Abstract
Early in development, murine B-lineage progenitor cells express two classes of IgG Fc receptors (FcγR) designated as FcγRII (CD32) and FcγRIII (CD16), but mature B lymphocytes only express FcγRII (CD32), which functions as an inhibitor of B-cell activation when it is induced to associate with mIgM. The functions of CD16 and CD32 on B-lineage precursor cells have not previously been investigated. To search for FcγR functions on developing B-lineage cells, normal murine bone marrow cells were cultured in the presence of 2.4G2, a rat monoclonal antibody that binds to CD16 and CD32, or in the presence of control normal rat IgG, and then the B-lineage compartment was analyzed for effects. Cultures that contained 2.4G2 showed enhanced growth and differentiation of B-lineage cells compared with control cultures. The enhancing effect of 2.4G2 also occurred when fluorescence-activated cell-sorted B-cell precursors (B220+, sIgM−, HSAhigh, FcγR+) from normal bone marrow were cocultured with BMS2, a bone marrow stromal cell line, but not when they were cultured in BMS2-conditioned media. The enhancement of B-lineage development induced by 2.4G2 was CD16-dependent and CD32-dependent, because 2.4G2 did not effect B-lineage growth or differentiation in cultures of bone marrow from mice in which either the gene encoding CD16 or CD32 had been disrupted. Analysis of fresh bone marrow from the CD16 gene-disrupted mice showed normal numbers and distribution of cells within the B-cell compartment, but in CD32 gene-disrupted mice, the B-cell compartment was significantly enlarged. These experiments provide several lines of evidence that the FcγR expressed on murine B-cell precursors can influence their growth and differentiation.
© 1998 by The American Society of Hematology.
Fc RECEPTORS DESIGNATED as FcγR bind to epitopes located in the constant region domains of IgG and are expressed on cells of hematopoietic lineages in which they mediate multiple physiological functions.1,2 Most previous studies of FcγR function have focused on mature cells and have identified a diversity of roles for FcγR on macrophages, polymorphonuclear leukocytes, natural killer (NK) cells, and lymphocytes. Although FcγR are expressed very early in hematopoietic cell ontogeny,3-7 the functional significance of these receptors on hematopoietic progenitor cells is unknown.
During murine lymphopoiesis, T- and B-lineage progenitor cells express FcγRIII (CD16) and FcγRII (CD32), but with the initiation of antigen receptor gene rearrangement, CD16 is downregulated in B cells and T cells, and CD32 is downregulated in T cells.8 These curious patterns of FcγR displayed on developing lymphoid cells raise the possibility that CD16 and CD32 may participate in some aspect of T- and B-cell development before the stage at which these lymphoid cells become antigen responsive.
That multiple functions have been associated with FcγR on mature myeloid and lymphoid cells can be partially accounted for by the structural heterogeneity of FcγR molecules. The chemical structures of members of the family of FcγR show interesting similarities and striking differences, characteristics that have important functional implications. There is 95% identity in the amino acid sequences of the ligand-binding, extracellular segments of FcγRII and FcγRIII, the two low-affinity IgG receptors expressed on lymphocytes, but the sequences of the transmembrane and cytoplasmic signaling segments are structurally unrelated.9,10 The cytoplasmic segment of CD16 associates with a homodimer of an activation signaling molecule termed FcRγ, which is a member of the CD3ζ chain family.11 The γ-chain contains an ITAM (immunoreceptor tyrosine activating motif)12 that, when tyrosine phosphorylated, transduces cellular activation signals. The cytoplasmic segment of CD32 contains a phosphorylable tyrosine in a 13 amino acid motif designated an ITIM (immunoreceptor tyrosine inhibitory motif) that inducibly associates with the tyrosine phosphatase SHP-1 and can inhibit ITAM-induced cellular activation.13 14
We were interested in the functional significance of FcγR on progenitor B-lineage cells because, in a previous study,5FcγR on murine fetal prothymocytes had been shown to influence the rate of differentiation to fully mature, immunocompetent thymocytes. In those experiments, the addition of an anti-FcγR monoclonal antibody (MoAb; 2.4G2) to fetal thymic organ cultures resulted in a dose-dependent acceleration in thymocyte maturation to αβ TCR+, HSAdim, CD3bright T cells. 2.4G2 recognizes an epitope in the extracellular segment of the FcγR and does not distinguish between CD16 and CD32. Acceleration of thymocyte maturation was also induced by the addition of soluble recombinant FcγR to the fetal thymic organ cultures. This combination of findings raised the possibility that FcγR on pro-T cells normally interacted with alternative non-Ig ligand(s) and that this interaction had the effect of modulating the rate of differentiation of prothymocytes to mature T cells. Experimental blockade of this putative interaction, by the addition of anti-FcγR MoAb or soluble recombinant FcγR to the thymic organ cultures, resulted in a faster rate of T-cell maturation. In support of the alternative ligand hypothesis was our finding that soluble recombinant FcγR bound strongly to a small population of CD44−, CD3− thymic stromal cells.5 Additional evidence for a role of CD16 in modulating the maturation of prothymocytes was the finding that overexpression of CD16 on prothymocytes in mice expressing an FcRγ-chain transgene driven by a CD2 promoter was associated with a decreased rate of differentiation to mature thymocytes.15
Because FcγR are also expressed on B-lineage precursor cells16 and because we had observed that soluble recombinant FcγR could bind to normal murine bone marrow stromal cells and to BMS2, a murine bone marrow stromal cell line,5we considered the possibility that FcγR on B-cell precursors might influence the development of B-lineage cells. In the studies reported below, we found that the addition of 2.4G2 accelerated B-lineage lymphopoiesis in cultures of bone marrow from normal mice, but not in cultures of bone marrow from CD16 or CD32 knockout mice. These studies provide experimental evidence that FcγR on developing B-lineage cells can modulate B-cell development.
MATERIALS AND METHODS
Cultures of bone marrow from normal mice.
C57Bl6/129-CD16 gene-disrupted mice (CD16−/−) were constructed by Dr Sjef Verbeek (Department of Immunology, University of Utrecht, Utrecht, The Netherlands).17C57Bl6/129-CD32 gene-disrupted mice were purchased from Taconic (Germantown, NY). Caged mice were maintained in a horizontal laminar flow cabinet and provided with sterile food and water. Femoral and tibial bone marrow samples were taken from 6- to 9-week-old mice, and a single-cell suspension was prepared in Hanks’ balanced salt solution (HBSS) following a previously described procedure,18 with modifications. After three washes, 2 × 105 cells/mL were resuspended in RPMI 1640 supplemented with 10% bovine calf serum, 0.2 mmol/L L-glutamine, 0.1 mmol/L essential and nonessential amino acids, 0.1 mmol/L Na-pyruvate, 5 × 10−5 mol/L 2-βME, and an antibiotic-antimycotic. All cell culture reagents used in this study, unless otherwise specified, were purchased from GIBCO/BRL (Grand Island, NY). In some experiments, bone marrow cells in 24-well plates (#3424; Costar, Cambridge, MA) were cultured in the presence of 10 U/mL recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), 20 U/mL recombinant interleukin-5 (rIL-5), and 10 U/mL rIL-3 (Genzyme, Cambridge, MA).18 In other experiments, a media formulation containing IL-7 was used to promote the growth of B cells.19 Viability of the cultured cells was determined by Trypan blue dye exclusion.
MoAbs.
The MoAbs used in the fluorescence-activated cell sorting (FACS) analyses were labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or cyanine 5.18 (Cy5). They included RB6-8C5 (a rat IgG2b reactive with the granulocyte marker, Gr-1), 6B2 (a rat IgG2a antimurine CD45R/B220), Mac-1 (a rat IgG2b antimurine CD11b), c-kit (a rat IgG2b antimurine CD117), S7 (a rat IgG antimurine CD43), and M1/69 (rat IgG2b antimurine CD24, HSA; all purchased from Pharmingen, San Diego, CA); and antimouse IgM (a hamster IgG; purchased from Jackson ImmunoResearch, West Grove, PA). In bone marrow culture experiments, 2.4G2, a rat IgG2b antimurine FcγRII/RIII,20 was added to the cultures (25 μg/mL). In some experiments, a control rat IgG (purified rat IgG2b; Pharmingen) was added; in other experiments, the control was cytokines alone. Both antibodies had previously been dialyzed against phosphate-buffered saline (PBS) and filtered under sterile conditions before addition to the cultures. Endotoxin was not detected in 2.4G2 MoAbs by the Lumulus amebocyte lysate assay (Pyrochrome, Cape Cod, MA).
In some experiments, we used these MoAbs to determine the size of the B-lineage subpopulations as defined by Wasserman et al.27
Flow cytometric analysis.
Cells were suspended at a concentration of 107 cells/mL in HBSS buffer containing 10% bovine calf serum, 10 mmol/L HEPES, and 0.02% Na-azide (FACS staining buffer). Cells were stained in 50 μL of conjugated Abs for 40 minutes at 4°C, washed three times, fixed in 2% paraformaldehyde in PBS (pH 7.3), and analyzed on a Becton Dickinson FACS Vantage (Becton Dickinson, Mountain View, CA) equipped with four decade logarithmic amplifiers. Forward and side scatter and two or three fluorescence parameters were collected on 10,000 cells and the data were analyzed on a VAX 4000 computer equipped with DESK software.16 For sorting pro/pre-B cells (B220+, IgM−), 2 × 107cells/mL were stained with anti-B220 and anti-IgM in FACS staining buffer. Cells were collected on a Coulter Epics 753 flow cytometer (Coulter, Hialeah, FL) and analyzed using Coulter Elite software (University of Iowa College of Medicine Flow Cytometry Facility, Iowa City, IA). Reanalysis of the sorted populations showed a purity greater than 98%.
Cocultures of progenitor B cells with stromal cell lines.
Sorted progenitor B cells (B220+, IgM−, HSAhi, 2 × 105 in 2.0 mL) were cultured for 3 days with the stromal cell line BMS2 generously provided by Dr P.W. Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK). The BMS2 stromal cell line was maintained in complete media and plated into 6-well plates (Costar 3406) at a density of 1 × 105 cells per 6-cm2 well and expanded to 80% confluence to support pre–B-lymphocyte growth.21 22
RESULTS
B-lymphopoiesis and eosinophilopoiesis are induced in bone marrow cells cultured with IL-3, IL-5, and GM-CSF.
After 72 hours of culture in the presence of GM-CSF, IL-3, and IL-5, the B220+ population had increased approximately 1.5-fold, and about 43% of the cultured cells expressed B220 (Table 1). The number of B220+/IgM+ cells had increased approximately threefold, and these accounted for about one third of the cultured cells (Table 1). Microscopic examination of Wright-Giemsa–stained smears showed that bone marrow cells freshly isolated from normal, 6- to 9-week-old mice contain less than 5% eosinophils, but in the presence of GM-CSF, IL-3, and IL-5 this had increased to approximately 20% by 72 hours of culture. This cytokine protocol was being used because we were investigating the role of FcR in eosinophilopoiesis when the initial observation of the effect of 2.4G2 on B-cell development was made. Under the conditions of culture used, virtually all the Gr-1+ cells present at 72 hours were eosinophils.23 Neutrophils were not detected by Wright-Giemsa microscopic examination of smears. In addition, uncommitted progenitors and stromal cells (c-kit+ cells) account for approximately 46% of the cultured cells at 72 hours. The percentage of cells expressing T-cell markers at 72 hours was 5% or less.
Effect of 2.4G2 on B-Lymphopoiesis in C57BL6/129+/+, CD16−/−, and CD32−/−
| . | Fresh Bone Marrow . | Bone Marrow Culture at 72 h . | ||||
|---|---|---|---|---|---|---|
| Cytokines . | Cytokines +2.4G2 . | |||||
| No. . | %-150 . | Total No. . | % . | Total No. . | % . | |
| B220+ cells | ||||||
| wt+/+ | 22,000 ± 9,200 | 32 ± 3 | 33,285 ± 8,741 | 43 ± 3 | 48,000 ± 3,390 | 48 ± 4 |
| CD16−/− | 26,414 ± 13,480 | 38 ± 6 | 14,538 ± 3,800-151 | 49 ± 11 | 16,071 ± 1,739-152 | 47 ± 10 |
| CD32−/− | 43,950 ± 11,855-151 | 40 ± 14 | 28,923 ± 5,519 | 28 ± 6 | 23,259 ± 12,419 | 28 ± 9 |
| B220+/IgM+ cells | ||||||
| wt+/+ | 6,893 ± 3,378 | 12 ± 2 | 21,895 ± 6,159 | 34 ± 4 | 30,255 ± 3,331 | 39 ± 5 |
| CD16−/− | 6,437 ± 2,163 | 10 ± 0.2 | 8,511 ± 1,131-151 | 34 ± 3 | 10,240 ± 814-152 | 36 ± 6 |
| CD32−/− | 16,755 ± 4,391-151 | 19 ± 8 | 18,193 ± 3,325 | 16 ± 5 | 14,308 ± 8,530 | 16 ± 6 |
| . | Fresh Bone Marrow . | Bone Marrow Culture at 72 h . | ||||
|---|---|---|---|---|---|---|
| Cytokines . | Cytokines +2.4G2 . | |||||
| No. . | %-150 . | Total No. . | % . | Total No. . | % . | |
| B220+ cells | ||||||
| wt+/+ | 22,000 ± 9,200 | 32 ± 3 | 33,285 ± 8,741 | 43 ± 3 | 48,000 ± 3,390 | 48 ± 4 |
| CD16−/− | 26,414 ± 13,480 | 38 ± 6 | 14,538 ± 3,800-151 | 49 ± 11 | 16,071 ± 1,739-152 | 47 ± 10 |
| CD32−/− | 43,950 ± 11,855-151 | 40 ± 14 | 28,923 ± 5,519 | 28 ± 6 | 23,259 ± 12,419 | 28 ± 9 |
| B220+/IgM+ cells | ||||||
| wt+/+ | 6,893 ± 3,378 | 12 ± 2 | 21,895 ± 6,159 | 34 ± 4 | 30,255 ± 3,331 | 39 ± 5 |
| CD16−/− | 6,437 ± 2,163 | 10 ± 0.2 | 8,511 ± 1,131-151 | 34 ± 3 | 10,240 ± 814-152 | 36 ± 6 |
| CD32−/− | 16,755 ± 4,391-151 | 19 ± 8 | 18,193 ± 3,325 | 16 ± 5 | 14,308 ± 8,530 | 16 ± 6 |
P values were calculated by comparison with the wild-type.
Percentage of the lymphoid gate.
P < .05.
P < .001.
2.4G2 accelerates the differentiation of progenitor B cells in bone marrow cultures.
To determine if B-lymphopoiesis is influenced by FcγR interactions, bone marrow cells were cultured in the presence of IL-3, IL-5, and GM-CSF and 25 μg/mL of the anti-FcγR MoAb (2.4G2) to engage the FcγRs CD16 and CD32. As shown in Table 1, cultures that contained 2.4G2 had approximately 50% more B220+ cells and B220+/IgM+ cells compared with cultures not containing 2.4G2. To further examine the effect of 2.4G2 on B-lymphopoiesis, additional markers were assessed by FACS analysis.
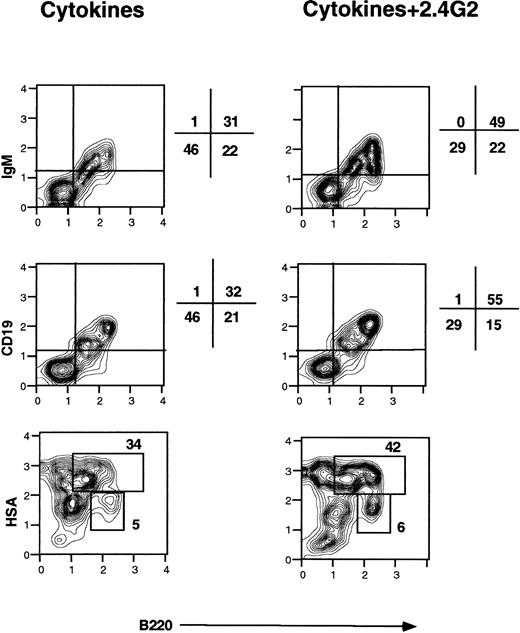
Figure 1 shows a representative flow cytometric analysis of bone marrow cells after 3 days of culture with cytokines in the presence or absence of 2.4G2. In addition to the significant increment in the size of the B220+/IgM+ population induced by treatment with 2.4G2, Fig 1 shows that bone marrow cells cultured with cytokines and 2.4G2 showed an enhanced expression of CD19 and HSA and that the level of B220 on individual cells was increased. In data not shown, the B220+/mIgM+ cells did not express mIgD, CD23, or CD25. Taken together, these findings indicate that 2.4G2 promoted differentiation of B-cell precursors to the stage of the immature B cell.24 25
Effect of 2.4G2 on B220+ cells in bone marrow cultures treated with IL-3, IL-5, and GM-CSF. Bone marrow cells (2 × 105 cells per well) were cultured with cytokines for 3 days in the presence of 2.4G2 (25 μg/mL) or with cytokines alone. The analysis shown was performed after setting a lymphocyte gate based on orthogonal and forward scatter. The figure shows one representative experiment of three with similar results.
Effect of 2.4G2 on B220+ cells in bone marrow cultures treated with IL-3, IL-5, and GM-CSF. Bone marrow cells (2 × 105 cells per well) were cultured with cytokines for 3 days in the presence of 2.4G2 (25 μg/mL) or with cytokines alone. The analysis shown was performed after setting a lymphocyte gate based on orthogonal and forward scatter. The figure shows one representative experiment of three with similar results.
Effect of 2.4G2 on the growth and differentiation of bone marrow B-lineage cells containing disrupted CD16 or CD32 genes.
Normal pro/pre-B cells are known to express FcγRIIβ1(CD32) and FcγRIII(CD16), but FcγRIII is detected only during the developmental stages before the expression of mIgM (Hagen et al, submitted for publication). In contrast, FcγRII is present throughout B-cell ontogeny, even to the plasma cell stage.16 To determine whether the 2.4G2-triggered acceleration of B-lineage development in vitro depended on the expression of CD16 and/or CD32, experiments were conducted with bone marrow cells obtained from mice in which the CD16 gene or the CD32 gene had been disrupted. The CD16−/− mice do not produce the α-chain subunit of FcγRIII, but they do produce the γ-chain subunit that is also a subunit of other immunoreceptors.17 The CD32−/− mice do not produce CD32 β chains.26 Following the same protocol as that described above, bone marrow cells from CD16−/− or CD32−/− mice were cultured with and without 2.4G2. We found that, in the bone marrow cultures from these mice, there was no significant effect of 2.4G2 on B-lineage growth or differentiation (Table 1). By 72 hours of culture, there was a decrease in the total number of B220+ cells, but not of B220+/IgM+ cells, in the samples from CD16−/− and CD32−/− mice. Thus, the loss of B-lineage cells in the cultures from CD16−/− and CD32−/− mice appears to have been selective for cells at stages before the expression of mIgM. The absence of an effect of 2.4G2 in the bone marrow cultures from CD16−/− and CD32−/−mice suggests that FcγR may play a previously unsuspected role in B-lymphopoiesis.
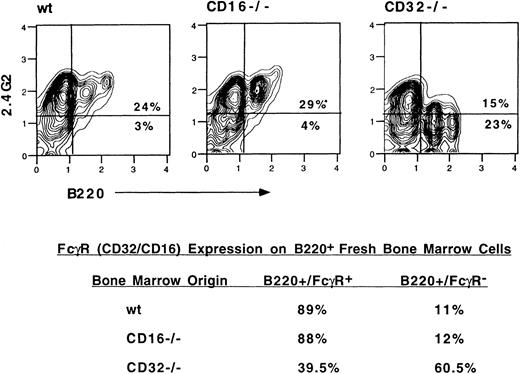
It was possible that the absence of any effect of 2.4G2 on the B-lineage cells from CD16−/− and CD32−/− mice and the decrease in B220+ cells in the cultures of bone marrow from these mice were reflections of some defect in the culture system. We thought that this possibility was unlikely because, in the cultures of the CD16−/− marrow, we found the same high level of eosinophil production as in the bone marrow cultures from normal mice. This indicted that myelopoiesis was proceeding normally in those cultures. However, because IL-7–containing media is the more conventional method to promote B-lymphopoiesis in vitro, we conducted additional experiments incorporating IL-7 into the cultures. The presence of IL-7 promoted a more robust level of B-lymphopoiesis (Table 2). Under these conditions of culture, the presence of 2.4G2 resulted in further increases in B220+ cells and B220+/IgM+ cells. To further explore this consideration, we examined B-lineage cells from normal, CD16−/−, and CD32−/− mice for their expression of FcγR as detected by FACS analysis of 2.4G2-binding cells. As shown in Fig 2, almost 90% of the B220+cells in the bone marrow from normal background strain mice bind 2.4G2, but only 40% of the B220+ cells from CD32−/− bind 2.4G2. These data suggest that normally CD16 is coexpressed with CD32 on some B-lineage cells, because, in its absence in CD16−/− mice, 2.4G2 continues to detect approximately the same percentage of B220+ cells. However, the data also suggest that CD32 is expressed on a large fraction of B220+ cells that do not coexpress CD16, because, in the CD32−/− mice, there is a large fraction of B220+ cells that are not detected with 2.4G2. In the CD32−/− mice, the vast majority of the B220+ cells that bind 2.4G2 belong to the low-density B220+ population, indicating that CD16 is expressed on the less mature B-lineage cells. This finding agrees well with data on CD16 and CD32 transcript expression in a large panel of B-lineage tumor cell lines and FACS-sorted normal B-lineage subpopulations (Hagen et al, submitted for publication).
Effect of IL-7 and 2.4G2 on B-Lymphopoiesis in C57BL6/129
| . | Total No. of B220+ Cells . | No. of B220+/IgM+ Cells . |
|---|---|---|
| −IL-7 | ||
| Control* | 30,831 ± 9,302 | 18,197 ± 4,570 |
| +2.4G2 | 44,737 ± 13,804 | 27,774 ± 10,259 |
| (1.54 SI) | (1.52 SI) | |
| +IL-7 | ||
| Control* | 99,560 ± 11,623 | 44,045 ± 6,448 |
| +2.4G2 | 128,660 ± 5,006 | 60,295 ± 3,867 |
| (1.29 SI) | (1.36 SI) |
| . | Total No. of B220+ Cells . | No. of B220+/IgM+ Cells . |
|---|---|---|
| −IL-7 | ||
| Control* | 30,831 ± 9,302 | 18,197 ± 4,570 |
| +2.4G2 | 44,737 ± 13,804 | 27,774 ± 10,259 |
| (1.54 SI) | (1.52 SI) | |
| +IL-7 | ||
| Control* | 99,560 ± 11,623 | 44,045 ± 6,448 |
| +2.4G2 | 128,660 ± 5,006 | 60,295 ± 3,867 |
| (1.29 SI) | (1.36 SI) |
Values were determined after 72 hours of culture using the cytokines and conditions described in the text.
Abbreviation: SI, stimulation index.
In some experiments, control cultures contained isotype-matched rat antibody of irrelevant specificity; in others, antibody was omitted.
FcγR expression on B220+ cells. Flow cytometric analysis of fresh bone marrow cells from wild-type, CD16−/−, and CD32−/− mice. The lower part of the figure shows the percentage of B220+ cells binding 2.4G2.
FcγR expression on B220+ cells. Flow cytometric analysis of fresh bone marrow cells from wild-type, CD16−/−, and CD32−/− mice. The lower part of the figure shows the percentage of B220+ cells binding 2.4G2.
2.4G2 enhances the differentiation of progenitor B lymphocytes cocultured with stromal cells.
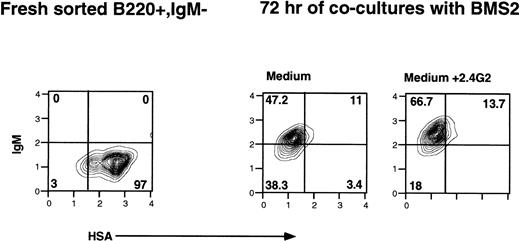
Because CD16 and CD32 can be expressed on many different cell types in bone marrow, the experiments and data presented above, although showing a requirement for CD16 and CD32 in the enhancement induced by 2.4G2, did not address on which cells the CD16 and CD32 expression was required. Because the previous studies with fetal thymocytes5 suggested that it was the FcγR on the prothymocyte that was required for the 2.4G2-triggered promotion of thymocyte maturation, we investigated whether 2.4G2 would enhance the maturation of purified pro-B cells (B220+, IgM−, FcγR+) cultured with a bone marrow stromal cell line that supports B-lineage development in vitro.21 Experiments were performed using FACS-sorted normal B-cell precursors (B220+, IgM−, FcγR+, CD43+, HSAhi) cultured with BMS2, a murine bone marrow stromal cell line. As shown in Fig 3, during 3 days of cocultures with BMS2 cells, the pro-B cells underwent further differentiation, as reflected by the shift from HSAhi to HSAlo and the shift from IgM− to IgM+. However, in the cultures that contained 2.4G2, there was a statistically significant enhancement of IgM expression (Table 3). This was manifested by both an increase in the percentage of IgM+ cells and a higher level of IgM per cell (Table 3). When the purified pro-B cells were cultured alone in normal media or in BMS2-conditioned media, the pro-B cells were nonviable after overnight culture (data not shown).
The effect of 2.4G2 on differentiation of B-lineage precursor cells (B220+, IgM−, HSAhi). Fresh FACS-sorted B220+, IgM− bone marrow cells (left panel) were cocultured with bone marrow stromal cell line BMS2. IgM and HSA expression on B220+ cells after 72 hours of culture without (middle panel) or with 2.4G2 (25 μg/mL; right panel). Cultures with 2.4G2 showed an enhanced percentage of cells with IgM expression and increased amounts of IgM per cell.
The effect of 2.4G2 on differentiation of B-lineage precursor cells (B220+, IgM−, HSAhi). Fresh FACS-sorted B220+, IgM− bone marrow cells (left panel) were cocultured with bone marrow stromal cell line BMS2. IgM and HSA expression on B220+ cells after 72 hours of culture without (middle panel) or with 2.4G2 (25 μg/mL; right panel). Cultures with 2.4G2 showed an enhanced percentage of cells with IgM expression and increased amounts of IgM per cell.
Effect of 2.4G2 on Purified Progenitor B Cells (B220+, IgM−) Cocultured With Bone Marrow Stromal Cells (BMS2) for 72 Hours
| Source of Progenitor B Cells . | 2.4G2 Added . | % HSAlo . | MFI . | %IgM+ . | MFI . |
|---|---|---|---|---|---|
| wt+/+ | No | 75 ± 13 | 77 ± 9 | 58 ± 6 | 127 ± 4 |
| Yes | 73 ± 17 | 81 ± 10 | 76 ± 7 | 140 ± 7 | |
| P = .014* | P = .032 | ||||
| CD16−/− | No | 71 ± 17 | 75 ± 14 | 64 ± 9 | 123 ± 16 |
| Yes | 67 ± 7 | 71 ± 14 | 67 ± 15 | 129 ± 7 |
| Source of Progenitor B Cells . | 2.4G2 Added . | % HSAlo . | MFI . | %IgM+ . | MFI . |
|---|---|---|---|---|---|
| wt+/+ | No | 75 ± 13 | 77 ± 9 | 58 ± 6 | 127 ± 4 |
| Yes | 73 ± 17 | 81 ± 10 | 76 ± 7 | 140 ± 7 | |
| P = .014* | P = .032 | ||||
| CD16−/− | No | 71 ± 17 | 75 ± 14 | 64 ± 9 | 123 ± 16 |
| Yes | 67 ± 7 | 71 ± 14 | 67 ± 15 | 129 ± 7 |
Abbreviation: MFI, mean fluorescence intensity.
P values were calculated by comparison between medium with or without 2.4G2.
As was found in the experiments with cultures of unfractionated bone marrow cells, the enhancing effect of 2.4G2 on the purified pro-B cells cocultured with BMS2 cells was CD16-dependent. The data in Table 3 show that the B-precursors purified from CD16−/−bone marrow were not influenced by the presence of 2.4G2 when cocultured with BMS2 stromal cells. Although the B-cell precursors from CD16−/− bone marrow did differentiate to HSAlo, IgM+ cells when cocultured with BMS2 cells, differentiation was not affected by the presence of 2.4G2. In comparable experiments performed with purified pro-B cells from CD32−/− bone marrow, the presence of 2.4G2 did not influence B-cell differentiation (data not shown).
Because the data from the in vitro experiments with normal bone marrow (Fig 1 and Table 1) suggested that FcγR could influence B-cell development, it was of interest to consider the in vivo situation. To begin to address this issue, we measured the expression of B220, CD19, and HSA and determined the sizes of the Hardy fractions A, B, C, D, E, and F27 in fresh bone marrow samples from CD16−/−, CD32−/− and normal control mice of the C57Bl6/129 background strain. The results of these experiments are shown in Table 4. No differences were seen between the CD16−/− and normal background control samples, but in the CD32−/− samples there was a significant increase in total numbers of B-lineage cells (CD19+) and in the size of the Hardy fractions D through F.
B-Cell Phenotype of Fresh Bone Marrow Cells From C57BL6/129 wt+/+, CD16−/−, and CD32−/− Mice
| Origin of Bone Marrow . | Absolute No. (×10−3/mL) . | ||||
|---|---|---|---|---|---|
| B220+ . | CD19+ . | B220+HSA+ . | Fr. A-C . | Fr. D-F . | |
| wt+/+ | 315 ± 46 | 210 ± 49 | 265 ± 69 | 37 ± 19 | 251 ± 95 |
| CD16−/− | 287 ± 69 | 228 ± 75 | 230 ± 26 | 27 ± 15 | 186 ± 50 |
| CD32−/− | 612 ± 473-150 | 445 ± 1003-151 | 591 ± 493-150 | 19 ± 8 | 452 ± 713-151 |
| Origin of Bone Marrow . | Absolute No. (×10−3/mL) . | ||||
|---|---|---|---|---|---|
| B220+ . | CD19+ . | B220+HSA+ . | Fr. A-C . | Fr. D-F . | |
| wt+/+ | 315 ± 46 | 210 ± 49 | 265 ± 69 | 37 ± 19 | 251 ± 95 |
| CD16−/− | 287 ± 69 | 228 ± 75 | 230 ± 26 | 27 ± 15 | 186 ± 50 |
| CD32−/− | 612 ± 473-150 | 445 ± 1003-151 | 591 ± 493-150 | 19 ± 8 | 452 ± 713-151 |
P values were calculated by comparison with the wild-type.
P < .001.
P < .05.
DISCUSSION
The major finding of the present study is that CD16 and CD32, the low-affinity FcγR expressed on normal murine pro/pre-B cells as well as on many other hematopoietic cells, can influence the growth and differentiation of developing B-lineage cells. These findings identify a possible role for FcγR in B-cell ontogeny, and they parallel the original findings of Sandor et al5 that FcγR on prothymocytes can influence T-cell ontogeny. In these studies, the presence of anti-FcγR antibody (2.4G2), but not control rat IgG antibody, in cultures of normal bone marrow cells enhanced the growth of B-lineage precursor cells (B220+/HSAhi/mIgM−) and promoted their differentiation to more mature levels (B220+/HSAlo/mIgM+). Because these mIgM+ cells did not express mIgD, CD23, or CD25 (data not shown), their phenotype is characteristic of the stage that has been defined as the immature B cell.24 25 Although modest in magnitude, the enhancing effect of 2.4G2 in vitro is reproducible and, as shown above, occurs in several different experimental formats. Furthermore, the expansion of the B-lineage compartment in the bone marrow of CD32−/− mice suggests that our in vitro findings have an in vivo relevance.
There are several possible mechanisms that could account for these findings. One possibility is that 2.4G2 binds to FcγR on B-lineage precursor cells and directly stimulates the cells to proliferate and differentiate. Because 2.4G2 binds to both FcγRII (CD32) and FcγRIII(CD16), the enhancing effect of 2.4G2 could be dependent on CD32, CD16, or both. The data from experiments with bone marrow from CD16−/− and CD32−/−mice indicate a requirement for both CD16 and CD32. Because CD16 and CD32 are coexpressed only during the early stages of B-lymphopoiesis, it is possible that 2.4G2 triggers an interaction between CD16 and CD32 on B-cell progenitors that results in enhanced B-lymphopoiesis. Alternatively, the 2.4G2-induced enhancement might be mediated by a sequential mechanism in which an initial signal involving both CD16 and CD32 on the progenitors was followed by a second signal through the solitary CD32 on the more differentiated cells.
Another possibility is that the enhancement effect is mediated indirectly by FcγR+ cells that are not B-lineage precursors. Because CD32 and CD16 are expressed on other types of hematopoietic cells,28 it was possible that, when 2.4G2 bound to CD32 and/or CD16 on non–B-lineage cells in the cultures, those cells promoted the growth and differentiation of the B-lineage cells. Although not formally eliminated as a possible mechanism, an indirect effect seems unlikely, because enhancement occurred in the experiments in which FACS-sorted B-cell precursors were cocultured with cells of BMS2, a murine bone marrow stromal cell line. In these experiments, a requirement for the presence of both CD16 and CD32 was demonstrated by using FACS-sorted B-cell precursors from CD16−/− and CD32−/−mice.
A third possible mechanism to account for the enhanced differentiation induced with 2.4G2 is that the anti-FcγR antibody blocks interaction between FcγR on the B-cell precursors and an alternative, non-Ig ligand on bone marrow stromal cells. In the experimental system we used, the cultured bone marrow cells are supported by components of the media, including the added GM-CSF, IL-3, and IL-5,18 and by soluble factors produced by cells in the culture, particularly by the hematopoietic stromal cells.21 Several observations suggest that direct contact of the precursor B cells and stromal cells is required for the enhancing effect of 2.4G2. In previous studies,5 it was shown that soluble, recombinant FcγR binds to the surface of BMS2 cells, a finding that implies the presence of an alternative, non-Ig counter-receptor for FcγR on BMS2 cells. It has also been shown that normal murine bone marrow stromal cells bind soluble, recombinant FcγR.8 The presence of a receptor for FcγR would provide a receptor:counter-receptor couple that could mediate direct interaction between B-lineage precursors and stromal cells. A requirement for direct contact between the B-precursors and stromal cells is also favored by the finding that, although the murine bone marrow stromal cell line BMS2 supports the growth of B-lineage precursors, BMS2-conditioned media does not. In data not shown, we found that the conditioned medium generated by culturing BMS2 cells for 24 hours in cytokines plus 2.4G2 was unable to support the survival of FACS-sorted normal B-precursors. Nonetheless, the mechanism that mediates 2.4G2 triggered enhancement of B-lymphopoiesis in this model is unknown and awaits further investigation. There are interesting parallels between developing T and B cells with regards to the expression of FcγR. In both lineages the onset of rearrangement of genes that encode antigen receptors coincides with the downregulation of CD1629-31 (Hagen et al, manuscript submitted). Thus, with the exception of some classes of γδ T cells, CD16 is not expressed on mature T cells32or mature B cells. This strongly implies that CD16 mediates its function(s) at the early stages of T- and B-lymphopoiesis. The other interesting parallel is that the presence of anti-FcγR antibody in fetal thymus organ cultures5 and cultures of pro/pre-B cells accelerates growth and differentiation of T- and B-lineage cells, respectively. This is particularly interesting in view of the report33 that human macrophage differentiation is also accelerated in vitro by antibodies directed to FcγRI (CD64), the high-affinity IgG receptor expressed on monocyte precursors. Based on previous studies, we proposed that experimental blockade of the interaction between FcγR on prothymocytes and a putative alternative ligand on stromal cells accelerates maturation of the prothymocyte. If correct, this model predicts that overexpression of FcγR would retard prothymocyte development. This prediction has been confirmed for T-lineage development by Flamand et al15 in mice constructed with a CD16 γ-chain transgene driven by a CD2 promoter.
Curiously, the patterns of expression of CD16 and CD32 on progenitor and immature T and B cells show a striking concordance, whereas their display on adult T and B cells is discordant. This is interesting, because the ligand-binding properties of CD16 and CD32, although distinct, are predicted to have considerable overlap based on the 95% identity in amino acid sequences of their extracellular, ligand-binding segments.34 Because CD16 and CD32 are structurally unrelated in their transmembrane and cytoplasmic segments, different cellular consequences follow the binding of ligand by CD16 and CD32. The cytoplasmic segment of the ligand-binding α-chain of CD16 associates with a homodimer of the FcR γ-chain, an ITAM-containing signal transducing molecule.12,35,36 It is known from the study of CD16 on other cells that the binding of ligand by CD16 triggers a cascade of activation events mediated through specific tyrosine kinases. Disappearance of the ITAM-containing CD16 from the developing T and B cells coincides with the acquisition of antigen-responsiveness that is mediated through another set of ITAM-containing molecules, the TCR and BCR, respectively. Although CD16 is not expressed on adult T and B cells, ligands for CD16 still are present in the host and CD16 continues to be expressed on other cells, such as NK cells. Thus, the developmental window during which FcγRIII (CD16) is expressed on B-precursors allows it to influence their development only before antigen-specific clonal commitment. In contrast, CD32 is present on progenitor and mature B-lineage cells, but it does not associate with the FcR γ-chain. When induced to associate with mIgM on mature B-lymphocytes, CD32 blocks B-cell activation.37 The molecular basis of the blockade of B-cell activation has been shown to be an ITIM,12 located in the cytoplasmic segment of CD32 that functions to inhibit phosphorylations triggered by the ITAM-containing immunoreceptors TCR, BCR, and FcR.13 Whether CD32 mediates an inhibitory function in progenitor T- and B-lineage cells has not been investigated. In view of the inhibitory effects mediated by CD32 in mature cells of several hematopoietic lineages,13 it is interesting that fas-dependent programmed cell death is induced in developing and mature murine eosinophils by aggregation of CD32, but not CD16, on their cell surface.38
Finally, the occurrence of FcγR on T- and B-cell precursors and their ability to influence lymphopoiesis has implications for certain diseases. For example, patients with multiple myeloma39 and human immunodeficiency virus (HIV)40 develop high circulating levels of soluble FcγR. Conceivably, these soluble receptors could competitively inhibit the generation of signals through FcγR on developing lymphoid cells, thereby uncoupling lymphopoiesis. In addition, circulating IgG immune complexes in certain immunological disorders might avidly bind to FcγR on developing lymphoid cells, thereby triggering pathologic signaling and altered immune function.
ACKNOWLEDGMENT
The authors gratefully acknowledge the excellent secretarial assistance of Vicki Brown.
Address reprint requests to Richard G. Lynch, MD, Department of Pathology, College of Medicine, 1117 Medical Laboratories, University of Iowa, Iowa City, IA 52242; e-mail: richard-lynch@uiowa.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal