To the Editor:
Genetic hemochromatosis (GH) is a common HLA-linked recessive disorder characterized by progressive parenchymal iron loading and the appearance of clinical manifestations in the fifth decade of life, predominantly in males. HFE has been recently identified as the candidate gene, with most patients being homozygous for a Cys-282 → Tyr (C282Y) mutation and others being compound heterozygotes for C282Y and a second mutation, His-63 → Asp (H63D).1 Homozygosity for C282Y is found in more than 90% of North European patients,2 but in only 64% of severely iron-loaded Italian individuals.3 This finding may suggest that various genetic iron overload syndromes exist in addition to the HFE-related one.
Fifteen years ago, we described cases of juvenile GH suggesting that this was a distinct disease entity.4 In the juvenile condition, males and females appear to be equally affected. Patients present with hypogonadotropic hypogonadism and, unless proper treatment is started, die early because of cardiac dysfunction. We now provide further evidence that the juvenile condition is clinically and genetically distinct from the classical adult disorder.
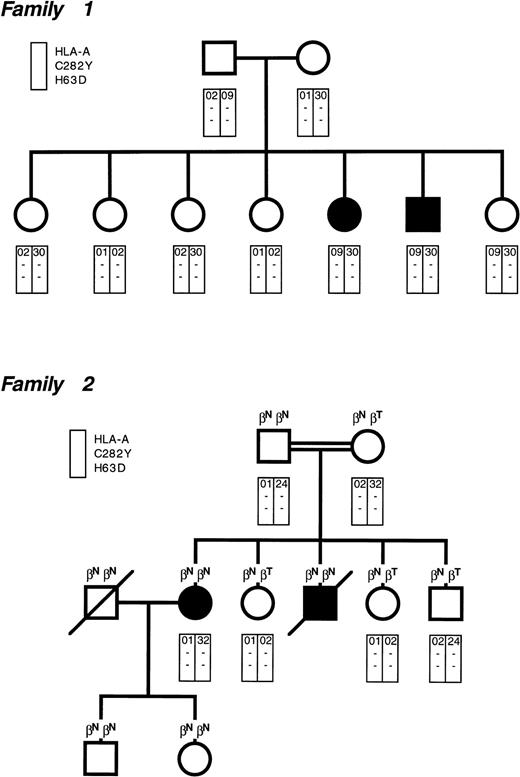
The pedigrees of our two Italian families with juvenile GH are shown in Fig 1. The clinical features of family 1 were reported in 1983,4 whereas family 2 has never been described. Of the four affected individuals, three presented with hypogonadotropic hypogonadism at 14 to 21 years of age. The affected male of family 2 presented with cardiac failure at 20 years of age and died at 21 years of age of congestive cardiomyopathy.
Pedigree of the two families with juvenile genetic hemochromatosis. Circles denote female family members and squares denote male family members. Solid symbols indicate probands. In family 2, the parents were consanguineous (first cousins). Each rectangle indicates the HLA-A locus and the presence (+) or absence (−) of the C282Y and H63D mutations. All individuals but the probands had normal body iron status. Some individuals in family 2 were heterozygous for β-thalassemia (βNβT), whereas the remaining ones had normal β-gene (βNβN).
Pedigree of the two families with juvenile genetic hemochromatosis. Circles denote female family members and squares denote male family members. Solid symbols indicate probands. In family 2, the parents were consanguineous (first cousins). Each rectangle indicates the HLA-A locus and the presence (+) or absence (−) of the C282Y and H63D mutations. All individuals but the probands had normal body iron status. Some individuals in family 2 were heterozygous for β-thalassemia (βNβT), whereas the remaining ones had normal β-gene (βNβN).
There is no relationship in family 1 between HLA-A antigens and iron overload. In fact, although the two probands are HLA identical, the youngest sibling has the same HLA constellation, but a fully normal body iron status at 30 years of age. Family 2 is not informative with respect to HLA linkage, but helps to exclude any interaction between hemochromatosis and β-thalassemia.
All of the family members we examined using the approach of Lynas5 were found to be negative for the C282Y and H63D mutations in the HFE gene (Fig 1). In a study on 7 Italian patients belonging to 5 unrelated families with juvenile GH, Camaschella et al6 have independently excluded the HFE gene as responsible for this condition. Segregation analysis of 6p markers closely associated with HFE showed, in fact, that juvenile GH is unlinked to 6p and thus genetically distinct from HFE.
Both patients of family 1 and the young lady of family 2 underwent regular phlebotomies. Based on the amount of iron mobilized by bleedings, we estimated that these subjects had body iron stores ranging from 220 to 329 mg/kg of body weight at the time of diagnosis at 17 to 21 years of age. Once the three patients became iron deficient at the end of the intensive phlebotomy program, they underwent regular venesections to maintain serum ferritin levels less than 100 μg/L. At the time of our writing, they have been observed in this way for 7 to 16 years. Based on phlebotomy requirements for maintenance of normal iron balance, the rate of estimated iron accumulation (positive iron balance) ranged from 3.2 to 3.9 mg/d. This was clearly higher than the rate of 0.8 to 1.6 (1.2 ± 0.3) mg/d found in 5 adult males who are homozygous for the C282Y mutation. This remarkable difference in iron overprocurement (1 to 2 v 3 to 4 mg/d) suggests completely different pathogenetic mechanisms.
In conclusion, juvenile GH is an autosomal recessive condition clinically and genetically different from the classical adult disorder. HLA-typing and HFE-based genotyping are useless, and diagnosis must rely on conventional evaluation of body iron status. Identification of the candidate gene through positional cloning could unveil a new crucial protein of iron metabolism in humans.
ACKNOWLEDGMENT
Supported by grants from IRCCS Policlinico S. Matteo, Pavia, and MURST, Rome, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal