Abstract
Factor Va (FVa), derived from plasma or released from stimulated platelets, is the essential protein cofactor of the prothrombinase complex. Plasma-derived factor V (FV) is synthesized by the liver, whereas the source of the platelet-derived cofactor has not been unambiguously identified. Megakaryocytes, platelet precursors, are known to synthesize platelet proteins and to endocytose proteins from plasma (ie, fibrinogen) and then package these proteins into -granules. To determine which mechanism accounts for FV presence in platelets, two patients heterozygous for FVLeiden who underwent allogeneic transplantation from homozygous FV wild-type donors (bone marrow [BM] or liver) were studied. Patient JMW, whose skin biopsy specimen showed heterozygous FVLeiden, received a BM transplant from a wild-type homozygous FV donor as analyzed from posttransplant peripheral blood cells. Patient FW, whose native liver is heterozygous for FVLeiden, received a homozygous wild-type FV liver. Because each individual has two distinct genetic pools of factor V in liver and megakaryocytes, it was possible to determine whether secretable platelet-derived FV was normal or contained the FVLeiden mutation. Platelet-derived FVa released from thrombin-activated platelets from a normal individual, an individual heterozygous for the FVLeiden mutation, and the two patients was incubated with phospholipid vesicles and activated protein C (APC). Western blotting analyses using a monoclonal antibody that allows distinction between platelet-derived FVa and FVaLeiden subsequent to APC-catalyzed cleavage were then performed. Based on the accumulation of proteolytic fragments derived from APC-induced cleavage, analyses of platelet-derived FVa from JMW demonstrated both normal FVa and FVaLeiden consistent with a plasma-derived origin of the secretable platelet-derived FVa. Western blotting analyses of the APC-cleaved platelet-derived FVa from FW showed a wild-type phenotype, despite the presence of a FVLeiden allele in her megakaryocyte genome, also consistent with a plasma origin of her secretable platelet-derived FVa. Platelets do not appear to endocytose the plasma cofactor, because a 35-hour incubation of platelet-rich plasma with 125I-factor V showed no specific association/uptake of the radiolabeled ligand with the platelet pellet. Collectively, these results show for the first time that the majority of secretable platelet-derived factor V is endocytosed by megakaryocytes from plasma and is not exclusively synthesized by these cells, as previously believed.
© 1998 by The American Society of Hematology.
BLOOD COAGULATION factor Va is a heterodimer composed of a heavy chain (molecular weight [Mr] = 105,000) and a light chain (Mr = 74,000) which arises from limited proteolysis of the single-chain procofactor factor V (Mr = 330,000).1 Factor Va is the cofactor required in vivo for the rapid generation of thrombin catalyzed by the prothrombinase complex, a 1:1, Ca2+-dependent complex that is composed of the serine protease factor Xa, and factor Va, bound to an appropriate cellular membrane surface.2 The cofactor plays a central role in this enzymatic complex and profoundly influences the amount of thrombin generated.3 Clinically, this is underscored by the observation that deficiencies of the procofactor factor V can lead to severe hemorrhage and possibly death,4 whereas inefficient inactivation of the cofactor (ie, factor VaLeiden; Arg506 → Gln) by activated protein C (APC) can lead to venous thrombosis.5,6 Interestingly, recent studies using gene knockout technology indicate that factor V deficiency in mice is embryonic-lethal, suggesting that a complete deficiency in humans may lead to a similar fate.7
Factor V, the precursor of factor Va, is distributed between two blood pools. Approximately 80% of the total factor V circulates in plasma (7 μg/mL; 20 nmol/L) as the single-chain procofactor, and the remaining 20% is found within the α-granules of platelets (≈4,600 to 14,000 molecules/platelet) in a partially proteolyzed state exhibiting significant cofactor activity after its release by a variety of agonists.8,9 Plasma-derived factor V is synthesized in the liver10,11; however, the origin of the platelet-derived cofactor has not been unambiguously defined. Because platelets retain only limited biosynthetic capacity,12 platelet-derived factor V might originate from plasma through endocytosis, like fibrinogen, albumin, and IgG,13-15 or be synthesized by the precursor of platelets, the megakaryocyte, like platelet factor 4, β-thromboglobulin, and von Willebrand factor (vWF).16 17
A few studies have suggested that megakaryocytes may synthesize factor V. Chiu et al,18 using isolated guinea pig megakaryocytes, demonstrated biosynthesis of factor V by in vitro incubation with radiolabeled amino acids and isolation of biosynthesized factor V. Gewirtz et al,19 using freshly isolated human megakaryocytes and megakaryocytes cloned from their colony-forming units, showed that these cell populations both bind and synthesize factor V. Expression of these traits appears to be related to cell maturation, because cellular binding of factor V appears earlier than their ability to synthesize the protein.19 In addition, this same group of investigators was able to demonstrate factor V mRNA in both megakaryocytes and platelets using reverse transcriptase-polymerase chain reaction (RT-PCR).20However, it is unclear from these studies if the amount of factor V synthesized by megakaryocytes is sufficient to account quantitatively for the amount of factor V contained within platelets and, therefore, does not rule out endocytosis of factor V from plasma by megakaryocytes as a potential mechanism.
To elucidate more conclusively whether platelet-derived factor V is taken up by megakaryocytes or is synthesized by these cells, two patients, each heterozygous for the factor VLeidenmutation, who underwent allogeneic transplantation from a wild-type factor V donor (bone marrow [BM] or liver) were studied. Our results provide the first clear evidence that secretable platelet-derived factor V is predominately taken up from plasma by megakaryocytes before platelet formation.
MATERIALS AND METHODS
Materials and Reagents
Tris[hydroxymethyl]aminomethane (Trizma-Base), L-α-phosphatidyl-L-serine [bovine brain] (PS), L-α-phosphatidylcholine [egg yolk] (PC), Tween-20, 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), prostaglandin E1 (PGE1), heparin (bovine lung), and glycine were purchased from Sigma (St Louis, MO). Nitrocellulose membrane sheets (0.45 μm) were purchased from Bio-Rad (Hercules, CA). The chemiluminescent substrate, Luminol, Reflection autoradiography film, and 125I were purchased from DuPont, NEN Research Products (Boston, MA). Crystallized bovine serum albumin was purchased from ICN ImmunoBiologicals (Aurora, OH). The α-thrombin inhibitor, hirudin, was obtained from Calbiochem (La Jolla, CA). The fluorescent α-thrombin inhibitor, dansylarginine N-(3-ethyl-1,5-pentanediyl)amide (DAPA),21 and human APC were purchased from Haematologic Technologies Inc (Essex Junction, VT). Phospholipid vesicles composed of 75% (% wt/wt) PC and 25% (%wt/wt) PS (PCPS) were prepared as previously described,22 and their concentration determined by phosphorous assay.23
Patient Information
Patient no. 1.
JMW is a 52-year-old woman diagnosed with acute myelogenous leukemia in 1995. After several cycles of intensive chemotherapy she entered a hematologic remission but relapsed within 6 months. She then underwent BM transplantation (BMT), and her donor was an HLA-identical brother. Her four hospital courses were complicated by four venous thromboses, always in the circulatory bed in which a central venous catheter had been inserted. Neither her parents nor any of her seven siblings gave a history of thrombosis. Nevertheless, because of this history of catheter-induced thrombosis, she underwent evaluation for a hypercoagulable state, including tests of the lupus anticoagulant, protein C, protein S, anti–thrombin III, and APC resistance. Both with standard coagulation tests and with factor V–deficient plasma, the patient was found to display APC resistance. Posttransplantation peripheral blood leukocytes were then tested by PCR (35 cycles) for factor VLeiden and found to be homozygous wild type, whereas DNA obtained from a skin biopsy performed to confirm the diagnosis of skin graft-versus-host disease showed her to be heterozygous for factor VLeiden. In addition, incubation of her plasma (posttransplant; 1:10 dilution with HBS [20 mmol/L HEPES, 0.15 mol/L NaCl, pH 7.4]) with 10 μmol/L PCPS vesicles and 2 nmol/L APC,24 followed by Western blotting for factor Va with αHFVaHC#17,25 demonstrated an APC-induced cleavage pattern consistent with a heterozygous factor VLeiden phenotype (data not shown). Thus, JMW’s plasma-derived factor V is an equal mixture of wild-type factor V and factor VLeiden. Her leukocytes, megakaryocytes, and platelets, derived from the marrow transplant donor, express a homozygous wild-type factor V genotype with no evidence of factor VLeiden allelic expression remaining due to retention of the recipient’s marrow. Our inability to detect a factor VLeiden allele after PCR (35 cycles) of DNA extracted from her peripheral blood cells argues strongly against any significant contribution of recipient cells to her marrow megakaryocytes subsequent to BMT.
Patient no. 2.
FW is a 35-year-old woman postorthotopic liver transplantation for Budd-Chiari syndrome, who presented with left arm swelling 1 month post liver transplant. An ultrasound of the arm showed partially obstructing thrombi in the distal jugular/subclavian junction of the bronchopulmonary vein. The patient was placed on heparin for proper anticoagulation. A workup for risk factors contributing to hypercoagulability showed normal plasma levels of plasminogen, fibrinogen, functional antithrombin, functional protein C, free protein S antigen, and a normal activated protein C resistance test. A genotypic analysis from the patient’s peripheral blood cells showed that the patient was heterozygous for factor VLeiden. Because of the discrepant results between the patient’s APC resistance assay (2.2 with normal being > 2.0) and the presence of heterozygous factor VLeiden from the patient’s peripheral blood, further analyses were performed. DNA was extracted from histologic specimens from the patient’s native liver and from the donor’s gall bladder. Results revealed homozygous wild-type FV in the genome of the donor gall bladder, and FVLeiden in FW’s original liver. In addition, incubation of her plasma (posttransplant; 1:10 dilution with HBS) with 10 μmol/L PCPS vesicles and 2 nmol/L APC,24 followed by Western blotting for factor Va with αHFVaHC#17,25 showed an APC-induced cleavage pattern consistent with a homozygous factor V wild-type phenotype (data not shown). Thus, FW’s plasma-derived factor V is normal, wild-type whereas her leukocytes and megakaryocytes are heterozygous for factor VLeiden.
DNA Extraction From Tissue Blocks
Formalin-fixed paraffin-embedded tissue (PET) blocks were used as the DNA source.26 After thorough cleaning of the microtome and installation of a new disposable knife, 15- to 20-μm sections providing a 1- to 2-cm tissue area were cut from a paraffin block containing no tissue, followed by sectioning of the PET block. Sections were deparaffinized subsequently with xylene:ethanol (1:1, vol/vol). Deparaffinized tissue sections were incubated in 100 μL Proeinase K solution (6 μg Proteinase K, 10 mmol/L Tris HCl pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.01% gelatin) at 37°C for 1 to 3 hours, and then at 100°C for 10 minutes. After microcentrifugation at 14,000 rpm for 10 minutes, the supernatant containing the DNA was transferred to a clean microfuge tube for PCR analysis.
Isolation of Platelets
Platelets were isolated from consenting individuals essentially as described previously.24 Briefly, 26 mL of blood were collected into a 30-mL syringe (4 syringes, ≈100 mL of blood) containing 4 mL of ACD (0.022 mol/L citrate, 0.014 mol/L dextrose, final concentrations) and 5 μmol/L PGE1 (final concentration). The blood in each syringe was transferred to a 50-mL conical polypropylene centrifuge tube, everted twice, divided into two tubes, and centrifuged (190g, 15 minutes) at ambient temperature to obtain platelet-rich plasma (PRP). The PRP (≈7.5 mL) from two centrifuge tubes was combined and centrifuged at 1,100g for 15 minutes at ambient temperature. The platelet-poor plasma (PPP) was removed and the remaining platelet pellet was gently resuspended in a small volume (≈10 mL) of PPP. All platelets from the same donor were pooled (≈30 to 40 mL) to generate a platelet concentrate. This platelet concentrate was then shipped from either Seattle, WA (JMW) or Philadelphia, PA (FW) to Burlington, VT, via express mail (18 to 36 hours). Upon receipt, the platelet suspensions were placed immediately at 37°C and platelets were isolated as previously described.27,28 Platelets were counted on a Coulter counter (Coulter Electronics, LTD, Hialeah, FL) and brought to a final platelet concentration of 1 × 109/mL in 5 mmol/L HEPES-Tyrode’s (0.14 mol/L NaCl, 2.7 mmol/L KCl, 12 mmol/L NaHCO3, 0.42 mmol/L NaH2PO4 · H2O, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 5 mmol/L dextrose) buffer pH 7.4 for all experiments. Control studies performed on site and detailed in previous studies24 indicated that the time of shipment and the presence of PGE1 had no influence on the rate or mechanism of platelet-derived factor Va inactivation by APC.
APC-catalyzed inactivation and proteolysis of platelet-derived factor Va and factor VaLeiden.
The inactivation of platelet-derived factor Va by APC was performed in the presence of PCPS vesicles. Platelet-derived factor Va release and activation was accomplished by platelet incubation (1 × 109/mL) with 50 nmol/L α-thrombin (5 NIH U/mL) for 5 minutes at ambient temperature, followed by the addition of 60 nmol/L hirudin. Activated platelets were removed from platelet-derived factor Va by gentle centrifugation (1,100g, 5 minutes) and PCPS vesicles (20 μmol/L) were then added. In all experiments, the initial concentration of platelet-derived factor Va was donor dependent, and ranged from 1.0 nmol/L to 3.0 nmol/L.
Functional analyses and Western blotting.
After platelet activation with α-thrombin (50 nmol/L, 5 minutes), the inactivation of platelet-derived factor Va/VaLeiden was initiated immediately by APC addition (0.25 nmol/L). To monitor inactivation of the cofactor, samples of the reaction mixture were withdrawn at various time intervals and placed in a prothrombinase assay using purified protein components with saturating amounts of factor Xa (5 nmol/L) and PCPS vesicles (20 μmol/L) as the membrane surface as previously described.3,28 At the same time intervals, samples of the reaction mixture were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by addition of 62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.001% bromophenol blue (final concentrations). After heating at 95°C for 3 minutes, SDS-PAGE analysis was performed on 5% to 15% gradient slab gels according to the method of Laemmli.29 Following electrophoresis, the proteolytic fragments resulting from APC-catalyzed cleavage of factor Va were transferred to nitrocellulose using electroblotting techniques as described by Towbin et al.30 Transfer was performed at 500 mA for 2 hours at 4°C.31 Nitrocellulose was blocked with 5% nonfat dry milk in 20 mmol/L Tris, 0.15 mol/L NaCl, 0.05% Tween-20 (TBS-T) at pH 7.4. The platelet-derived factor Va antigen (≈50 ng/lane) was probed with a mouse anti-human factor Va heavy-chain IgG monoclonal antibody αHFVaHC#17,25 which recognizes an epitope between amino acids 307-506 in the factor Va heavy chain. The secondary antibody used was a horse anti-mouse IgG coupled to horseradish peroxidase (HRP; Southern Biotechnologies, Birmingham, AL). Detection of factor Va was performed by enhanced chemiluminescence (Western Blot Chemiluminescence Detection Kit; Dupont NEN, Boston, MA) by exposure of blots (5 to 30 seconds) to Reflection autoradiography film and developed in a Kodak M35A X-OMAT processor (Eastman Kodak, Rochester, NY).
125I-labeling of factor V and incubation with PRP.
Plasma-derived factor V was isolated by immunoaffinity chromatography as described.32 Factor V was radioiodinated using the IODO-GEN (Pierce, Rockford, IL) transfer technique and characterized as previously detailed.31125I-factor V was greater than 97% precipitable with 10% trichloroacetic acid, remained as a single-chain protein as determined by SDS-PAGE and autoradiography, could be activated by thrombin to yield the characteristic heavy and light chains, and expressed a specific radioactivity of 3,000 to 5,000 cpm/ng (0.2 to 0.4 mol of iodine/mol of factor V). The labeled protein was stored in 50% glycerol/2 mmol/L CaCl2 at −20°C.
To assess factor V uptake by platelets, 125I-factor V (used as a trace, 200,000 cpm/mL; 0.1 nmol/L) was incubated with PRP (factor V = 20 nmol/L; platelet concentration = 2 to 3 × 108/mL) in the presence or absence of 5 μmol/L PGE1 for 12, 21, and 35 hours. Specific binding/internalization of 125I-factor V was determined as previously described33 after layering of 0.5 mL of the PRP-125I-factor V mixture over 0.5 mL n-butyl phthalate and centrifugation at 12,000g, 2 minutes. Nonspecific binding/entrapment was determined using a 25-fold molar excess of cold factor V (1.0 μmol/L, final) added to the PRP mixture.
RESULTS
Studies were initiated to determine whether factor V in platelet α-granules originates through endogenous synthesis in the megakaryocyte, through uptake from plasma, or through both mechanisms. To differentiate between these processes, we studied the inactivation of platelet-derived factor Va by APC on synthetic phospholipid vesicles from two patients heterozygous for factor VLeiden who underwent allogeneic transplantation from homozygous factor V wild-type donors (BM or liver). Patient JMW, whose skin biopsy sample revealed heterozygous factor VLeiden, underwent BMT and subsequent DNA analyses indicated that her peripheral blood cells now exclusively express the wild-type factor V genotype, because factor VLeiden allelic expression was ablated completely before transplant. Patient FW, whose marrow cell population showed heterozygous factor VLeiden, underwent liver transplantation, and DNA analyses on a sample of the new liver indicated that her factor V was homozygous wild type. Therefore, if platelet-derived factor V is exclusively synthesized by megakaryocytes, then platelet-derived factor Va from JMW should be homozygous wild type whereas that derived from FW should be heterozygous for the factor VLeiden mutation. Alternatively, if platelet-derived factor V is endocytosed by megakaryocytes (and/or platelets), then platelet-derived factor Va from JMW should be a mixture of wild type and factor VaLeiden and FW should be homozygous wild type.
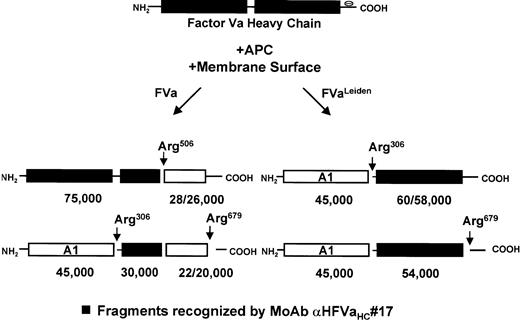
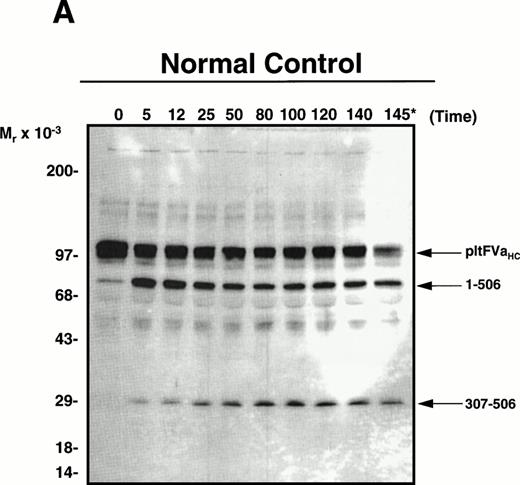
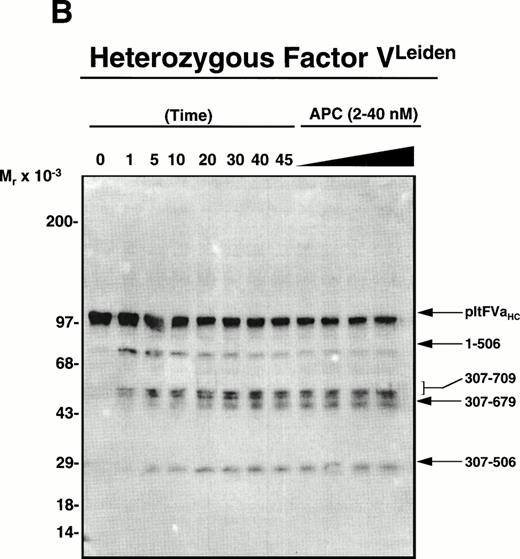
Wild-type factor Va versus factor VaLeiden is easily distinguished based on the heavy-chain products observed subsequent to APC-induced cleavage using monoclonal antibody αHFVaHC#17, which is specific for an epitope located between amino acids 307-506 within the factor Va heavy chain (see Fig 1).25 Thus, platelets isolated from a normal individual, a heterozygous factor VLeiden individual, and the two transplant patients were incubated with thrombin as described in Materials and Methods to both activate the platelet and to release and activate the platelet-derived cofactor. After centrifugation to remove platelets, phospholipid vesicles were added to the supernatant to provide an appropriate membrane surface required for the APC-catalyzed inactivation of the cofactor. Following the addition of APC to initiate the reaction, samples were removed at selected time intervals and prepared for SDS-PAGE/Western blotting to detect the proteolytic fragments accompanying inactivation. As shown in Fig2A, APC initially cleaved normal platelet-derived factor Va within the heavy chain at Arg506 generating an Mr = 75,000 intermediate, which was further cleaved at Arg306generating a fragment migrating at Mr = 30,000. The inactivation of platelet-derived factor Va by APC derived from an individual heterozygous for factor VLeiden is depicted in Fig 2B. As expected, APC cleaved the normal pool of platelet-derived factor Va initially at Arg506, followed by cleavage at Arg306; however, the mutant pool of platelet-derived factor Va, which lacks the cleavage site at Arg506, was initially cleaved by APC at Arg306 generating Mr = 60/58,000 fragments, followed by cleavage at Arg679, generating a fragment that migrates at Mr = 54,000 and is resistant to any further APC-induced proteolysis.
Schematic representation of membrane-bound factor Va/VaLeiden heavy-chain inactivation by APC. Normal plasma factor Va is inactivated following three ordered and sequential cleavages in the heavy chain at Arg506, Arg306, and Arg679.45 Cleavage at Arg506, which gives rise to an Mr = 75,000 fragment and an Mr = 28/26,000 doublet, is necessary to optimally expose the site at Arg306. Further cleavage at Arg306yields an Mr = 45,000 fragment and an Mr = 30,000 fragment.45 Individuals with the Arg506→ Gln mutation (factor VLeiden) no longer have a cleavage site at position 506, which slows the rate of cleavage at Arg306.46 Cleavage at Arg306 yields an Mr = 45,000 fragment and an Mr = 60/58,000 doublet. Further cleavage of the Mr = 60/58,000 doublet at Arg679 yields an Mr = 54,000 fragment. Fragments that are recognized by the monoclonal antibody (HFVaHC#17) used in this study are indicated by the shaded boxes.25
Schematic representation of membrane-bound factor Va/VaLeiden heavy-chain inactivation by APC. Normal plasma factor Va is inactivated following three ordered and sequential cleavages in the heavy chain at Arg506, Arg306, and Arg679.45 Cleavage at Arg506, which gives rise to an Mr = 75,000 fragment and an Mr = 28/26,000 doublet, is necessary to optimally expose the site at Arg306. Further cleavage at Arg306yields an Mr = 45,000 fragment and an Mr = 30,000 fragment.45 Individuals with the Arg506→ Gln mutation (factor VLeiden) no longer have a cleavage site at position 506, which slows the rate of cleavage at Arg306.46 Cleavage at Arg306 yields an Mr = 45,000 fragment and an Mr = 60/58,000 doublet. Further cleavage of the Mr = 60/58,000 doublet at Arg679 yields an Mr = 54,000 fragment. Fragments that are recognized by the monoclonal antibody (HFVaHC#17) used in this study are indicated by the shaded boxes.25
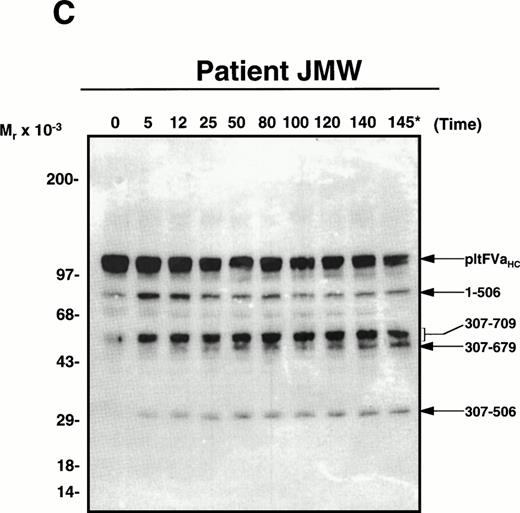
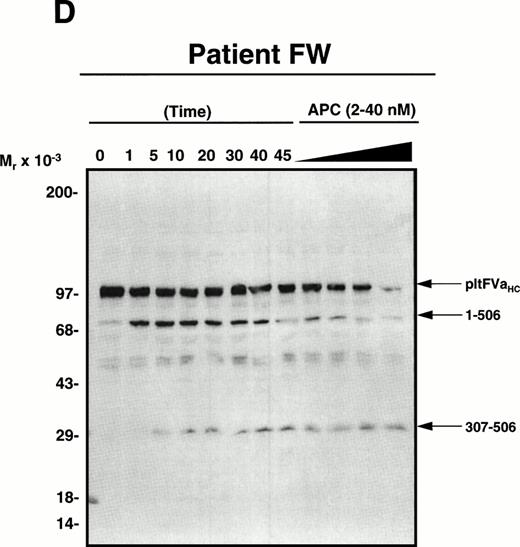
APC-catalyzed inactivation of platelet-derived factor Va and factor VaLeiden bound to phospholipid vesicles. Platelets (1 × 109/mL) were treated with 5 NIH U/mL (50 nmol/L) of -thrombin for 5 minutes to both activate the platelets and release and activate the platelet-derived factor V. Hirudin (60 nmol/L) was then added to inhibit thrombin. The activated platelets were immediately removed from suspension by gentle centrifugation (1,100g, 5 minutes), and PCPS vesicles (20 μmol/L) were added to the supernatant to provide an appropriate alternate anticoagulant surface. APC (0.25 nmol/L) was then added. At selected time points, samples of the reaction mixture were withdrawn and subjected to SDS-PAGE using a 5% to 15% gradient gel. After transfer to nitrocellulose, fragments were visualized using a monoclonal antibody, HFVaHC#17, as described.25 Each of the panels represents the inactivation of secretable platelet-derived factor Va on phospholipid vesicles by APC: (A) normal platelet-derived factor Va, (B) platelet-derived factor Va derived from a heterozygous factor VLeiden individual, (C) platelet-derived factor Va derived from JMW, and (D) platelet-derived factor Va derived from FW. The time course of inactivation by APC is given at the top of each immunoblot. In (A) and (C), 145* indicates the platelet factor Va/APC mixture after a 145-minute incubation, with an additional 20 nmol/L APC incubated for 5 minutes. In (B) and (D), following a 45-minute incubation with 0.25 nmol/L APC, platelet-derived factor Va was incubated with increasing concentrations of APC (2, 10, 20, 40 nmol/L) for an additional 15 minutes, which is indicated in the last four lanes of these immunoblots. The position of the molecular weight markers are indicated at the left of the immunoblots. Arrows to the right of the immunoblots represent residue numbers corresponding to factor Va fragments derived from APC-induced cleavage.
APC-catalyzed inactivation of platelet-derived factor Va and factor VaLeiden bound to phospholipid vesicles. Platelets (1 × 109/mL) were treated with 5 NIH U/mL (50 nmol/L) of -thrombin for 5 minutes to both activate the platelets and release and activate the platelet-derived factor V. Hirudin (60 nmol/L) was then added to inhibit thrombin. The activated platelets were immediately removed from suspension by gentle centrifugation (1,100g, 5 minutes), and PCPS vesicles (20 μmol/L) were added to the supernatant to provide an appropriate alternate anticoagulant surface. APC (0.25 nmol/L) was then added. At selected time points, samples of the reaction mixture were withdrawn and subjected to SDS-PAGE using a 5% to 15% gradient gel. After transfer to nitrocellulose, fragments were visualized using a monoclonal antibody, HFVaHC#17, as described.25 Each of the panels represents the inactivation of secretable platelet-derived factor Va on phospholipid vesicles by APC: (A) normal platelet-derived factor Va, (B) platelet-derived factor Va derived from a heterozygous factor VLeiden individual, (C) platelet-derived factor Va derived from JMW, and (D) platelet-derived factor Va derived from FW. The time course of inactivation by APC is given at the top of each immunoblot. In (A) and (C), 145* indicates the platelet factor Va/APC mixture after a 145-minute incubation, with an additional 20 nmol/L APC incubated for 5 minutes. In (B) and (D), following a 45-minute incubation with 0.25 nmol/L APC, platelet-derived factor Va was incubated with increasing concentrations of APC (2, 10, 20, 40 nmol/L) for an additional 15 minutes, which is indicated in the last four lanes of these immunoblots. The position of the molecular weight markers are indicated at the left of the immunoblots. Arrows to the right of the immunoblots represent residue numbers corresponding to factor Va fragments derived from APC-induced cleavage.
As seen in Fig 2C, the APC-induced cleavage pattern of platelet factor Va derived from JMW is consistent with a heterozygous factor VaLeiden phenotype based on the accumulation of factor Va fragments migrating at Mr =30,000 and 54,000 to 60,000. These results clearly indicate that a significant portion of platelet-derived factor V must be endocytosed by megakaryocytes (and/or platelets) from plasma, because posttransplantation her plasma is the only source of the factor VLeidenprotein, because no factor VLeiden allele could be detected in DNA isolated from her marrow-derived cells after 35 cycles of PCR.
To more firmly establish the contribution that megakaryocyte endocytosis of plasma factor V makes to the platelet factor V pool, FW was studied. Western blotting analyses of the APC-cleaved platelet-derived factor Va from FW indicated a wild-type phenotype (Fig2D), despite the presence of a factor VLeiden allele in her marrow cell populations. As previously shown by our laboratory, normal platelet-derived factor Va can be initially cleaved by APC at either Arg506 or Arg306.24 28 It is evident from Fig 2A and D, both of which represent normal platelet-derived factor Va, that there was some initial cleavage of the cofactor at Arg306 which generated fragments migrating at Mr = 60/58,000. Because both of these factor Va molecules lack the factor VLeiden mutation, these fragments were subsequently cleaved by APC at Arg506 to generate a 30,000-dalton fragment. No accumulation of a 54,000-dalton fragment was observed (Fig 2D) since the cleavage site at position 506 remained intact, in contrast to that observed with platelet-derived factor VaLeiden (Fig 2B and C). In fact, prolonged exposure of the blot shown in Fig 2D did not allow detection of any 54,000-dalton factor VaLeiden subsequent to APC addition. Thus, because factor VLeiden protein was undetectable in FW’s platelet pool, the combined results are consistent with endocytosis of platelet-derived factor V by megakaryocytes (and/or platelets) from plasma and argues against synthesis as the primary and only mechanism responsible for the presence of factor V in platelets.
Studies investigating the nature of platelet fibrinogen have indicated that this protein is taken up by megakaryocytes through a process involving receptor-mediated endocytosis most likely involving GP IIb/IIIa.13-15,34,35 In addition, it was also established that platelets, as well as megakaryocytes, are involved in fibrinogen uptake.14 35 To establish whether platelets are involved in the endocytosis of factor V from plasma, single-chain, human plasma-derived factor V was radiolabeled with 125I and subsequently incubated with PRP (200,000 cpm/mL of PRP) from a normal donor. Nonspecific binding of 125I-factor V was assessed by incubating PRP with trace label (200,000 cpm/mL) plus a 25-fold excess of cold single-chain factor V (final concentration in PRP = 1.0 μmol/L). Even after a 35-hour incubation period, we were unable to detect any specific association of 125I-factor V with the platelet pellet (data not shown), suggesting that uptake of factor V most likely takes place exclusively in the megakaryocyte. However, these in vitro conditions may not adequately mimic the environment of the normal platelet circulating in vivo, a condition that may be more conducive to the uptake of factor V by platelets.
DISCUSSION
The purpose of this present study was to determine whether platelet-derived factor V originates from endogenous synthesis by megakaryocytes, endocytosis by megakaryocytes (and/or platelets), or through both mechanisms. Results from our present study provide strong evidence that platelet-derived factor V originates from plasma by endocytosis from the megakaryocyte and indicates that endogenous synthesis of the procofactor by megakaryocytes contributes little to the total pool of factor V contained within a platelet.
The mechanism of acquisition of factor V within megakaryocytes or platelets had been established previously as due to endogenous synthesis in the megakaryocyte. The biosynthesis of factor V has been shown in isolated guinea pig megakaryocytes18 and human megakaryocytes19 by in vitro incorporation of radiolabeled amino acids into the procofactor. In addition, specific mRNA for factor V has been identified in both human megakaryocytes and platelets by RT-PCR.20 Although each of these studies show that megakaryocytes may synthesize factor V, there has been no report to date demonstrating whether such synthesis accounts quantitatively for megakaryocyte- and platelet-derived factor V. In addition, neither of the studies provide any data showing that biosynthesis of factor V is the only mechanism of factor V acquisition in megakaryocytes or platelets. Thus, although these previous studies demonstrate some level of factor V synthesis by megakaryocytes, our present results clearly show that the principal mechanism by which platelets acquire factor V is via its endocytosis from plasma by megakaryocytes.
Interestingly, Gewirtz et al19 noted that while mature megakaryocytes can both bind and synthesize factor V, immature megakaryocytes do not appear to synthesize the procofactor. However, these same immature megakaryocytes contain large amounts of immunochemically detectable factor V, suggesting that the ability of these megakaryocytes to bind factor V develops early and in addition to binding factor V, megakaryocytes may also endocytose this protein early in development. Clearly, the data presented in the current study strongly support the latter mechanism. Additional studies are planned to determine quantitatively the extent to which megakaryocyte endocytosis of plasma factor V versus its endogenous synthesis contributes to the cofactor pool within the platelet.
Several studies have now clearly established that the platelet α-granule is a unique type of secretory granule whose contents can originate by endogenous synthesis in the megakaryocyte, or by endocytosis and pinocytosis at both the megakaryocyte and circulating platelet level.16,36 Platelet-specific proteins, like platelet factor 4 and β-thromboglobulin, appear to be synthesized solely by the megakaryocyte.16,36 However, the origin of platelet proteins which have plasma counterparts is less obvious. For example, albumin, IgG, and fibrinogen are endocytosed by megakaryocytes, and to a certain extent by platelets, and incorporated into α-granules.13-15 Alternatively, platelet and megakaryocyte vWF originates from endogenous synthesis and is independent of plasma vWF.17
The mechanism(s) by which factor V is endocytosed by megakaryocytes is currently unknown. George16 has hypothesized that the different origins of α-granular proteins are suggested by investigating their relative platelet-plasma concentration. For example, β-thromboglobulin and platelet factor 4, proteins that are known to be exclusively synthesized in the megakaryocyte, have greater concentrations in platelets than in plasma (platelet/plasma ratio >250,000).16 Alternatively, albumin and IgG, proteins, which are thought to enter megakaryocytes and/or platelets though fluid-phase endocytosis (pinocytosis), have much higher concentrations in plasma than in platelets (platelet/plasma ratios <0.07). A protein with an intermediate platelet to plasma ratio is fibrinogen (≈2.5). Recent data from several laboratories have established that fibrinogen enters megakaryocytes (and possibly platelets) through receptor-mediated endocytosis via glycoprotein (GP) IIb-IIIa.34,35 37 Interestingly, factor V has a platelet to plasma ratio of ≈60, much lower than that of β-thromboglobulin and platelet factor 4, and much higher than albumin and IgG. Thus, simply by looking at the concentration of factor V in platelets to that in plasma, it is tempting to hypothesize that factor V as well as fibrinogen enters megakaryocytes through receptor-mediated endocytosis.
Several studies investigating platelet IgG, which is taken up from plasma by fluid phase endocytosis, have shown that the concentration of platelet IgG mirrors the concentration and composition of plasma IgG in both normal subjects as well as in a wide range of abnormal plasma IgG concentrations.16 As with platelet IgG, the concentration of platelet-derived factor V also appears to mirror the concentration of plasma-derived factor V. Our laboratory has shown that platelet-derived factor V consistently comprises ≈20% of the total factor V contained in whole blood from normal donors as determined by radioimmunoassay.8 Although there have been several reports documenting factor V deficiency with respect to plasma factor V,4 unfortunately none of these studies simultaneously measured the factor V content in platelets. However, our laboratory has recently studied an individual with mild factor V deficiency. Our results indicate that the patient has ≈30% factor V activity in plasma, as determined by a clotting assay, and ≈37 % platelet factor Va activity, as determined by a purified prothrombinase assay (unpublished observations, September 1996). In addition, Weiss and Lages38 have recently described a patient with congenital factor V deficiency, whose plasma- and platelet-derived factor V concentrations were ≈14% and ≈12% of normal, respectively, both based on activity assays. Thus, like endocytosed platelet IgG, the concentration of platelet-derived factor V is also very sensitive to the concentration of its plasma counterpart and suggests an endocytotic mechanism of acquisition of the procofactor by megakaryocytes.
Additional insight into the nature of proteins endocytosed by megakaryocytes can be made by investigating the gray platelet syndrome (GPS). GPS is a rare congenital bleeding disorder in which megakaryocytes and platelets are deficient in α-granule secretory proteins.39 Studies into the GPS indicate that some α-granule proteins appear reduced to a lesser extent than other α-granule proteins, for example, albumin compared with platelet factor 4.39 Why this occurs is currently not known; however, it has been hypothesized that the functional abnormality in GPS may result from the defective targeting of endogenously synthesized secretory proteins to developing α-granules in megakaryocytes.39,40 Consistent with the notion that endocytosed proteins are affected to a lesser extent than endogenously synthesized proteins, Chenu and Delmas41 have demonstrated that levels of bone sialoprotein, which is endocytosed by megakaryocytes, were normal in platelets of a patient with GPS. Interestingly, our laboratory has shown that lysed platelets from a patient with GPS contain near-normal amounts of platelet-factor V antigen, again consistent with an endocytotic mechanism.42
Our combined data support endocytosis by megakaryocytes as the major mechanism by which platelet-derived factor V is acquired, because we could not show direct platelet uptake of factor V from plasma. Since platelet-derived factor V is stored within the platelet as a partially proteolyzed molecule9 and is a different substrate for proteases24,28,43 and platelet kinases44 when compared with its plasma counterpart, we hypothesize that the platelet-derived molecule may be physically altered during endocytosis and packaging in the α-granule.
ACKNOWLEDGMENT
We acknowledge Rama Kudaravalli, PhD, for her expert technical assistance regarding DNA extraction and testing for the factor VLeiden mutation, and Ilka Warshawsky, MD, for her clinical service work. The Blood Drawing Services of the General Clinical Research Center at Fletcher Allen Health Care in Burlington, VT, are gratefully acknowledged as well.
Supported by Grant No. HL P01-46703, Project 4 (to P.B.T.).
Address reprint requests to Paula B. Tracy, PhD, Given C409, Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT 05405-0001; e-mail: ptracy@salus.med.uvm.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal