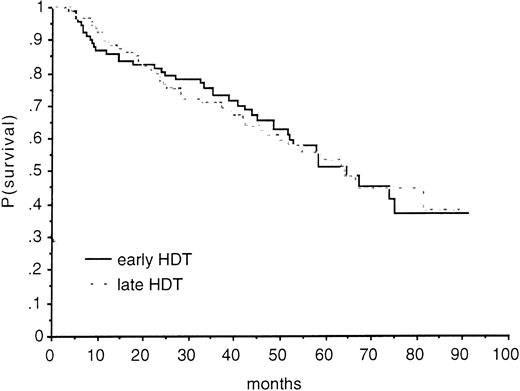
Forty-one and 42 patients died in the early and late HDT groups, respectively. There was no significant improvement in survival in the early HDT group compared with the late group (relative risk of death in the late HDT group, 1.02; 95% CI, 0.67 to 1.57; P = .92 by the log-rank test) (Fig 2). Because of the different isotype distribution between the two groups (Table 1), adjusted comparison for IgA was performed but did not modify the results.
OS according to treatment group. At 24 months, the estimated survival rate was 80% (95% CI; range, 72% to 88%) in the early HDT group and 78% (range, 70% to 86%) in the late group; at 36 months, it was 73% (range, 64% to 82%) and 71% (range, 62% to 80%). At 48 months, these figures were 66% (range, 56% to 76%) and 61% (range, 51% to 71%), respectively.
OS according to treatment group. At 24 months, the estimated survival rate was 80% (95% CI; range, 72% to 88%) in the early HDT group and 78% (range, 70% to 86%) in the late group; at 36 months, it was 73% (range, 64% to 82%) and 71% (range, 62% to 80%). At 48 months, these figures were 66% (range, 56% to 76%) and 61% (range, 51% to 71%), respectively.
EFS and TWiSTT.
At the reference date, relapse or death had occurred in 58 patients of the early HDT group and the median EFS was 39 months (95% CI; range, 29 to 48). In the late HDT group, the median interval between randomization and VMCP failure or death (post-CCT EFS) was 13 months (95% CI; range, 9.4 to 17.6) (83 events including 2 deaths on VMCP). The median interval between randomization and post-HDT relapse or death (post-HDT EFS) was 50 months (95% CI; range, 37.5 to 63.2).
During the median follow-up of 58 months, the average TWiSTT results were 27.8 months (95% CI; range, 23.8 to 31.8) and 22.3 months (95% CI; range, 16.0 to 28.6) in the early and late HDT groups, respectively (Fig 3).
Partitioned Kaplan-Meier survival curves according to treatment group, ie, early HDT group (top plot) and late HDT group (bottom plot). Each plot displays the Kaplan-Meier estimations of time to OS, EFS, and time to end-of-treament, either conventional chemotherapy (CCT) or transplantation (HDT), since randomization. Note that two EFS were considered in the late HDT group (after conventional chemotherapy, “post-CCT” and after transplantation, “post-HDT”). The areas between these curves and the vertical line at 58 months, which corresponds to the median follow-up of the whole cohort, represent estimates of the mean durations between these events, namely treatment duration (either CCT [▧] or HDT [⊞]), time without symptoms and treatment toxicity (TWISTT [▧]), and time between relapse and death (▨). All patients were included in the analysis on an intent-to-treat basis. IFN was not taken into account because it was usually maintained only when well tolerated.
Partitioned Kaplan-Meier survival curves according to treatment group, ie, early HDT group (top plot) and late HDT group (bottom plot). Each plot displays the Kaplan-Meier estimations of time to OS, EFS, and time to end-of-treament, either conventional chemotherapy (CCT) or transplantation (HDT), since randomization. Note that two EFS were considered in the late HDT group (after conventional chemotherapy, “post-CCT” and after transplantation, “post-HDT”). The areas between these curves and the vertical line at 58 months, which corresponds to the median follow-up of the whole cohort, represent estimates of the mean durations between these events, namely treatment duration (either CCT [▧] or HDT [⊞]), time without symptoms and treatment toxicity (TWISTT [▧]), and time between relapse and death (▨). All patients were included in the analysis on an intent-to-treat basis. IFN was not taken into account because it was usually maintained only when well tolerated.
Completion of allocated treatment.
In the early HDT group, two patients died on VAMP, because of progressive disease in one, and infectious complications in the other. HDT was performed within a median of 4 months after randomization.
In the late HDT group, the median number of VMCP courses was 8 (range, 1 to 20). Thirty-six patients had progressive (n = 11) or resistant (n = 25) disease. Fifty-eight were considered as responders, of whom 2 committed suicide and 45 relapsed. Among the 81 patients who should have received HDT, 7 did not because of myeloma-related deaths on VAMP in 6 and lethal heart amyloidosis in 1. One additional patient could not be engrafted because of accidental thawing of PBSC. The median interval between randomization and HDT was 21.3 months.
During post-HDT relapse, patients received various CCT regimens. A second high-dose regimen was performed in 10 and 4 patients in the early and late HDT groups, respectively.
Treatment-related mortality (TRM) and hematopoietic engraftment.
In the early HDT group, TRM (including all deaths that occurred in the absence of overt myeloma relapse) during the first year after transplantation was 10% (9 of 89). Patients died because of pulmonary infection (4 cases), veno-occlusive disease (2 cases), brain hemorrhage (2 cases), and sudden death (1 case). For the 80 remaining patients, engraftment (leukocytes >1,000/μL and platelets >25,000/μL) occurred after a median of 12 days. Of note, one patient died 4 years after transplantation from acute myeloid leukemia.
In the late HDT group, TRM during the first year after transplantation was 14% (10 of 73). Patients died of infectious complications (n = 6), veno-occlusive disease (n = 2), or hemorrhage (n = 2). For the 63 surviving patients, engraftment occurred at a median of 12 days.
Response to therapy.
At six months after HDT, 78 patients in the early HDT group were in remission, including 17 in CR, 40 with MRD, and 21 in PR. Three patients achieved a minimal response.
In the late HDT group, 58 patients (62%) responded to VMCP (and CHOP). Among these, 5 achieved CR, 15 had MRD, and 38 were in PR. At analysis, 81 patients had reached requirement for HDT (Fig 1). Seven died on VAMP (including 1 from heart amyloidosis). After HDT, 60 had achieved a good response, including 8 who were in CR and 21 in MRD, and 6 had a resistant disease.
Among the 81 patients in the late group who had reached requirement for HDT, 45 patients were responders to VMPC (and CHOP) and were intended to be transplanted at relapse whereas 36 patients had a progressive or a resistant disease (Fig 1). Two-year survival since VAMP beginning were 59% (95% CI; 43% to 75%) and 57% (41% to 73%) for those who had a relapsing disease and for those who had a primary refractory disease, respectively.
Interferon treatment.
IFN was used in 60% of CCT-induced remissions and in 56% of posttransplant remissions of patients in the early HDT group. Median duration of IFN therapy was 13.7 months for patients in the early HDT group and 11.6 months in the late HDT group (P=.91).
DISCUSSION
Some controversies remain with respect to the role of HDT and autologous transplantation in the management of MM, including its benefit as compared with standard therapy, indications according to patients’ age and clinical status, modalities, and optimal timing. A recent randomized trial in part solved some of these issues, providing evidence that intensive treatment yields better results than conventional treatment.14
The main issue addressed by this study was the optimal timing of HDT and autotransplantation. Thus, PBSC collection was systematically performed after enrollment in an intent-to-treat all patients with HDT. In the early HDT group, 89 (of 91) patients actually received HDT, whereas in the late group, 81 (of 94) reached requirement for HDT, of whom 73 could be transplanted. Median intervals from randomization to transplantation were 4 and 21 months, respectively.
Analyzed on an intent-to-treat basis, OS was similar in the two groups, with a median duration exceeding 5 years. The triangular sequential design permitted termination of the trial on confident evidence of the absence of difference in survival.17 Main baseline patient characteristics were similar in the two groups except for myeloma isotype distribution, with more IgA MM in the late HDT group and more Bence Jones (and IgG) diseases in the early group. However, in contrast to some series18 but in accordance with others,19 there was no prognostic role of IgA isotype. Indeed, analysis of pre-enrollment parameters showed that β2 microglobulin, hemoglobin, calcium and creatinine serum levels, but no isotype, affected OS (data not shown). Adjusted comparison for IgA and other prognostic covariates eliminated potential bias due to any imbalance.
Median EFS in the early HDT group was 39 months, whereas in the late HDT group median time from randomization to death or standard chemotherapy failure (post-CCT EFS) was 13 months. However, the clinical significance of comparing these EFS is questionable, because patients in the late HDT group were systematically treated by HDT. The comparison of post-HDT EFS is also not very meaningful because of the different design of the two treatments including only one step in one and two in the other. Thus, we also evaluated time without symptoms, treatment (ie, chemotherapy), and treatment toxicity, adapted from the TWiST recently used in breast cancer and in human immunodeficiency virus infection.20 21 As shown in Fig 3, the distribution of the periods spent with or without chemotherapy was different between the two groups and the latter was longer in the early HDT group. This suggests a clinical benefit for early treatment but the repercussion on quality of life of each strategy may be variably appreciated by patients and physicians facing the choice between early or late HDT.
This trial confirms the value of autologous PBSC as the source of hematopoietic stem cells for HDT in myeloma patients.15 In this study, designed in 1989, PBSC were successfully collected in 92% of enrolled patients, although mobilizations were performed using chemotherapy alone, without the systematic use of G-CSF, the interest of which in increasing stem cell yield (but not tumoral cell contamination) is now well documented.22 PBSC collection was performed early in the disease course, which is crucial to avoid chemotherapy-induced hematopoietic stem cell damage with increasing risks of collection failure, delay in engraftment, and occurrence of myelodysplasia.23 Of note, however, one patient died from acute myeloid leukemia 4 years posttransplantation and additional follow-up is obviously needed in both arms, especially from the point of view of detecting secondary malignancies.
In this multicenter study, PBSC transplantation alone (without any hematopoietic growth factor) supported a highly intensive treatment, and TRM during the first posttransplant year were 9% and 14% in the early and late groups, respectively. Lower TRM rates were reported after transplantation following high-dose melphalan (HDM) alone or combined with TBI 850 cGy,14 but TRM within 12 months of first treatment was 7% in the series of patients treated with tandem transplants in one single institution recently reported by Vesole et al18 and Barlogie et al.24 In their series, median OS was estimated at 62 months, and whether two successive HDT regimens of relatively low intensity would improve OS compared with one more intensive treatment is open to question. Patients’ age was limited to 56 years in the present study. In older patients, HDM is feasible with stem cell support,24 but the increased risks incite investigators to reduce the intensity of the treatment, and actual benefit compared with standard therapy remains to be established.
In the present series, as in others,10-14,18 no discernible plateau was observed in OS curves, indicating that cure may be achieved in only a few, if any, patients. Several strategies are currently under evaluation to improve outcome of HDT and autografting. These include improvement of tumor mass reduction through multiple high-dose therapies,24,25 reinfusion of tumor-free grafts, which may be achieved by positive stem cell selection,26 27 and innovative options to eradicate minimal residual disease, especially stimulating autologous immunity to myeloma. We are presently evaluating sequential HDT and selected CD34+ PBSC in a randomized trial.
ACKNOWLEDGMENT
We are indebted to the patients who participated in the trial, to the attending physicians who referred their patients to our centers, to M. Bargis-Touchard and K. Le Moal for secretarial assistance, and to C. Chastang for statistical support.
APPENDIX
The following investigators also participated in the trial: A. Bisagni, D. Bouscary, M.C. Quarre: Hôpital Cochin, Paris, France; F. Beaujan, X. Chevalier, M. Kuentz, C. Rieux: Hôpital Henri-Mondor, Créteil, France; P. Bourgeois, J.P. Marre, S. Rozenberg: Hôpital Pitié-Salpétrière, Paris, France; M. Le Porrier, X. Troussard: Centre Hospitalier, Caen, France; A.M. Penit: Centre A. Baclesse, Caen, France; M.F. Kahn, E. Palazzo: Hôpital Bichat-Beaujon, Paris, France; T. André, M. Schlienger, Hôpital Tenon, Paris, France; Y. Kerneis, G. Philippe: Centre Hospitalier, Pontoise, France; S. Brechignac, P. Brice, J.P. Clauvel, C. Hennequin, J.P. Marolleau: Hôpital Saint Louis, Paris, France; A. Delmer: Hôtel Dieu, Paris, France; M. Janvier: Centre R. Huguenin, Saint Cloud, France; J. Dumont, J.M. Cosset, A. Fourquet, J.R. Vilcoq: Institut Curie, Paris, France; M. Abgrall: Hôpital Morvan, Brest, France; B. Pignon: Centre Hospitalier, Reims, France; D. Clerc: Hôpital Bicêtre, Paris, France; F. Lioté, J.M. Zini: Hôpital Lariboisière, Paris, France; B. Varet: Hôpital Necker, Paris, France.
J.-P.F. and P.R. have contributed equally to this work.
The other investigators who participated in the study are listed in the Appendix.
Address reprint requests to Jean-Paul Fermand, MD, Immuno-Haematology Unit, Hôpital Saint-Louis, 1, avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail:immuno-hem@chu-stlouis.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Partitioned Kaplan-Meier survival curves according to treatment group, ie, early HDT group (top plot) and late HDT group (bottom plot). Each plot displays the Kaplan-Meier estimations of time to OS, EFS, and time to end-of-treament, either conventional chemotherapy (CCT) or transplantation (HDT), since randomization. Note that two EFS were considered in the late HDT group (after conventional chemotherapy, “post-CCT” and after transplantation, “post-HDT”). The areas between these curves and the vertical line at 58 months, which corresponds to the median follow-up of the whole cohort, represent estimates of the mean durations between these events, namely treatment duration (either CCT [▧] or HDT [⊞]), time without symptoms and treatment toxicity (TWISTT [▧]), and time between relapse and death (▨). All patients were included in the analysis on an intent-to-treat basis. IFN was not taken into account because it was usually maintained only when well tolerated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3131/5/m_blod42130003x.jpeg?Expires=1769103791&Signature=cwX4yECiK3QbswGaEqpOXC323U5LfncY50f7cGGc~0GguI4o58Qp0F3I2Dk5uarxaO5nLzS8icDsI9CC1g7r5Gj4uLJx2JQEkybpERsvYT63yuZkYd6dC-NNt5VIgNq~jKHEfQLraNsBraMf3Lska2EwuquOvX1dBwNA8ZnodM2aNqat32kRZy8Xf3R1Z~ijkOAzMU8u2-MkF~8QfAmIZMzmTfV0BnByYY98tpoYDGE2hK1PiNX29T-k5wvW7DNapY0abWUnF6YQd5YHnpyK0C-FCMEe8LZG7ayFSxL-JaopYdsNryz4e1nbMOhEmDVglFHseMh7wR3yo8YCOtRVGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal