Abstract
von Willebrand factor (vWF) is frequently used as a biochemical marker for endothelial cells (ECs). Despite this, little is known about the relative level of expression and regulation of this hemostatic factor in ECs in different vascular beds in vivo. In the present study, we used quantitative reverse transcription polymerase chain reaction and in situ hybridization analysis to study vWF gene expression in murine tissues. Large differences in the level of vWF mRNA were observed when comparing highly vascularized tissues, with the lung and brain containing 5 to 50 times higher concentrations of vWF mRNA than the kidney and liver. In this regard, ECs of small vessels and some microvessels in the lung and brain expressed abundant vWF mRNA, whereas ECs of similar sized vessels in the liver and kidney expressed relatively low levels. In general, significantly higher levels of vWF mRNA and antigen were demonstrated in ECs of larger vessels compared with microvessels and in venous ECs compared with arterial ECs. Although intraperitoneal administration of endotoxin (or tumor necrosis factor-) increased plasma vWF levels, it had variable effects on the steady-state level of vWF mRNA in murine tissues (ie, it decreased vWF mRNA in many tissues, increased it in others, and had little effect on still others). These results indicate that vWF is differentially expressed and regulated in ECs present in different tissues and within the same vascular bed.
© 1998 by The American Society of Hematology.
VON WILLEBRAND FACTOR (vWF) is a multimeric plasma glycoprotein that mediates platelet adhesion to the subendothelium at sites of vascular injury.1 It does so by forming a link between specific platelet membrane receptors and constituents of the subendothelial connective tissue. In addition, vWF binds to and stabilizes blood coagulation factor VIII in the circulation.2 vWF is synthesized from an 8.7-kb mRNA and appears to be expressed exclusively in endothelial cells (ECs)3 and megakaryocytes.4 In vitro data suggest that, in the blood vessel, vWF is both constitutively secreted by ECs5 and stored within intracellular granules in both ECs (Weibel-Palade bodies)6 and platelets (α-granules).7 Although vWF is commonly used as an immunohistochemical marker for ECs,3 8 little is known about the relative level of expression of vWF in ECs of the same or different vascular beds in vivo, and little information is available to demonstrate that all ECs actually produce it in vivo.
It is now apparent that ECs in different vascular beds may develop specialized functions associated with their vascular location. Two examples include the synthesis of tissue-type plasminogen activator (t-PA)9 and vWF.10 For example, t-PA has been reported to be differentially expressed in ECs of different size and location,11 whereas preliminary data suggest that the concentration of vWF antigen differs considerably in the thoracic aorta, inferior vena cava, and pulmonary artery of pigs.10Differential expression of vWF mRNA also was demonstrated in ECs isolated from the porcine pulmonary artery, lung, and aorta.12 These data suggest that the synthesis and regulation of vWF gene expression may vary in ECs from different locations. The generality of this observation among species and between vascular beds is largely unknown. In addition, it is not clear whether the level of vWF synthesis by a specific population of ECs is fixed and reflects an intrinsic property of the cell or if it can be modulated in response to various circulating factors. vWF behaves as an acute phase protein, because its plasma concentration may increase severalfold in response to a variety of physiological and pathophysiological conditions.13 14 The identities of the tissue and type of ECs within it that are responsible for this increase in plasma vWF are unknown.
In this study, we used quantitative reverse transcription-polymerase chain reaction (RT-PCR), in situ hybridization, and immunohistochemistry to examine vWF mRNA and antigen in various vascular beds of the mouse. We demonstrate that the expression levels of vWF mRNA and antigen differ significantly between ECs in different murine tissues and in venous and arterial ECs in the same tissues. Because endotoxin (lipopolysaccharide [LPS]) is known to modify the expression of a number of hemostatic genes13-17 and has been shown to elevate plasma vWF when administered to human volunteers,13 14 we also determined its effect on vWF. Our data show that murine plasma vWF is elevated by both LPS and tumor necrosis factor-α (TNF-α) and that LPS has a variable effect on vWF mRNA, downregulating it in many tissues but increasing it in others.
MATERIALS AND METHODS
Tissue preparation.
Two-month-old adult male CB6 mice (BALB/c/ByJ × C57BL6/J; Scripps Clinic Rodent Breeding Colony, La Jolla, CA), weighing 25 to 30 g, were used for all experiments. The mice were killed and various tissues were surgically removed and immersed in chilled 4% paraformaldehyde (Sigma, St Louis, MO). The tissues were fixed at 4°C overnight, embedded in paraffin blocks, and sectioned at 2- to 5-μm thickness using a microtome. The sections were then mounted onto polylysine slides (Superfrost/Plus; Fisher Scientific, Pittsburgh, PA) and stored at room temperature pending analysis. In some experiments, the mice were injected intraperitoneally with 50 μg LPS (2.0 mg/kg; Sigma) in saline (Baxter, Deerfield, IL), with 4 μg TNF-α (a kind gift of Richard Ulevitch, The Scripps Research Institute, La Jolla, CA) in saline, or with saline vehicle alone. After various times, the mice were killed, their plasma was collected, and their tissues were processed as described above.
RNA preparation.
Tissues were rapidly removed by standard dissection techniques, minced, and immediately frozen in liquid nitrogen. Total RNA was prepared from the frozen tissues by the acid guanidium thiocyanate-phenol-chloroform method, as previously described,18 and then treated with RNase-free DNase (Promega, Madison, WI) for removal of contaminating genomic DNA.19 RNA was quantitated by measuring absorption at 260 nm, and the integrity of the RNA was assessed by monitoring 18S and 28S ribosomal RNA by electrophoresing 10 μg of total RNA in 1.2% agarose/formaldehyde gels.
Quantitative RT-PCR.
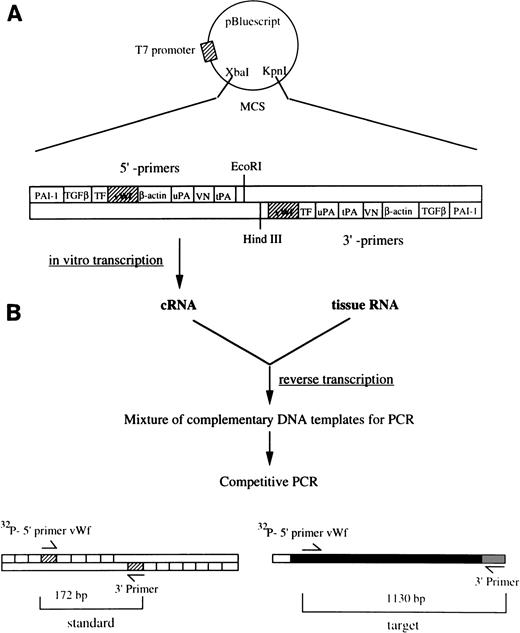
We have developed a quantitative RT-PCR method15 and modified it to determine the concentration of vWF mRNA in murine tissues. This approach is outlined in Fig 1. Briefly, a synthetic DNA template was constructed by cloning upstream and downstream sequences for vWF and other hemostatic genes into the multiple cloning site of the vector pBluescript II KS+ (Stratagene, La Jolla, CA). The sequences of upstream primers for vWF (5′-ATGATGGAGAGGTTACACATC-3′, position 4015-4035) and β-actin (sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′, position 886-910) followed by their corresponding downstream primers (vWF, 5′-GGCAGTTGCAGACCCTCCTTG-3′, position 5124-5144; β-actin, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′, position 1210-1234). A complementary RNA (cRNA) standard was transcribed off the linearized (Kpn I) synthetic template from the T7 promoter, using the Riboprobe Gemini II in vitro transcription system (Promega). RT-PCR, using either 104 or 105 molecules of cRNA for vWF and 107 for β-actin, was performed as described previously.15,20 The possibility that PCR products were generated from contaminating genomic DNA was ruled out by amplifying each sample using primers corresponding to the sequence of intron 28 of murine vWF gene (sequence information was kindly provided by Dr D. Ginsburg, University of Michigan, Ann Arbor, MI).21 Aliquots (20 μL) of the PCR products were electrophoresed through 2% agarose gels containing ethidium bromide. The appropriate bands corresponding to the standard cRNA product (172 bp for vWF and 293 bp for β-actin) and the target mRNA product (1,130 bp for vWF and 349 bp for β-actin) were excised from the gels, and the incorporated radioactivity (see below, riboprobe preparation) was determined using a scintillation counter. A standard curve for the cRNA standard was constructed and used to extrapolate the number of molecules of vWF mRNA per microgram of total tissue RNA. The estimation of the concentration of vWF mRNA was calculated as follows:
where 8,800 is the number of nucleotides in full-length vWF mRNA, 321 is the average molecular weight of one base, and 6.023 × 1023 is Avogadro’s number. Variations in sample loading were assessed by measuring β-actin mRNA by RT-PCR.
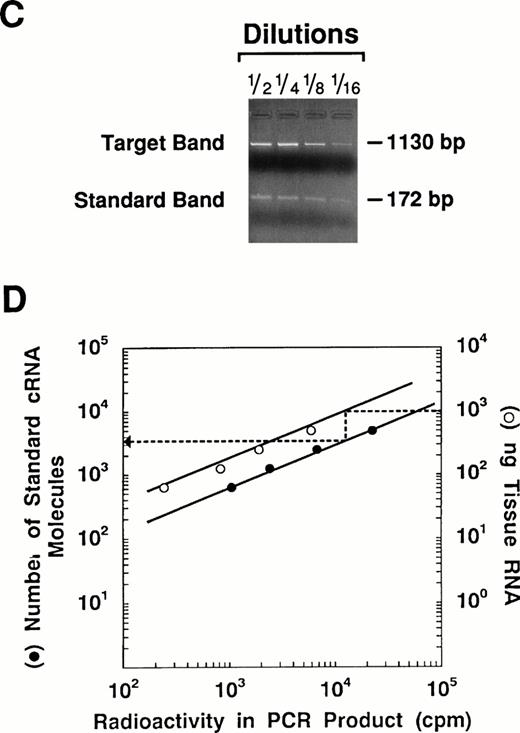
Quantitation of vWF mRNA by competitive RT-PCR. The approach used to quantitate vWF mRNA in murine tissues is shown in this diagram. (A) The synthetic plasmid used in these experiments is shown. It contains upstream and downstream sequences from several hemostatic genes including vWF. The plasmid was linearized with Kpn I and used as a template for in vitro transcription with T7 RNA polymerase. The resulting cRNA standard was then used as a competitor during RT-PCR. (B) One microgram of total tissue RNA and a fixed amount of the cRNA standard were mixed and then reverse transcribed into cDNA. The resulting mixture thus contained cDNA transcribed from both tissue mRNA and the standard cRNA. Serial twofold dilutions of the DNA mixture were amplified (30 cycles) using the vWF-specific primers containing 1 × 106 cpm of 32P-end labeled sense primer. (C) The PCR reaction mixture was electrophoresed through 2% agarose gels and photographed. The size difference between the PCR products permits easy separation of the standard (172 bp) from the target (1,130 bp). The appropriate bands for each PCR product were cut out of the gel and the radioactivity in each was determined using a scintillation counter. (D) The cpm in the PCR products obtained from the cRNA standard was plotted against the number of cRNA standard molecules used for RT-PCR using a double logarithmic scale. The cpm in the PCR product from the target mRNA was plotted against the amount of target RNA used for RT-PCR. Because the reaction rates for the cRNA standard and target mRNA should be the same, the number of vWF mRNA molecules in the tissue can be determined by extrapolation using the standard cRNA curve.
Quantitation of vWF mRNA by competitive RT-PCR. The approach used to quantitate vWF mRNA in murine tissues is shown in this diagram. (A) The synthetic plasmid used in these experiments is shown. It contains upstream and downstream sequences from several hemostatic genes including vWF. The plasmid was linearized with Kpn I and used as a template for in vitro transcription with T7 RNA polymerase. The resulting cRNA standard was then used as a competitor during RT-PCR. (B) One microgram of total tissue RNA and a fixed amount of the cRNA standard were mixed and then reverse transcribed into cDNA. The resulting mixture thus contained cDNA transcribed from both tissue mRNA and the standard cRNA. Serial twofold dilutions of the DNA mixture were amplified (30 cycles) using the vWF-specific primers containing 1 × 106 cpm of 32P-end labeled sense primer. (C) The PCR reaction mixture was electrophoresed through 2% agarose gels and photographed. The size difference between the PCR products permits easy separation of the standard (172 bp) from the target (1,130 bp). The appropriate bands for each PCR product were cut out of the gel and the radioactivity in each was determined using a scintillation counter. (D) The cpm in the PCR products obtained from the cRNA standard was plotted against the number of cRNA standard molecules used for RT-PCR using a double logarithmic scale. The cpm in the PCR product from the target mRNA was plotted against the amount of target RNA used for RT-PCR. Because the reaction rates for the cRNA standard and target mRNA should be the same, the number of vWF mRNA molecules in the tissue can be determined by extrapolation using the standard cRNA curve.
Riboprobe preparation.
A HindIII/Kpn I fragment of the murine vWF cDNA containing exon 2821 was subcloned into the vector pSP73 (Promega). This vector was linearized and used as a template for in vitro transcription of radiolabeled antisense or sense riboprobes using SP6 or T7 RNA polymerase (Promega), respectively, in the presence of [35S]UTP (>1,200 Ci/mmol; Amersham Corp, Arlington Heights, IL). Templates were removed by digestion with RQ1 DNase (Promega) for 15 minutes at 37°C, and the riboprobes were purified by phenol extraction and ethanol precipitation.
In situ hybridization.
In situ hybridizations were performed as described previously.22 After hybridization, the slides were dehydrated by immersion in a graded alcohol series containing 0.3 mol/L NH4Ac, dried, coated with NTB2 emulsion (Kodak, Rochester, NY; 1:2 in water), and exposed in the dark at 4°C for 4 to 12 weeks. Slides were developed for 2 minutes in D19 developer (Kodak), fixed, washed in water, and counterstained with hematoxylin and eosin. Parallel sections were analyzed using a sense probe as the control for nonspecific hybridization.
Immunohistochemistry and immunofluorescence.
Paraffin-embedded tissues were deparaffinized using xylene (3× for 5 minutes), treated with 2% hydrogen peroxide to quench endogenous peroxidase activity, and rehydrated. The sections were then permeabilized by sequential treatment with 0.2%, 0.5%, and 1.0% (vol/vol) Triton X-100 in Tris-buffered saline (TBS; 1× for 10 minutes each). For vWF, sections were incubated at 37°C for 8 minutes with prewarmed 0.23% (wt/vol) pepsin (2,830 U/mg; Worthington Biochemical Corp, Freehold, NJ) in 0.01 N HCl to unmask tissue antigens. After pepsin treatment, the tissue sections were rinsed with distilled water, washed with 0.2% Triton-TBS (1× for 5 minutes) and then incubated with 10% (wt/vol) normal goat serum for 30 minutes. Incubations with primary rabbit antibodies (5 or 10 μg/mL of antimouse albumin [Accurate Chemical & Scientific Corp, Westbury, NY]), 1:100 or 1:200 dilution of antihuman vWF IgG (Dakopatts, Glostrup, Denmark), or 10 μg/mL of preimmune (normal) rabbit IgG (Sigma) as a control in TBS containing 0.1% bovine serum albumin were performed in a humidified box for 1 hour at 37°C. The slides were then washed and treated sequentially with biotinylated goat antirabbit IgG (30 minutes; Zymed Laboratories, South San Francisco, CA), streptavidin-peroxidase conjugate (20 minutes; Zymed Laboratories), and aminoethylcarbazole chromagen containing 0.03% hydrogen peroxide (5 to 10 minutes; Zymed Laboratories).
After rinsing in distilled water for 3 minutes, the slides were counterstained with Gill-modified hematoxylin for 20 seconds, rinsed well with tap water, dried overnight, and mounted in Aquamount (Lerner Lab, Pittsburgh, PA). For immunofluorescent staining, a fluorescent-labeled secondary antibody was used instead of the biotinylated antibody. The slides were incubated for 20 minutes with Cy-3–conjugated goat antirabbit IgG (1:300 dilution; stock in 50% glycerol; Jackson Immunoresearch Lab, West Grove, PA) in TBS containing 0.05% Tween-20. The slides were washed with 0.02% Triton-TBS (3× for 5 minutes), rinsed in tap water, dried overnight, and mounted with Vectashield mounting medium (Vector Laboratories, Inc, Burlingame, CA).
For histochemical staining with lectins, the cells were treated as described for antibodies. However, they were not permeabilized or pepsin treated, but were incubated instead with 10% NGS for 30 minutes at 25°C and then incubated overnight with biotinylated Lycopersicon esculentum (LEA) lectin (1 μg/mL; Vector Laboratories, Inc) at 4°C. Under these conditions, LEA lightly stained mesothelial cells (data not shown). Because the lectin was biotinylated, the slides could be washed and directly incubated with streptavidin-peroxidase and the chromogen thereafter. As a negative control, the lectins were preincubated first for 30 minutes at 37°C with NN′N′′ triacetylchitotriose (Sigma) in excess of 1:1,000 and then tested.
Determination of vWF antigen levels in murine plasma.
The concentration of vWF antigen in murine plasma was determined by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Asserachrom vWF Kit; Diagnostica Stago, Asnieres-Sur-Seine, France). The results are expressed as the percentage of vWF detected compared with that present in a human plasma calibration standard set at 100%.
RESULTS
Analysis of vWF mRNA in murine tissues.
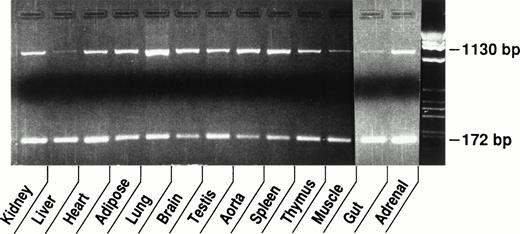
Experiments were performed to examine expression of the vWF gene in vivo. Total RNA was extracted from tissues of adult male CB6 mice and the level of vWF mRNA was determined by quantitative competitive RT-PCR using 1 μg of total tissue RNA and 104 molecules of cRNA standard (see Fig 1 and Materials and Methods). In these experiments, differences in the level of expression of vWF mRNA between murine tissues were apparent when the intensity of the target bands for vWF in the various tissues were compared (Fig 2). For example, the target band for vWF in the lung is the most intense, whereas those for vWF in the liver and muscle were the least intense. Thus, the concentration of vWF mRNA appears to be the highest in the lung and the lowest in the liver and muscle. These conclusions were confirmed and extended using the quantitative assay (Fig 3). For example, the typical lung was shown to contain approximately 0.22 pg of vWF mRNA/μg total RNA, followed by the spleen (0.16 pg/μg), aorta (0.13 pg/μg), and brain (0.11 pg/μg). Considerably lower levels of vWF mRNA were detected in the kidney (0.015 pg/μg), skeletal muscle (0.008 pg/μg), gut (0.005 pg/μg), and liver (0.004 pg/μg). The results shown in Figs 2 and 3thus demonstrate that the concentration of vWF mRNA in highly vascularized tissues of the mouse varies by greater than 50-fold.
Tissue distribution of vWF mRNA in murine tissues. Competitive RT-PCR was performed using 1 μg of RNA from each tissue and 1 × 104 molecules of cRNA standard. The top band (1,130 bp) is the PCR product derived from the endogenous vWF mRNA and the bottom band (172 bp) is from the cRNA standard. The difference in the expression of vWF mRNA among murine tissues can be approximated by comparing the intensity of the endogenous mRNA bands.
Tissue distribution of vWF mRNA in murine tissues. Competitive RT-PCR was performed using 1 μg of RNA from each tissue and 1 × 104 molecules of cRNA standard. The top band (1,130 bp) is the PCR product derived from the endogenous vWF mRNA and the bottom band (172 bp) is from the cRNA standard. The difference in the expression of vWF mRNA among murine tissues can be approximated by comparing the intensity of the endogenous mRNA bands.
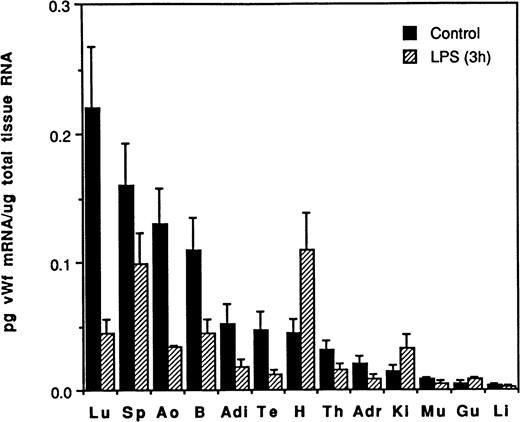
The effect of LPS on vWF mRNA in murine tissues. The concentration of vWF mRNA in tissues from control mice (▪) and mice treated with LPS for 3 hours (▨) was determined using the quantitative RT-PCR approach (Materials and Methods). The results are expressed as the mean concentration of vWF mRNA per microgram of total tissue RNA, with standard deviation (for 3 different mice) indicated by the error bars. Tissue abbreviations: Lu, lung; Sp, spleen; Ao, aorta; B, brain; Adi, adipose; Te, testis; H, heart; Th, thymus; Adr, adrenal; Ki, kidney; Mu, skeletal muscle; Gu, gut; Li, liver.
The effect of LPS on vWF mRNA in murine tissues. The concentration of vWF mRNA in tissues from control mice (▪) and mice treated with LPS for 3 hours (▨) was determined using the quantitative RT-PCR approach (Materials and Methods). The results are expressed as the mean concentration of vWF mRNA per microgram of total tissue RNA, with standard deviation (for 3 different mice) indicated by the error bars. Tissue abbreviations: Lu, lung; Sp, spleen; Ao, aorta; B, brain; Adi, adipose; Te, testis; H, heart; Th, thymus; Adr, adrenal; Ki, kidney; Mu, skeletal muscle; Gu, gut; Li, liver.
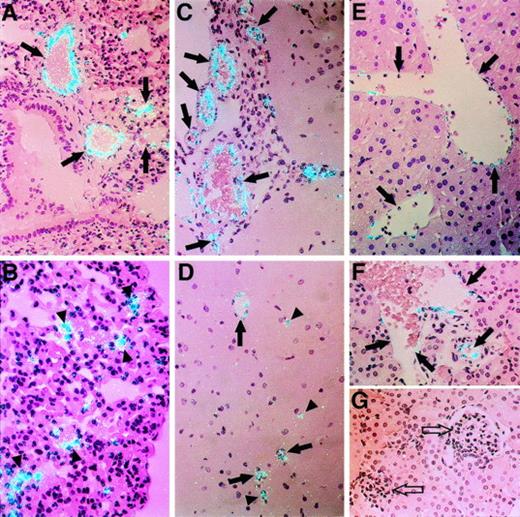
Although vWF mRNA was detected in ECs from all tissues examined by in situ hybridization, a wide range in the relative level of expression of this hemostatic factor was observed in ECs from different vascular beds (Figs 4 and5). For example, a relatively strong hybridization signal was detected in ECs of large vessels in the lung (Fig 4A) and brain (Fig 4C), two tissues that were shown to contain high amounts of vWF mRNA by quantitative RT-PCR (Fig 3). ECs of small vessels and some microvessels in both tissues also appeared to express abundant vWF mRNA (Fig 4B and D). In contrast, relatively weak signals for vWF mRNA were observed in ECs of large veins in the liver (Fig 4E) and kidney (Fig 4F). Little or no vWF mRNA was detected in sinusoidal ECs in the liver (Fig 4E) or in renal glomerular and peritubular ECs (Fig 4G). A relatively strong signal for vWF mRNA was detected in ECs of the endocardium in the heart, whereas there was a weak signal in ECs of vessels in the myocardium (data not shown). A strong hybridization signal was also apparent in megakaryocytes in the spleen (data not shown), which may contribute to the relatively high expression of vWF mRNA in this tissue as determined by RT-PCR (Fig 3). In general, the hybridization signal for vWF mRNA was lower in veins than arteries in these tissues. This differential expression of vWF was also observed between venous and arterial ECs of other tissues (Fig 5). However, in these instances, higher levels of vWF mRNA were frequently detected in venous ECs compared with arterial ECs (eg, in subcutaneous adipose tissues [Fig 5A] and in connective tissues, heart, kidney, and liver [data not shown]). It should be noted that the signal for vWF mRNA was similar in venous and arterial ECs in the spleen, lung, and brain (data not shown). The results shown in Figs 4 and 5 thus emphasize that the relative level of expression of vWF mRNA is not constant in ECs, but varies with the size, location, and type of the vessel. This variation is not constant, because the relationships between veins, arteries, and capillaries in terms of vWF mRNA appear to change from tissue to tissue.
Differential expression of vWF mRNA in ECs from different tissues. The relative level of expression of vWF mRNA in murine tissues was compared by in situ hybridization. (A) and (B) show the lung at an original magnification of × 250 (A) and × 400 (B). Arrows denote pulmonary arteries and veins. Arrowheads indicate microvascular ECs surrounding alveoli. (C) and (D) show brain (original magnification × 250). Arrows in (C) denote cerebral arteries and veins. Arrows in (D) indicate small vessels. Arrowheads denote microvessels in the cerebral cortex. (E) shows a section of liver (original magnification × 250). Arrows indicate hepatic veins. (F) and (G) show sections of kidney (original magnification × 250). Solid arrows denote renal arteries and veins; open arrows indicate glomeruli. All slides were exposed for 12 weeks.
Differential expression of vWF mRNA in ECs from different tissues. The relative level of expression of vWF mRNA in murine tissues was compared by in situ hybridization. (A) and (B) show the lung at an original magnification of × 250 (A) and × 400 (B). Arrows denote pulmonary arteries and veins. Arrowheads indicate microvascular ECs surrounding alveoli. (C) and (D) show brain (original magnification × 250). Arrows in (C) denote cerebral arteries and veins. Arrows in (D) indicate small vessels. Arrowheads denote microvessels in the cerebral cortex. (E) shows a section of liver (original magnification × 250). Arrows indicate hepatic veins. (F) and (G) show sections of kidney (original magnification × 250). Solid arrows denote renal arteries and veins; open arrows indicate glomeruli. All slides were exposed for 12 weeks.
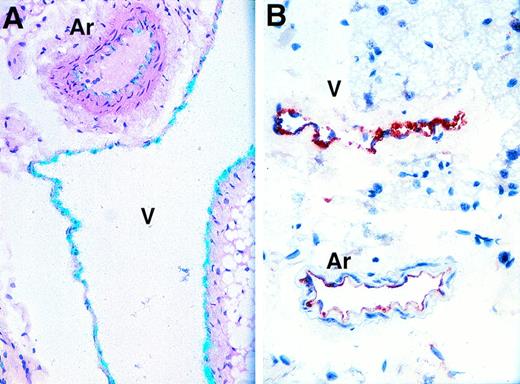
Differential expression of vWF mRNA and antigen in arteries and veins. Analysis of subcutaneous adipose tissue by in situ hybridization for vWF mRNA (A; original magnification × 250) or by immunohistochemistry for vWF antigen (B; original magnification × 250). The in situ hybridization slide was exposed for 8 weeks.
Differential expression of vWF mRNA and antigen in arteries and veins. Analysis of subcutaneous adipose tissue by in situ hybridization for vWF mRNA (A; original magnification × 250) or by immunohistochemistry for vWF antigen (B; original magnification × 250). The in situ hybridization slide was exposed for 8 weeks.
Analysis of vWF antigen in different vascular beds.
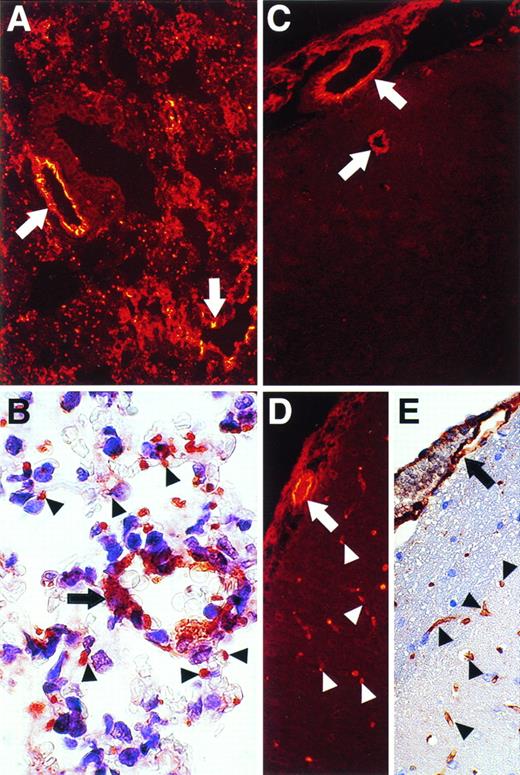
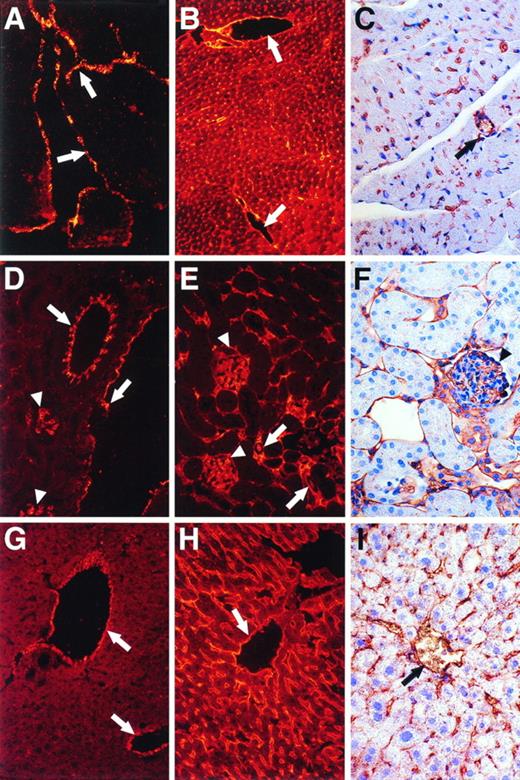
Immunohistochemical analysis also showed differential expression of vWF antigen in different murine tissues and in different types of vessels in the same tissues (Figs 5, 6, and7). For example, stronger staining for vWF antigen was occasionally observed in venous ECs compared with arterial ECs in adipose tissues (Fig 5B). Relatively strong staining was observed in ECs of large and small vessels in the lung and in microvessels distributed between bronchioles and alveoli (Fig 6A and B). In contrast, whereas ECs of large and small vessels in the brain expressed abundant vWF antigen (Fig 6C), microvascular ECs in the brain rarely expressed vWF antigen (Fig 6C). The presence of microvessels in the brain was readily demonstrated using antibodies to albumin (Fig 6D) or LEA-lectin (Fig 6E). Thus, the ECs of microvessels in the brain appear to express relatively low levels of vWF antigen compared with larger vessels. Little detectable vWF antigen was apparent in microvascular ECs in the myocardium (Fig 7; compare A through C), kidney (Fig 7; compare D through F), or liver (Fig 7; compare G through I), whereas strong staining was demonstrable in endocardial ECs (Fig7A) and in the larger vessels (Fig 7A, D, and G). However, positive staining for vWF antigen was apparent in glomerular ECs (Fig 7D, arrowheads). Importantly, abundant microvessels were shown in the kidneys and liver using antibodies to albumin (Fig 7B, E, and H) or by staining with LEA-lectin (Fig 7C, F, and I).
Immunohistochemical and/or immunofluorescent staining analysis for vWF and albumin antigens and for LEA lectin in the lung and brain. Sections of murine lung (A and B) and brain (C, D, and E) were analyzed for vWF antigen (A, B, and C), for albumin (D), and for LEA lectin (E). Staining was visualized by immunofluorescence (A, C, and D) or regular immunohistochemistry (B and E). Arrows denote larger vessels, whereas arrowheads denote microvascular ECs surrounding aveoli in the lung (B) or present in the brain (C, D, and E). Original magnifications: for (A), (C), (D), and (E), ×250; for (B), ×1,000.
Immunohistochemical and/or immunofluorescent staining analysis for vWF and albumin antigens and for LEA lectin in the lung and brain. Sections of murine lung (A and B) and brain (C, D, and E) were analyzed for vWF antigen (A, B, and C), for albumin (D), and for LEA lectin (E). Staining was visualized by immunofluorescence (A, C, and D) or regular immunohistochemistry (B and E). Arrows denote larger vessels, whereas arrowheads denote microvascular ECs surrounding aveoli in the lung (B) or present in the brain (C, D, and E). Original magnifications: for (A), (C), (D), and (E), ×250; for (B), ×1,000.
Immunohistochemical and/or immunofluorescent staining analysis for vWF and albumin antigens and for LEA lectin in the heart, kidney, and liver. Sections of murine heart (A, B, and C), kidney (D, E, and F), and liver (G, H, and I) were analyzed for vWF (A, D, and G) or albumin (B, E, and H) antigens and for LEA lectin (C, F, and I). Staining was visualized by immunofluorescence (A, B, D, E, G, and H; original magnification × 250) or immunohistochemistry (C, F, and I; original magnification × 400). Arrows in (A) denote endocardium. In other panels, arrows denote relatively large vessels, whereas arrowheads denote glomerular ECs.
Immunohistochemical and/or immunofluorescent staining analysis for vWF and albumin antigens and for LEA lectin in the heart, kidney, and liver. Sections of murine heart (A, B, and C), kidney (D, E, and F), and liver (G, H, and I) were analyzed for vWF (A, D, and G) or albumin (B, E, and H) antigens and for LEA lectin (C, F, and I). Staining was visualized by immunofluorescence (A, B, D, E, G, and H; original magnification × 250) or immunohistochemistry (C, F, and I; original magnification × 400). Arrows in (A) denote endocardium. In other panels, arrows denote relatively large vessels, whereas arrowheads denote glomerular ECs.
Effects of LPS on vWF gene expression in murine tissues.
Experiments were performed to determine whether the basal rate of vWF gene expression could be altered by various agents. Because LPS is known to modify the expression of a number of hemostatic genes,13-17 we examined the effect of LPS on the level of vWF mRNA in several murine tissues. Mice were injected with 50 μg LPS and tissues were removed 3 hours later and analyzed for vWF mRNA. Significant decreases in vWF mRNA were observed by 3 hours in all tissues examined except the heart, kidney, gut, and, perhaps, muscle and liver (Fig 3). For example, an 80% reduction of vWF mRNA was observed in the lung of LPS-treated mice, a 75% reduction in the aorta and testis, and a 65% reduction in the brain and adipose tissue. In contrast to these results, the expression of vWF mRNA was actually increased by LPS in the heart (2.5-fold) and kidney (2.2-fold).
The specific effect of LPS on vWF mRNA levels in ECs was also demonstrated by in situ hybridization. The level of vWF mRNA in ECs of large and small vessels in the lung (Fig 8) and brain (not shown) were dramatically decreased at 3 hours after LPS injection. The strong signal for vWF mRNA in ECs of the thoracic aorta also appeared to be decreased at 3 hours after LPS injection (not shown).
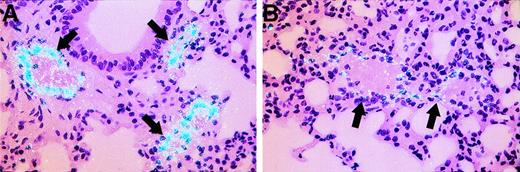
In situ hybridization analysis for vWF mRNA in tissues from control and LPS-treated mice. Tissue sections from lung of control mice (A) and mice treated with LPS for 3 hours (B) were hybridized for vWF mRNA as described in Materials and Methods. Arrows indicate pulmonary arteries or veins. Slides were exposed for 8 weeks. Original magnification × 400.
In situ hybridization analysis for vWF mRNA in tissues from control and LPS-treated mice. Tissue sections from lung of control mice (A) and mice treated with LPS for 3 hours (B) were hybridized for vWF mRNA as described in Materials and Methods. Arrows indicate pulmonary arteries or veins. Slides were exposed for 8 weeks. Original magnification × 400.
Effect of LPS on vWF antigen levels in murine plasma.
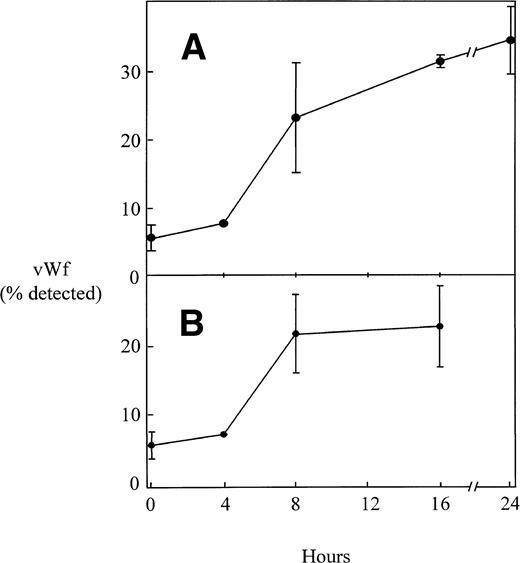
Administration of LPS to human volunteers has been shown to lead to an increase in plasma vWF concentration.13,14Figure 9A shows that LPS also induces plasma vWF in the mouse and does so with kinetics that are similar to those of the human studies.13,14 Many of the biological effects of LPS are mediated by the release of TNF-α and other mediators from activated mononuclear cells.16 20 Figure 9B demonstrates that administration of TNF-α also increases the concentration of plasma vWF in the mouse. The kinetics and magnitude of the effects of TNF-α are similar to those of LPS.
The effect of LPS and TNF- on vWF antigen in murine plasma. Mice were injected intraperitoneally with endotoxin (50 μg; A) or TNF- (4 μg; B). At various times, plasma was collected from individual mice and the concentration of vWF antigen was determined as described in Materials and Methods. The results are expressed as the mean percentage of vWF antigen detected, with the standard deviation for results from at least 3 different mice indicated by the error bars. The concentration of vWF in the plasma of mice treated for 8, 16, or 24 hours with either LPS or TNF was significantly higher than that in the plasma from untreated controls (P < .001).
The effect of LPS and TNF- on vWF antigen in murine plasma. Mice were injected intraperitoneally with endotoxin (50 μg; A) or TNF- (4 μg; B). At various times, plasma was collected from individual mice and the concentration of vWF antigen was determined as described in Materials and Methods. The results are expressed as the mean percentage of vWF antigen detected, with the standard deviation for results from at least 3 different mice indicated by the error bars. The concentration of vWF in the plasma of mice treated for 8, 16, or 24 hours with either LPS or TNF was significantly higher than that in the plasma from untreated controls (P < .001).
DISCUSSION
Although vWF is frequently used as a universal marker for ECs, it is not clear whether all ECs actually produce it, and little is known about its relative level of expression in different vascular beds in vivo. Previous immunohistochemical studies showed that vWF antigen was not universally distributed in the porcine vascular system23,24 and that different levels of vWF antigen were detected in arteries and veins. These immunological observations raised the possibility that porcine vWF gene expression also would vary at different anatomical locations, a hypothesis confirmed in Northern blotting and dot blotting experiments.12 Our RT-PCR and in situ hybridization studies in the mouse confirm and extend these observations. For example, high levels of vWF mRNA were present in lung, spleen, aorta, and brain, with low levels in liver, gut, skeletal muscle, and kidney (Figs 2 and 3). Differences in vascularization of the various tissues do not appear to account for these results, because two highly vascularized tissues (eg, kidney and liver; Fig 7) showed relatively low levels (Figs 2, 3, and 7). Rather, the results seem to reflect the differential expression of vWF mRNA from tissue to tissue. Thus, in some tissues, significantly higher levels of vWF mRNA and antigen were observed in venous ECs compared with arterial ECs (Fig 5). However, in other tissues, relatively high expression of vWF was detected in arteries (eg, in the thoracic aorta and in arteries in the lung, spleen, and brain; Figs 4 and 6). In general, we observed vWF expression in ECs of large and small vessels (Figs 6 and 7), but failed to detect it in ECs of most microvessels except those in the lung and brain (Figs 5, 6, and 7). These observations indicate that, in the mouse, like in the pig,12,23 24 vWF expression in ECs is heterogeneous throughout the vascular tree, varying with the size, location, and type of vessel.
The physiological significance of the heterogeneity in EC vWF expression remains to be determined. The role of subendothelial vWF in promoting local platelet-arterial wall interactions under high shear rate conditions is well established.25-28 It is tempting to speculate that the detection of vWF mRNA and antigen in some venous ECs (Fig 5) and in many large vessels compared with microvessels (Figs 5,6, and 7) implies a role in venous thrombosis as well. This speculation is perhaps premature, because the origin of subendothelial vWF has not been determined. Thus, it is unclear whether subendothelial vWF actually originates from the local endothelium or is deposited from plasma or platelets. Without this information, it is difficult to conclude that the differences in vWF synthesis observed here for the mouse and elsewhere in the porcine system23,24 are actually translated into local differences in the concentration of vWF in the subendothelium. In this regard, studies of pigs with severe vWF disease29 demonstrate that plasma vWF can correct the defect in platelet adhesion. Thus, in this model, endothelial and subendothelial vWF is apparently not necessary for the interaction between platelets and the vessel wall.
The observation that the liver is not a significant site of vWF synthesis (Figs 2, 3, 4, and 7) was somewhat unexpected, because the liver appears to be an important site of factor VIII synthesis.30-32 Factor VIII circulates in complex with vWF,2 and this interaction appears to prevent premature proteolytic cleavage and clearance of factor VIII.2 Thus, the low level of vWF synthesis in the liver raises the interesting possibility that the synthesis of these molecules may be spatially separated. In the lung, physiological hemostatic potential is considered to be high because of the high expression of both tissue factor (TF),33 an initiator of the extrinsic coagulation cascade, and of plasminogen activator inhibitor-1 (PAI-1),15 the primary inhibitor of plasminogen activation. The finding of high vWF (this study) further supports this hypothesis. The specialized structure of the alveolar unit for gas exchange may make it especially vulnerable to local injury caused by inflammation or mechanical stress. In this context, high expression of vWF in the lung could promote hemostatic processes that protect against local bleeding after vascular injury through adhesion of cells (eg, platelets) at the alveolar-capillary level in the lung. In the case of the brain, specialization in the vasculature is induced by the brain tissue environment (eg, the expression of blood-brain barrier properties34). Moreover, cerebral neurons express abundant amounts of TF33 and prothrombin,35 both of which are primary genes in the coagulation cascade. High expression of vWF in ECs of the cerebral vasculature may also contribute to the defense mechanism against local vascular injury in the brain. These considerations suggest that ECs in the cerebral vessels may have several tissue-specific functions that contribute to the regulation of cerebral circulation and the physiology of the central nervous system.
The heterogeneous expression of vWF in ECs from different vessels provides another example of vascular differentiation in an organ-specific manner, as previously reported for EC products such as t-PA,11 angiotensin-converting enzyme,36prostacyclin,37 fibronectin,38 certain cell receptors,39,40 and the lymphocyte-EC recognition system.41 These observations and others23,24,42suggest that ECs along the vascular tree are functionally heterogeneous, and our results are consistent with this possibility. The heterogeneous expression of these genes may reflect the local adaptation of these cells to perform distinct functions in specific tissues or may result from differences in local environmental conditions (eg, flow, blood pressure, and oxygen tension23) that alter the gene expression pattern of a multipotent EC. It is likely that both mechanisms function to regulate the tissue-specific expression of EC genes in vivo. For example, the finding that the different levels of vWF mRNA detected in various porcine vessels in vivo are maintained in ECs cultured from those vessels12suggests that the level of vWF mRNA is an intrinsic property of the cells. On the other hand, recent vascular transplant studies suggest that vascular bed-specific expression of vWF is programmed by the tissue microenvironment.42
The expression of the murine vWF gene in ECs was downregulated by LPS in many tissues (Figs 3 and 8), raising the possibility that LPS may also decrease the concentration of vWF in plasma. However, LPS actually increased plasma vWF when administered to mice (Fig 9) and to human volunteers,13,14,16 and it stimulates vWF release from cultured ECs.43 Thus, the LPS-mediated decreases in vWF gene expression in these tissues do not appear to lead to corresponding changes in plasma vWF. There are a number of possible explanations for this apparent disparity between tissue mRNA levels and plasma antigen concentration. For example, there were two exceptions to the LPS-mediated downregulation of vWF expression (ie, heart and kidney), and in these instances, vWF mRNA synthesis was actually stimulated by LPS (Fig 3). Whether the increased vWF gene expression in these tissues contributes to the observed increase in plasma vWF antigen remains to be demonstrated. The concentration of vWF mRNA in tissues was examined at relatively early times (3 hours) compared with the observed changes in plasma vWF antigen (8 hours). Thus, it is also possible that after the early decrease in vWF mRNA, vWF gene expression may increase again. Finally, it is likely that the increase in plasma vWF may also reflect the release of vWF antigen from ECs and/or platelets. Whatever the mechanism, the observation that administration of TNF-α also increases plasma vWF makes it likely that the effects of LPS are mediated by TNF-α and possibly other secondary mediators. We previously showed that the kidney is one of the more susceptible tissues to LPS-induced microthrombosis,15 and the upregulation of vWF expression in renal ECs by endotoxin may contribute in part to this susceptibility. Taken together, these results suggest that vascular ECs may influence hemostasis in a highly tissue-specific manner.
ACKNOWLEDGMENT
The authors thank Dr J. van Mourik for reading the manuscript and providing critical comments and Dr D. Ginsburg for providing sequence information on murine vWF. They also thank T. Thinnes for expert technical assistance and M. McRae for excellent secretarial assistance. This is manuscript no. 10105-VB from the Department of Vascular Biology.
Supported by National Institutes of Health Grant No. HL-47819 (to D.J.L.) and by grants from the Netherlands from the Foundation “De Drie Lichten,” the Netherlands Heart Foundation, the Spinoza Foundation, and the Dr. Hendrik Muller’s Vaderlandsch Foundation (to V.d.W.).
Presented in part at the VIIIth International Symposium on the Biology of Vascular Cells, August 30-September 4, 1994, Heidelberg, Germany.
Address reprint requests to David J. Loskutoff, PhD, Department of Vascular Biology, VB-3, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal