IN THE 1880s Elie Metchnikoff observed specialized phagocytic cells ingesting bacteria, and recognized the importance of phagocytosis as a defense mechanism in multicellular organisms.1 Neutrophils are one of the professional phagocytes in humans. They ingest bacteria into intracellular compartments called phagosomes, where they direct an arsenal of cytotoxic agents. Metchnikoff noted that “what substances within the phagocyte harm and destroy the microbes is quite undecided.” One hundred years on, Mims stated that “we are still profoundly ignorant of the ways in which polymorphs attempt to kill and then to digest the great variety of microorganisms that are ingested.”2 Our understanding is gradually increasing, but there are still a number of questions to be answered.
It was recognized at an early stage that cytoplasmic granules containing digestive and antibacterial compounds are emptied into the phagosome.3 Later, it was discovered that phagocytosing neutrophils undergo a burst of oxygen consumption4,5 that is caused by an NADPH oxidase complex that assembles at the phagosomal membrane. As reviewed by others,6-8 electrons are transferred from cytoplasmic NADPH to oxygen on the phagosomal side of the membrane, generating first superoxide plus a range of other reactive oxygen species. This oxidative burst is essential for killing of a number of microorganisms, as shown by the susceptibility to infections of individuals with chronic granulomatous disease (CGD), a genetic disease in which the NADPH oxidase is inactive.9-11
Much is known about the reactive oxygen species released into the extracellular surroundings when neutrophils respond to soluble stimuli. However, the enzymatic and chemical reactions involved in oxidant production are dependent on environmental conditions, which may vary markedly between the phagosome and the extracellular medium. Knowledge of the biochemistry within the phagosome is limited by its inaccessibility to standard detectors and scavengers. Consequently, the oxidant species directly responsible for killing bacteria are still open to speculation. This review focuses on what is known about the chemical composition of the phagosome, the nature and amount of the oxidants generated inside, and on recent information that helps clarify the importance of myeloperoxidase-derived oxidants in killing.
EXTRACELLULAR OXIDANT PRODUCTION BY NEUTROPHILS
Superoxide and hydrogen peroxide.
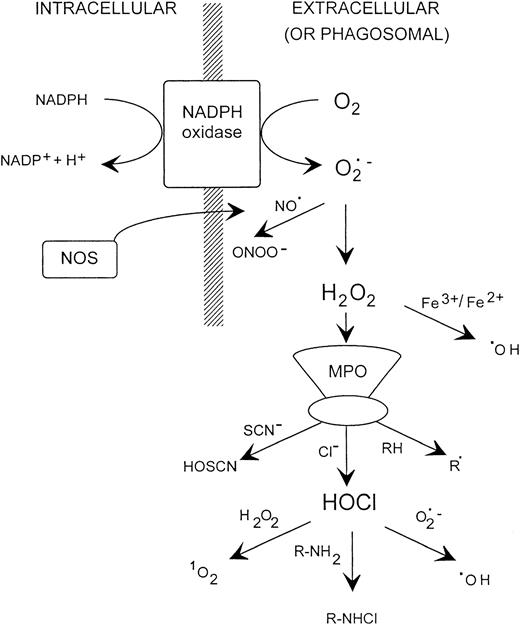
A variety of soluble and particulate stimuli induce extracellular superoxide production.5,12-14 Most of the oxygen consumed can be accounted for as hydrogen peroxide,15,16 which is formed from dismutation of the superoxide radical.7However, hydrogen peroxide is bactericidal only at high concentrations,17,18 and exogenously generated superoxide does not kill bacteria directly.19-21 Therefore, a variety of secondary oxidants have been proposed to account for the destructive capacity of neutrophils (Fig 1). Table 1 provides a summary of their properties.
Possible oxidant generating reactions with stimulated neutrophils. NOS, nitric oxide synthase; MPO, myeloperoxidase.
Possible oxidant generating reactions with stimulated neutrophils. NOS, nitric oxide synthase; MPO, myeloperoxidase.
Properties of Reactive Oxygen Species
| Superoxide: | Mild oxidant and reductant with limited biological activity; reduces some iron complexes to enable hydroxyl radical production by the Fenton reaction; inactivates iron/sulfur proteins and releases iron; limited membrane permeability. |
| Hydrogen peroxide: | Oxidizing agent; reacts slowly with reducing agents such as thiols; reacts with reduced iron and copper salts to generate hydroxyl radicals; reacts with heme proteins and peroxidases to initiate radical reactions and lipid peroxidation; membrane permeable. |
| Hydroxyl radical: | Extremely reactive with most biological molecules; causes DNA modification and strand breaks, enzyme inactivation, lipid peroxidation; very short range of action; generates secondary radicals, eg, from bicarbonate, chloride. |
| Singlet oxygen: | Electronically excited state of oxygen; reacts with a number of biological molecules, including membrane lipids to initiate peroxidation. |
| Hypochlorous acid: | Strong nonradical oxidant of a wide range of biological compounds, but more selective than hydroxyl radical; preferred substrates thiols and thioethers; converts amines to chloramines; chlorinates phenols and unsaturated bonds; oxidizes iron centers; crosslinks proteins; membrane permeable; in equilibrium with chlorine gas at low pH and hypochlorite at high pH. |
| Chloramines: | Milder and longer lived oxidants than HOCl; react with thiols, thioethers, iron centers; variable toxicity dependent on polarity and membrane permeability; chloramines of α-amino acids break down slowly to potentially toxic aldehydes. |
| Nitric oxide: | Reacts very rapidly with superoxide to give peroxynitrite; reaction with oxygen favored at high oxygen tension; forms complexes with heme proteins; inactivates iron/sulfur centers; forms nitrosothiols. |
| Peroxynitrite: | Unstable short lived strong oxidant with properties similar to hydroxyl radical; hydroxylates and nitrates aromatic compounds; reacts rapidly with thiols: breaks down to nitrate; interacts with bicarbonate to alter reactivity. |
| Superoxide: | Mild oxidant and reductant with limited biological activity; reduces some iron complexes to enable hydroxyl radical production by the Fenton reaction; inactivates iron/sulfur proteins and releases iron; limited membrane permeability. |
| Hydrogen peroxide: | Oxidizing agent; reacts slowly with reducing agents such as thiols; reacts with reduced iron and copper salts to generate hydroxyl radicals; reacts with heme proteins and peroxidases to initiate radical reactions and lipid peroxidation; membrane permeable. |
| Hydroxyl radical: | Extremely reactive with most biological molecules; causes DNA modification and strand breaks, enzyme inactivation, lipid peroxidation; very short range of action; generates secondary radicals, eg, from bicarbonate, chloride. |
| Singlet oxygen: | Electronically excited state of oxygen; reacts with a number of biological molecules, including membrane lipids to initiate peroxidation. |
| Hypochlorous acid: | Strong nonradical oxidant of a wide range of biological compounds, but more selective than hydroxyl radical; preferred substrates thiols and thioethers; converts amines to chloramines; chlorinates phenols and unsaturated bonds; oxidizes iron centers; crosslinks proteins; membrane permeable; in equilibrium with chlorine gas at low pH and hypochlorite at high pH. |
| Chloramines: | Milder and longer lived oxidants than HOCl; react with thiols, thioethers, iron centers; variable toxicity dependent on polarity and membrane permeability; chloramines of α-amino acids break down slowly to potentially toxic aldehydes. |
| Nitric oxide: | Reacts very rapidly with superoxide to give peroxynitrite; reaction with oxygen favored at high oxygen tension; forms complexes with heme proteins; inactivates iron/sulfur centers; forms nitrosothiols. |
| Peroxynitrite: | Unstable short lived strong oxidant with properties similar to hydroxyl radical; hydroxylates and nitrates aromatic compounds; reacts rapidly with thiols: breaks down to nitrate; interacts with bicarbonate to alter reactivity. |
Hydroxyl radicals and singlet oxygen.
Whether the hydroxyl radical is a major component of the neutrophil bactericidal arsenal has been controversial.22-26 There have been a large number of studies of isolated neutrophils, some of which have presented evidence for hydroxyl radical production.27-30 However, assays for this extremely reactive species rely on measuring secondary products and the use of inhibitors. They often lack specificity and reactions attributed to the hydroxyl radical may be caused by other oxidants such as superoxide or hypochlorous acid (HOCl).23 31
There are two potential mechanisms for hydroxyl radical production by neutrophils: the superoxide-driven Fenton reaction between hydrogen peroxide and an appropriate transition metal catalyst, and the reaction of HOCl with superoxide. The most definitive investigations of the Fenton mechanism have used spin traps to establish that neutrophils do not have an endogenous transition metal catalyst and that release of lactoferrin inhibits the reaction by complexing iron.25,32Myeloperoxidase limits the reaction further, even if iron is available, by consuming hydrogen peroxide.33 The overall conclusion is that the cells generate insignificant amounts of hydroxyl radical by this mechanism.23-25 This reaction may be more significant in vivo if target cells or molecules could provide iron to the neutrophils. Although most biological forms of iron are not catalytically active, neutrophils have been shown to produce hydroxyl radicals in the presence of proteolytically degraded transferrin25,34-36 or iron complexed to thePseudomonas aeruginosa siderophore pyochelin.37,38However, intracellular iron is not necessarily available and no enhanced hydroxyl radical production was observed when neutrophils ingested Staphylococcus aureus that had been preloaded with iron.35
Recently, more sensitive spin-trapping methods have detected myeloperoxidase-dependent hydroxyl radical formation by isolated neutrophils,25,39 presumably from HOCl and superoxide.40 Very little of the oxygen consumed by the cells has been measured as hydroxyl radicals, and whether this is sufficient to play a role in cytotoxicity is yet to be proven.
Hydroxyl radicals, including those generated by ionizing radiation, kill bacteria.41,42 However, they are not as efficient as their high reactivity might suggest.41 They have a limited radius of action, so even in the confined space of the phagosome, most are likely to react with other targets before reaching the bacterium. It has been proposed that secondary products from bicarbonate or chloride might be responsible for any biological activity.41 Czapski et al43 have observed that hydroxyl radical generating systems are more toxic to bacteria in the presence of chloride, and attributed this to a reaction between the two to produce HOCl. This would suggest that any hydroxyl radical generation from HOCl and superoxide would have little additional impact on the killing process, and may actually reduce toxicity by converting the extremely bactericidal HOCl to the more reactive, but less toxic, hydroxyl radical.
Singlet oxygen could theoretically be produced by neutrophils from the reaction of hydrogen peroxide with HOCl. Although it was initially proposed to be the source of the chemiluminescence of stimulated cells,44 subsequent studies measuring specific infrared chemiluminescence have failed to detect singlet oxygen production by neutrophils.45-47 Positive results were obtained with eosinophils, which generate hypobromous acid rather than HOCl, although the conversion of oxygen consumed was only 0.4%.48Steinbeck et al47 have used a singlet oxygen trap with neutrophils, and reported a surprisingly high 19% conversion of available oxygen to the singlet form. The significance of this finding to microbicidal activity and how it can be reconciled with the chemical findings require further investigation.
Myeloperoxidase and HOCl.
Most of the hydrogen peroxide generated by neutrophils is consumed by myeloperoxidase.12,49 Myeloperoxidase is a major constituent of the azurophilic cytoplasmic granules50 and a classical heme peroxidase that uses hydrogen peroxide to oxidize a variety of aromatic compounds (RH) by a 1-electron mechanism to give substrate radicals (R•)51-54(Fig 2). It is unique, however, in readily oxidizing chloride ions to the strong nonradical oxidant, HOCl.55 HOCl is the most bactericidal oxidant known to be produced by the neutrophil.5,56 Many species of bacteria are killed readily by a myeloperoxidase/hydrogen peroxide/chloride system.57 Bacterial targets include iron-sulfur proteins, membrane transport proteins,58 adenosine triphosphate (ATP)-generating systems,59 and the origin of replication site for DNA synthesis, which appears to be the most sensitive.60-62 Chloramines are generated indirectly through the reaction of HOCl with amines,63 and these are also bactericidal.64,65 Cell permeable chloramines, eg, monochloramine, can enhance the toxicity of HOCl, whereas protein chloramines have low toxicity. Other substrates of myeloperoxidase include iodide, bromide, thiocyanate, and nitrite.66-69 The corresponding hypohalous acids or nitrogen oxides that are produced vary in their bactericidal efficiency. Myeloperoxidase can also generate peroxides and hydroxylated derivatives of phenolics such as salicylate in superoxide-dependent reactions.31 70
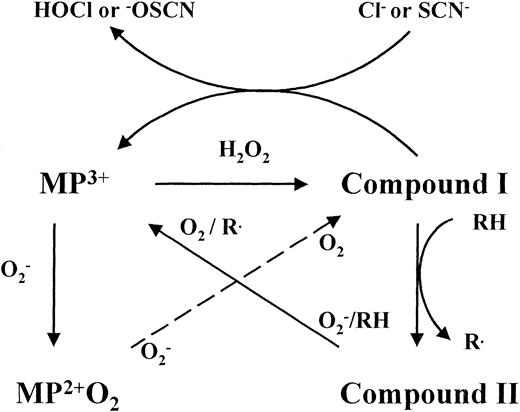
Reactions of myeloperoxidase. Ferric myeloperoxidase (MP3+) reacts with hydrogen peroxide to form the redox intermediate compound I, which oxidizes chloride or thiocyanate by a single 2-electron transfer to produce the respective hypohalous acids. Myeloperoxidase also oxidizes numerous organic substrates (RH) by two successive 1-electron transfers involving the enzyme intermediates compound I and compound II. Poor peroxidase substrates trap the enzyme as compound II and hypohalous acid production is inhibited unless superoxide is present to recycle the native enzyme. Superoxide can convert myeloperoxidase to compound III, which is turned over by a second reaction with superoxide. It has yet to be established whether the products of the latter reaction are compound I or MP3+ and hydrogen peroxide. Either way, the same net result is achieved.
Reactions of myeloperoxidase. Ferric myeloperoxidase (MP3+) reacts with hydrogen peroxide to form the redox intermediate compound I, which oxidizes chloride or thiocyanate by a single 2-electron transfer to produce the respective hypohalous acids. Myeloperoxidase also oxidizes numerous organic substrates (RH) by two successive 1-electron transfers involving the enzyme intermediates compound I and compound II. Poor peroxidase substrates trap the enzyme as compound II and hypohalous acid production is inhibited unless superoxide is present to recycle the native enzyme. Superoxide can convert myeloperoxidase to compound III, which is turned over by a second reaction with superoxide. It has yet to be established whether the products of the latter reaction are compound I or MP3+ and hydrogen peroxide. Either way, the same net result is achieved.
Because myeloperoxidase has the specialized ability to oxidize chloride, it is generally considered that its function is to generate HOCl. In in vitro systems with taurine or methionine added as a trap, from 28% to 70% of the hydrogen peroxide produced by neutrophils has been detected as HOCl.71 72 However, most experimental studies are performed in media without alternative myeloperoxidase substrates. The products formed in pathophysiological situations may be more varied.
Reactive nitrogen species.
There is considerable interest in nitric oxide and peroxynitrite as potential cytotoxic agents produced by inflammatory cells.73-77 It is well documented that murine macrophages generate nitric oxide in response to cytokines,78 but results have been contradictory and mostly negative for human neutrophils isolated from peripheral blood.79-84 The prevailing view is that reactive nitrogen species are important in human inflammation, and that in vitro studies have been negative because the conditions necessary for induction have not been elucidated. Nitric oxide synthase message has recently been detected in neutrophils isolated from urine passed during infection of the urinary tract,85 and in buffy coat neutrophils after exposure to inflammatory cytokines.86 Also, because both myeloperoxidase and HOCl can oxidize nitrite,69;87neutrophils may not need their own source of nitric oxide to generate reactive nitrogen oxides. These findings suggest that nitric oxide may be a significant player in the oxidative reactions of the neutrophil in vivo, but until human neutrophils can be induced experimentally to produce nitric oxide, the relevance of it, and its reaction with superoxide to produce peroxynitrite, cannot be assessed.
THE PHAGOSOME
The neutrophil makes tight contact with its target and the plasma membrane flows around the surface until the bacterium is completely enclosed.88 This minimizes the amount of extracellular fluid entering the phagosome with the bacterium, and means that the phagosome is initially a very small space (Fig 3). The exclusion of external medium sets up a new environment that will have an important influence on the biochemistry of oxidant production and bacterial killing. The major contributors to the chemical composition of the phagosome are the contents of the cytoplasmic granules that empty into it. Granule contents are released within seconds of ingestion and constitute a significant proportion of the phagosomal volume.3,89 There are at least four different classes of granules,90 and sequential release of the different types90 91 may provide a succession of different phagosomal environments.
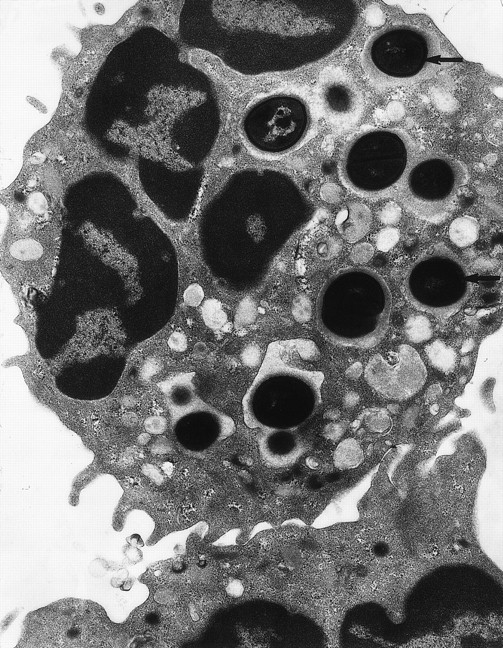
Transmission electron micrograph of S aureusinside the phagosome of a human neutrophil. Arrows pointed to examples of S aureus within phagosomes (original magnification × 15,000). (Courtesy of W.A. Day, Department of Pathology, Christchurch School of Medicine.)
Transmission electron micrograph of S aureusinside the phagosome of a human neutrophil. Arrows pointed to examples of S aureus within phagosomes (original magnification × 15,000). (Courtesy of W.A. Day, Department of Pathology, Christchurch School of Medicine.)
The large amount of degranulation into a small volume means that the initial protein concentration will be high (estimated 30% to 40% protein). This will decrease with time as the volume increases due to the osmotic influx of water associated with granule emptying and digestion of the bacterium. Ionic composition is unknown, and will depend on what is in the granules and also the activity of membrane pumps and channels that connect the phagosome to the neutrophil cytoplasm. The outward pumping of cytoplasmic chloride ions by stimulated neutrophils92 may be important for maintaining sufficient phagosomal chloride concentrations for HOCl production. Chloride is also necessary for azurophil degranulation,93and this may be a means of limiting myeloperoxidase release when chloride is depleted.
Phagosomal pH is under tight control. The oxidation of cytoplasmic NADPH to NADP+ and H+, and the transfer of reducing equivalents across the membrane to phagosomal oxygen, results in acidification of the cytoplasm.94 The dismutation of the superoxide anion, with its associated consumption of protons, would make the phagosome considerably alkaline. There is a transient increase in pH to 7.8 to 8.0 in the first few minutes after phagosome formation.95,96 However, activation of the oxidase is accompanied by activation of an Na+/H+antiport, an H+-ATPase, and an H+ conductance mechanism97 so that proton pumping from the cytoplasm into the phagosome restricts this increase and the pH decreases to approximately 6.0 after an hour.95 96
OXIDANT PRODUCTION IN THE PHAGOSOME
Taking into account the physical and chemical characteristics discussed above, what is known about the oxidants produced and the ability of myeloperoxidase to function in the phagosome? During phagocytosis, neutrophils consume a similar amount of oxygen as with strong soluble stimuli, yet release only small amounts of superoxide or hydrogen peroxide in the surroundings.14,98,99 However, there is convincing cytochemical evidence that superoxide100,101 and hydrogen peroxide13,102,103 are generated intraphagosomally and around ingested bacteria. In the presence of heme enzyme inhibitors, hydrogen peroxide detected in the medium can account for most of the oxygen consumed.104 105
On the assumption that ingestion of 15 to 20 bacteria gives maximal oxygen consumption, we have calculated that superoxide should be formed in the phagosomal space at the extraordinarily high rate of 5 to 10 mmol/L per second.106 Based on granule numbers, the myeloperoxidase released should reach a concentration of 1 to 2 mmol/L. Generation of large amounts of HOCl would be expected. However, the enzymology of myeloperoxidase is complex (Fig 2)49 and the efficiency of HOCl production is strongly dependent on conditions. Activity is decreased at high pH and at high hydrogen peroxide and chloride concentrations.107,108 Numerous physiological and pharmacological compounds that act as poor peroxidase substrates and reversibly inactivate the enzyme also inhibit HOCl production.109,110 It is likely that these substrates could modulate HOCl production in vivo. Superoxide reacts with myeloperoxidase107 to form a complex (Compound III) that lies outside the normal catalytic cycle. Superoxide can also reactivate myeloperoxidase that has become reversibly inhibited through compound II formation.108
We have developed a kinetic model of the phagosome, incorporating the known reactions of myeloperoxidase, hydrogen peroxide and superoxide, and their rate constants, to address how myeloperoxidase acts in the phagosomal environment (manuscript in preparation). Predictions from the model are consistent with direct spectral observation107 that superoxide initially reacts with the myeloperoxidase to convert it to compound III. To see significant peroxidase activity or HOCl generation, the compound III must turn over. Although this has been proposed to occur via reaction with hydrogen peroxide,108 this mechanism is much too slow to give any significant HOCl production. For myeloperoxidase to continue to function after the first few seconds, a reaction between compound III and superoxide must be invoked. Such a reaction has been proposed,111 and studies with purified myeloperoxidase provide further evidence for it.31 Myeloperoxidase can then handle the high rates of formation of superoxide and hydrogen peroxide such that neither builds up beyond micromolar concentrations, and the majority of the oxygen consumed is converted to HOCl. This system appears to be reasonably robust, with realistic variations in superoxide flux, myeloperoxidase release, phagosomal volume, and hydrogen peroxide scavenging by the cytoplasm making little difference to the efficiency of HOCl formation.
Until recently, evidence that HOCl is formed in the phagosome has been indirectly based on the incorporation of 36Cl or radiolabeled iodide into organic material during the ingestion of bacteria.112-115 More definitive evidence has come from recent measurements of chlorotyrosine and chlorinated fluorescein as specific markers of HOCl production. Hazen et al116 trapped tyrosine within red blood cell ghosts and showed that it became chlorinated when the ghosts were phagocytosed. In a related study, we have recovered ingested bacteria from neutrophil phagosomes and shown that protein hydrolysates contain chlorotyrosine that was not present in the isolated neutrophils or bacteria.117 Hurst et al have recently followed up earlier studies of bleaching of fluorescein attached to ingested latex beads118 to show that this is caused by chlorination.119 They calculated that at least 12% of the oxygen consumed was converted to HOCl within the phagosome.
The kinetic modeling has enabled assessment of why it might be advantageous for the neutrophil to produce superoxide rather than hydrogen peroxide directly. If superoxide is removed from the system, we find that the HOCl production becomes sensitive to fluctuations in oxidant flux or the amount of myeloperoxidase released into the phagosome. Under some conditions HOCl production is enhanced but without superoxide to regenerate the native enzyme from compound II, myeloperoxidase becomes prone to inhibition by electron donors that readily reduce compound I but not compound II. We speculate that substrates such as tryptophan and nitrite could be present in the phagosome and impair HOCl production by this mechanism. So for the neutrophil to maintain its myeloperoxidase activity without stringent environmental requirements, there would be a clear advantage in generating superoxide.
Experiments have not been performed with appropriate substrates to establish whether myeloperoxidase-derived oxidants other than HOCl are produced intraphagosomally. However, studies using an antibody against nitrotyrosine suggest that a nitrating agent can be formed when bacteria are ingested by cytokine-treated buffy coat neutrophils.86
CONTRIBUTION OF OXIDANTS TO BACTERIAL KILLING BY NEUTROPHILS
Oxidative and nonoxidative mechanisms.
Efficient control of a multitude of microorganisms is so important for host survival that the neutrophil does not rely on a single antimicrobial weapon. This review concentrates on oxidative mechanisms, but as discussed elsewhere,120-122 this is complemented by nonoxidative killing by granule proteins that are released into the phagosome. The mechanism that predominates may vary depending on the microbial species, its metabolic state, and the prevailing conditions.61
Optimal killing of many species of bacteria requires products from the oxidative burst. This is best exemplified in CGD, where affected individuals have an impaired or completely absent oxidative burst and suffer from recurrent and life-threatening infections.9,10The strains of bacteria that are killed poorly in vitro are responsible for the infections that are characteristic of CGD.10 Normal neutrophils tested in anaerobic environments, or in the presence of the NADPH oxidase inhibitor diphenyleneiodonium, are also impaired in their ability to kill these bacteria.123-126 Other species are killed normally, either because they are catalase-negative and able to supply an alternative source of hydrogen peroxide,127 128or because they can be disposed of effectively by nonoxidative mechanisms.
Myeloperoxidase and HOCl.
Myeloperoxidase appears critical for oxidative killing in experimental systems. Neutrophils isolated from the blood of myeloperoxidase-deficient individuals kill a variety of microorganisms poorly,129-131 and inhibitors of myeloperoxidase such as azide, cyanide, and salicylhydroxamic acid impair killing by normal cells.106,130,132,133 Neutrophil cytoplasts that lack granule enzymes but generate hydrogen peroxide only kill bacteria if they are coated with myeloperoxidase before ingestion.134
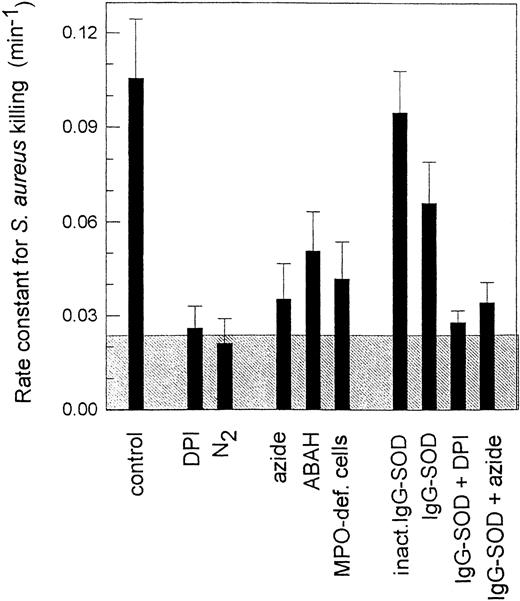
Measurements of rates of killing of S aureus by neutrophils isolated from human blood reinforce the importance of myeloperoxidase.106 126 Inhibition of the oxidative burst with diphenyleneiodonium, or removal of oxygen, decreases the rate constant for killing by 80%, enabling separation of the oxidative and nonoxidative components (Fig 4). Killing rates are substantially decreased in the presence of the myeloperoxidase inhibitors azide and 4-aminobenzoic acid hydrazide, and with myeloperoxidase-deficient neutrophils. Only the oxidative component is affected, and is six times slower when myeloperoxidase is not active. These results indicate that, at least with S aureus, the normal mechanism for oxidative killing uses myeloperoxidase. Direct killing by hydrogen peroxide, or other alternative oxidative mechanisms, are poor substitutes.
Rate constants for killing of S aureus by human neutrophils. Opsonized bacteria were mixed with neutrophils in a 1:1 ratio. Numbers of extracellular and viable intracellular bacteria were measured at 0, 10, 20, and 30 minutes, and from these independent first-order rate constants for phagocytosis and killing were measured. Superoxide dismutase was conjugated to IgG (IgG-SOD) and attached to the bacteria through binding to the protein A on their surface. ABAH, the myeloperoxidase inhibitor 4-aminobenzoic acid hydrazide. The shaded area represents the contribution of nonoxidative killing measured in the presence of diphenyleneiodonium (DPI) or anaerobically (N2). The data are taken from Hampton,117 and show the mean and SD of at least three experiments.
Rate constants for killing of S aureus by human neutrophils. Opsonized bacteria were mixed with neutrophils in a 1:1 ratio. Numbers of extracellular and viable intracellular bacteria were measured at 0, 10, 20, and 30 minutes, and from these independent first-order rate constants for phagocytosis and killing were measured. Superoxide dismutase was conjugated to IgG (IgG-SOD) and attached to the bacteria through binding to the protein A on their surface. ABAH, the myeloperoxidase inhibitor 4-aminobenzoic acid hydrazide. The shaded area represents the contribution of nonoxidative killing measured in the presence of diphenyleneiodonium (DPI) or anaerobically (N2). The data are taken from Hampton,117 and show the mean and SD of at least three experiments.
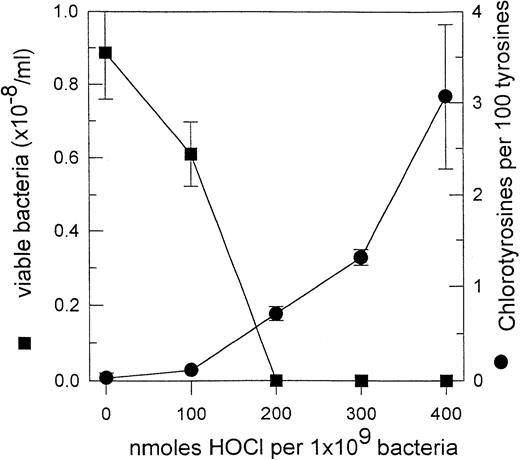
Although HOCl stands out as the prime candidate for the lethal agent produced by myeloperoxidase, there is currently insufficient evidence to exclude other products of the enzyme. We recently observed that the fraction of tyrosyl residues converted to chlorotyrosine in phagocytosed S aureus (0.5% ± 0.2%, SEM of 10 experiments) was similar to that for S aureus treated with a lethal amount of HOCl (Fig 5). This suggests that enough HOCl is generated in the phagosome for it to be responsible for killing. A similar conclusion was reached by Jiang et al119 measuring fluorescein chlorination. Inhibition of killing of Candida pseudohyphae by scavengers of HOCl and chloramines also supports the involvement of chlorinated oxidants.135 However, more direct evidence is necessary to confirm this role for HOCl.
Chlorotyrosine formation and loss of viability for S aureus exposed to reagent HOCl. Bacteria (1 × 108/mL) were treated with a range of concentrations of HOCl and then analyzed for tyrosine and chlorotyrosine content,165 and the number of remaining viable colony-forming units. The results are taken from Hampton.117 The means and SD of three experiments are reported.
Chlorotyrosine formation and loss of viability for S aureus exposed to reagent HOCl. Bacteria (1 × 108/mL) were treated with a range of concentrations of HOCl and then analyzed for tyrosine and chlorotyrosine content,165 and the number of remaining viable colony-forming units. The results are taken from Hampton.117 The means and SD of three experiments are reported.
Role of superoxide.
Neutrophils must generate superoxide to kill oxidatively. Its role could simply be as a precursor of hydrogen peroxide, or it could participate directly in the killing process. Distinguishing between these possibilities experimentally is complicated by the difficulty of getting sufficient superoxide dismutase (SOD) into the phagosome to scavenge all the superoxide generated. Adding SOD to phagocytosing neutrophils136 or modifying the expression of SOD in target bacteria137-142 has generally had little effect, but this could be because the SOD did not gain access to the phagosome. The few studies where this has been achieved indicate a direct role for superoxide in killing. Johnston et al136 showed that the killing of S aureus was impeded when SOD-coated latex beads were co-ingested with the bacteria. The accessibility problem has also been overcome by attaching SOD to the surface of S aureus.106 The SOD was covalently crosslinked to IgG that then bound to protein A in the cell wall. The bacteria were ingested normally, but the rate constant for killing was decreased by 30% (Fig 4). This represents a decrease in rate of oxidative killing to almost a half. SOD had no effect in the presence of peroxidase inhibitors, which suggests that it acts on a myeloperoxidase-dependent process.
The effect of SOD could be explained on the basis of its inhibiting hydroxyl radical production.136 If the route to hydroxyl radicals was via superoxide and HOCl, this could also explain the apparent involvement of a myeloperoxidase-dependent process. However, as argued above, the hydroxyl radical is unlikely to be a major player in the phagosome. An alternative explanation, which is consistent with the modeling studies of oxidant production, is that superoxide prevents reversible inactivation of myeloperoxidase, thereby optimizing killing by HOCl. More direct analyses are needed before firm conclusions can be drawn on the mechanism.
In the context of superoxide having a direct role in killing, it is of interest that Mycobacterium tuberculosis,143,Nocardia asteroides,144,Helicobacter pylori,145 and Actinobacillus pleuropneumoniae146 all secrete SOD. Antibodies to the superoxide dismutase of N asteroides enhanced both bacterial killing by neutrophils147 and clearance upon inoculation of mice.148 It is possible that this surface-associated superoxide dismutase could slow down intraphagosomal killing and be a factor in their pathogenicity.
MYELOPEROXIDASE DEFICIENCY
Although myeloperoxidase deficiency affects at least 1 in 4,000 people, these people are not unduly prone to infections.10 Only occasional increased susceptibility to Candida infection has been noted, and doubts have even been raised about whether myeloperoxidase has a role in bacterial killing.6 149 This contrasts dramatically with CGD, where the NADPH oxidase is absent. In CGD, common pathogens including S aureus cause life-threatening problems. Yet in vitro tests show markedly impaired oxidative killing for both types of neutrophil. On this basis it would be reasonable to expect individuals with CGD and myeloperoxidase deficiency to be similarly compromised in their ability to handle certain microorganisms. The key question is: what compensates for the defect in oxidative killing and prevents infections in myeloperoxidase deficiency?
The usual explanation is that an alternative oxidative killing mechanism operates as a backup. Myeloperoxidase-deficient neutrophils do consume more oxygen than normal130,150 and show extended superoxide and hydrogen peroxide production,150,151 along with increased phagocytosis152 and degranulation.153 These changes can be attributed to a lack of myeloperoxidase-dependent autoinactivation of neutrophil functions. One possibility is that sufficient hydrogen peroxide builds up in the absence of myeloperoxidase to kill directly or via hydroxyl radicals.154 However, myeloperoxidase-deficient cells release only slightly more hydrogen peroxide than normal, because of consumption by catalase,150 and since the hydroxyl radical production that has been detected in neutrophils is myeloperoxidase-dependent39 it should be diminished in deficient cells. We found that oxidative killing of S aureus by normal cells in the presence of azide was no better than with myeloperoxidase-deficient neutrophils, which accumulate less peroxide.106 Indeed, the difference in oxidative killing between cells lacking myeloperoxidase and NADPH-oxidase activity was so slight as to raise the possibility of whether there is a significant oxidative component independent of myeloperoxidase. The nonoxidative killing capacity of myeloperoxidase-deficient cells may be slightly enhanced,106,132 and it is possible to select in vitro conditions where these cells kill normally.61 However, CGD cells also kill normally under these conditions.
In our opinion, any slow oxidative killing that has been measured in vitro with myeloperoxidase-deficient cells does not provide a convincing explanation for the benign nature of myeloperoxidase deficiency and there is a need to look beyond killing by isolated neutrophils. One consideration is that NADPH oxidase is expressed in a number of inflammatory cells, including macrophages and eosinophils,155 whereas only neutrophils and monocytes have myeloperoxidase. CGD will affect a wider spectrum of cells than myeloperoxidase deficiency and this could contribute to its greater severity. Another possibility is that cytokines encountered by neutrophils as they move to a site of inflammation, or attachment to the endothelium, activate processes that assist killing. Both can enhance the oxidative burst.156,157 They may also activate neutrophils to express nitric oxide synthase.85 86 If so, a plausible scenario would be for peroxynitrite, generated from superoxide and nitric oxide, to act as a backup defense that abrogated the need for myeloperoxidase. Peroxynitrite might also be produced if nitric oxide from adjacent endothelial or mononuclear cells gained access to the neutrophil phagosome.
Alternatively, an aspect of pathogen clearance other than killing ability may distinguish the two enzyme deficiencies. One proposal is that neutrophil oxidants, but not myeloperoxidase, are critical for digestion rather than killing.158 A crucial phase of inflammation is the removal of neutrophils along with their ingested bacteria. Neutrophils become apoptotic once they have undergone phagocytosis, and oxidase products are implicated in the process.159,160 A critical step is the expression of surface markers such as phosphatidylserine that target the cells for ingestion and removal by macrophages.161 We have recently found that normal but not CGD neutrophils expose phosphatidylserine after stimulation with phorbol myristate acetate (Fadeel et al, manuscript submitted). However, myeloperoxidase-deficient cells or cells treated with azide exposed as much phosphatidylserine as normal cells (M.B. Hampton, C.C. Winterbourn, in preparation). Thus, the process requires hydrogen peroxide generation but not myeloperoxidase-derived oxidants. This mechanism could explain the different outcomes in myeloperoxidase-deficiency and CGD. Clearance of myeloperoxidase-deficient neutrophils by macrophages would be normal, even if their bacteria were killed more slowly. In contrast, CGD neutrophils would not be ingested, and their accumulation could give rise to the characteristic granulomas of the disease. A mouse model of chronic granulomatous disease has recently been developed.162-164 Neutrophils from these animals were defective not only in killing but also in their ability to dispose of dead microorganisms. Further studies with gene knockout models should help to test the proposals outlined above and bridge the gap between in vitro studies and clinical profiles.
CONCLUSION
In the century since Metchnikoff observed phagocytic cells ingesting bacteria, considerable progress has been made toward understanding the mechanisms involved in killing. However, there is still controversy and disagreement among researchers over some fundamental issues. HOCl appears as the most likely mediator of oxygen-dependent bacterial killing in the neutrophil phagosome. Chlorinated markers indicate that HOCl is generated in lethal amounts; however, analysis of the enzymology of myeloperoxidase has shown that a number of other reactions may occur, and it is not known whether the specific prevention of HOCl production affects bacterial killing. Superoxide is integral to many of the activities, and the ability of superoxide dismutase to inhibit killing suggests that superoxide is important in the physiological function of myeloperoxidase. Elucidating the biochemistry of the phagosome may ultimately lead to an understanding of how some pathogens can survive in such a harsh environment, and will assist in the development of therapies to attenuate the inflammatory pathologies where neutrophils unleash their destructive potential against host tissue.
Supported by the Health Research Council of New Zealand.
Address reprint requests to Christine C. Winterbourn, PhD, Department of Pathology, Free Radical Research Group, Christchurch School of Medicine, PO Box 4345, Christchurch, New Zealand; e-mail: ccw@chmeds.ac.nz.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal