Abstract
All trans-retinoic acid (ATRA) syndrome is a life-threatening complication of uncertain pathogenesis that can occur during the treatment of acute promyelocytic leukemia (APL) by ATRA. Since its initial description, however, no large series of ATRA syndrome has been reported in detail. We analyzed cases of ATRA syndrome observed in an ongoing European trial of treatment of newly diagnosed APL. In this trial, patients 65 years of age or less with an initial white blood cell count (WBC) less than 5,000/μL were initially randomized between ATRA followed by chemotherapy (CT) (ATRA→CT group) or ATRA with CT started on day 3; patients with WBC greater than 5,000/μL received ATRA and CT from day 1; patients aged 66 to 75 received ATRA→CT. In patients with initial WBC less than 5,000/μL and allocated to ATRA→CT, CT was rapidly added if WBC was greater than 6,000, 10,000, 15,000/μL by days 5, 10, and 15 of ATRA treatment. A total of 64 (15%) of the 413 patients included in this trial experienced ATRA syndrome during induction treatment. Clinical signs developed after a median of 7 days (range, 0 to 35 days). In two of them, they were in fact present before the onset of ATRA. In 11 patients, they occurred upon recovery from the phase of aplasia due to the addition of CT. Respiratory distress (89% of the patients), fever (81%), pulmonary infiltrates (81%), weight gain (50%), pleural effusion (47%), renal failure (39%), pericardial effusion (19%), cardiac failure (17%), and hypotension (12%) were the main clinical signs, and 63 of the 64 patients had at least three of them. Thirteen patients required mechanical ventilation and two dialysis. A total of 60 patients received CT in addition to ATRA as per protocol or based on increasing WBC; 58 also received high dose dexamethasone (DXM); ATRA was stopped when clinical signs developed in 30 patients. A total of 55 patients (86%) who experienced ATRA syndrome achieved complete remission (CR), as compared with 94% of patients who had no ATRA syndrome (P= .07) and nine (14%) died of ATRA syndrome (5 cases), sepsis (2 cases), leukemic resistance (1 patient), and central nervous system (CNS) bleeding (1 patient). None of the patients who achieved CR and received ATRA for maintenance had ATRA syndrome recurrence. No significant predictive factors of ATRA syndrome, including pretreatment WBC, could be found. Kaplan Meier estimates of relapse, event-free survival (EFS), and survival at 2 years were 32% ± 10%, 63% ± 8%, and 68% ± 7% in patients who had ATRA syndrome as compared with 15% ± 3%, 77% ± 2%, and 80% ± 2% in patients who had no ATRA syndrome (P= .05, P = .003, and P = .03), respectively. In a stepwise Cox model that also included pretreatment prognostic variables, ATRA syndrome remained predictive for EFS and survival. In conclusion, in this multicenter trial where CT was rapidly added to ATRA in case of high or increasing WBC counts and DXM generally also used at the earliest clinical sign, the incidence of ATRA syndrome was 15%, but ATRA syndrome was responsible for death in only 1.2% of the total number of patients treated. However, occurrence of ATRA syndrome was associated with lower EFS and survival.
© 1998 by The American Society of Hematology.
ACUTE PROMYELOCYTIC leukemia (APL) is a specific type of acute myeloid leukemia (AML) characterized by the morphology of blast cells (M3 in the French-American-British classification of AML,1,2 the t(15;17) translocation,3 which fuses the PML gene on chromosome 15 to the retinoic acid receptor (RAR) alpha gene on chromosome 17,4,5 and by a coagulopathy combining disseminated intravascular coagulation (DIC) and fibrinolysis.6,7 Until recently, intensive chemotherapy (CT), usually combining an anthracycline and cytosine arabinoside (AraC), was the only effective treatment for APL.8-11
All trans-retinoic acid (ATRA) can differentiate APL blasts in vivo and in vitro.12-14 Treatment by ATRA followed by anthracycline-AraC CT has improved the outcome of APL by slightly improving the complete remission (CR) rate, but more importantly by reducing the incidence of relapse.15-21
ATRA is usually well tolerated, but a few major side effects can be observed, ATRA syndrome being the most important of them. Frankel et al22 gave the first description of this syndrome in nine of 35 (25%) newly diagnosed APL patients they treated with ATRA. Signs occurred after 2 to 21 days of treatment and were generally associated with increasing white blood cell (WBC) count and combined fever, weight gain, dyspnea, pleural effusion, and pulmonary infiltrates on chest radiograph and, in some patients, renal failure, hypotension, and pericardial effusion. Five of the nine patients required transfer to an intensive care unit and mechanical ventilation, and three patients died.
In the present report, we analyzed characteristics and outcome of cases of ATRA syndrome that occurred in 413 patients with newly diagnosed APL treated by ATRA in a multicenter European trial (APL 93 trial).
PATIENTS AND METHODS
Patients with newly diagnosed APL who were included in the APL 93 trial and developed ATRA syndrome during ATRA treatment form the basis of this study.
Diagnosis of APL.
Inclusion criteria were as follows: (1) diagnosis of APL, based on morphological criteria,1 2 (2) age 75 years or less, and (3) informed consent. Diagnosis had to be subsequently confirmed by the presence of t(15;17) or PML-RARα gene rearrangement. In the absence of t(15;17) and if no analysis of the rearrangement could be made, review of initial marrow slides by an independent morphologist was mandatory.
APL 93 trial.
Objectives of the APL 93 trial were to assess the optimal timing of ATRA treatment (before or combined with CT) and the role of maintenance therapy.
Induction treatment was stratified on age and initial WBC count. Patients aged ≤65 years with WBC less than 5,000/μL were randomized between ATRA followed by CT (ATRA→CT group) and ATRA plus CT (ATRA + CT). In the ATRA→CT group, patients received ATRA 45 mg/m2/d orally until CR or for a maximum of 90 days. After CR achievement, they received a course of Daunorubicin (DNR) 60 mg/m2/d for 3 days and AraC 200 mg/m2/d for 7 days (course I). However,course I was added to ATRA if WBC raised above 6,000/μL, 10,000/μL, or 15,000/μL by days 5, 10, and 15 of ATRA treatment, respectively, as from our experience, patients were at risk of ATRA syndrome above those thresholds.21 CT was also to be immediately added if clinical signs of ATRA syndrome developed, irrespective of the WBC count.
Patients randomized to the ATRA + CT group received the same combination of ATRA and CT, but CT course I was started on day 3 of ATRA treatment. Patients with WBC >5,000/μL at presentation or aged 66 to 75 and with WBC less than 5,000/μL were not randomized and received ATRA plus CT course I from day 1 (high WBC group) and the same treatment as in the ATRA→CT group (elderly group), respectively.
Patients who achieved CR received two chemotherapy consolidation courses, including course II (identical to course I) and course III, consisting of DNR 45 mg/m2/d for 3 days and AraC 1 g/m2 every 12 hours for 4 days. The elderly group, however, only received course II. Patients were then offered a second randomization testing both intermittent ATRA (45 mg/m2/d, 15 days every 3 months) and continuous CT with 6 mercaptopurine (90 mg/m2/d orally) and methotrexate (15 mg/m2/week orally), both scheduled for 2 years, using a(2x2) design. Randomization was performed through a centralized telephone procedure.
Diagnosis and treatment of the ATRA syndrome.
In the absence of biological diagnostic criteria of ATRA syndrome, diagnosis of ATRA syndrome was made on clinical grounds by the association of at least three of the following signs, in the absence of other causes: fever, weight gain, respiratory distress, lung infiltrates, pleural or pericardial effusion, hypotension, and renal failure.22
When ATRA syndrome was suspected, recommendation was made to immediately start treatment with dexamethasone (DXM) (10 mg/12 hours intravenously) for at least 3 days. Furthermore, addition of the first course of CT was scheduled, if CT had not already been started. Discontinuation of ATRA was recommended if the patient had received at least 20 days of ATRA. In patients who had received less than 20 days of ATRA, immediate discontinuation of the drug was also recommended if ATRA syndrome was life-threatening and did not rapidly improve with CT and DXM.
Statistical methods.
Predictive factors of ATRA syndrome and the prognostic value of ATRA syndrome on event-free survival (EFS), relapse and survival, were analyzed. The following pretreatment parameters were studied: age, sex, WBC count, platelet count, absolute number of circulating blasts, M3 subtype (classical M3vmicrogranular variant M3), and fibrinogen level. The χ2 test, Fisher’s exact test, and a Cox model were used. Relapse, EFS, and survival curves were estimated by the Kaplan Meier method and compared by the log rank test.23-25
RESULTS
Incidence and clinical features of the ATRA syndrome in APL 93 trial.
Between April 1, 1993 and December 31, 1996, 439 patients from 93 centers were entered in the APL 93 trial. Diagnosis of APL was confirmed in 413 cases by the presence of t(15;17) translocation (352 cases), PML-RARα rearrangement (30 cases), and review of initial bone marrow slides (31 cases where cytogenetic analysis was a failure and molecular analysis could not be performed).
Of the 413 patients, 64 (15%, 95% exact confidence interval [CI]: 12% to 19%) experienced ATRA syndrome during induction treatment. In two of them, with WBC counts of 186,000 and 38,000/μL upon admission, respectively, dyspnea, hypoxia, and pulmonary infiltrates were already present, before the onset of ATRA. Worsening of those signs occurred within hours on ATRA onset. In the other patients, signs developed after 1 to 35 days (median, 7 days) of ATRA treatment. They occurred later than day 14 of ATRA treatment in 21 (33%) patients. In 11 of those 21 patients, ATRA syndrome was observed upon recovery from the phase of aplasia that followed the addition of CT (indicated because of high WBC counts at diagnosis or during treatment).
Twenty-four (16%) of the 151 patients included in the ATRA→CT group and the elderly group, ie, who presented with less than 5,000 WBC/μL and were initially treated with ATRA alone, experienced ATRA syndrome. ATRA syndrome in 22 of them was preceded by an increase in WBC, which fell in 18 cases (75% of the 24 patients) within our criteria for adding CT. In the other groups (ATRA + CT and high WBC groups), the correlation between WBC counts and occurrence of the ATRA syndrome was difficult to determine because CT had been systemically rapidly added to ATRA, resulting in a decrease in WBC counts.
Clinical features of ATRA syndrome observed in the 64 patients included respiratory distress in 57 patients (89%), pulmonary infiltrates in 52 (81%), fever in 52 (81%), weight gain in 32 (50%), pleural effusion in 30 (47%), renal failure in 25 (39%), pericardial effusion in 12 (19%), cardiac failure in 11 (17%), and hypotension in 8 (12%). All but one of the patients had at least three of those clinical signs, and no other explanation to those signs (especially infection). The last patient had only unexplained fever and pericardial effusion, but this rapidly resolved with intravenous DXM. He was therefore considered to have ATRA syndrome and included in the series.
Outcome of ATRA syndrome.
Sixty of the 64 patients who developed ATRA syndrome received CT, based on WBC counts >5,000/μL at diagnosis, randomization to the ATRA + CT group, or WBC increase with ATRA (above 6,000, 10,000, 15,000/μL by day 5, 10, or 15 of ATRA treatment, respectively, according to protocol guidelines). In 53 of the patients, addition of CT had been made before the onset of clinical signs of ATRA syndrome, whereas in seven, CT was added when symptoms of ATRA syndrome occurred. In three of those seven patients, addition of CT should, in fact, have been made earlier according to protocol guidelines, because of increasing WBC counts. Finally, four patients did not receive CT even when ATRA syndrome occurred. In two of them, WBC had increased above thresholds defined for starting CT before ATRA syndrome developed.
After ATRA syndrome was suspected, 58 of the 64 patients also received intravenous DXM during a median of 6 days (range, 3 to 23 days). The duration of DXM treatment was greater than 10 days in nine patients, due to very slow improvement of signs of ATRA syndrome in five cases, or very progressive tapering of the doses in the remaining four cases. In 30 of the 64 patients, ATRA was stopped when ATRA syndrome developed after 2 to 26 days of ATRA treatment. Thirteen patients required mechanical ventilation and two hemodialysis.
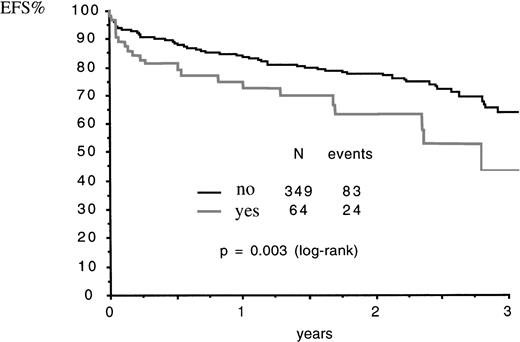
Fifty-five (86%) of the patients who experienced ATRA syndrome achieved CR and nine (14%) had early death, after 3 to 21 days of ATRA (median, 17 days), from ATRA syndrome in five cases, from CNS bleeding in one patient (who had moderate ATRA syndrome), from sepsis after resolution of ATRA syndrome in two cases, and from leukemic resistance in the remaining case. Fatal cases due to ATRA syndrome included one of the four cases and one of the six cases where addition of CT and DXM, respectively, was not made, and one of the four cases where addition of CT was delayed. The CR rate was not significantly lower in patients who experienced the ATRA syndrome than in patients who had no ATRA syndrome (86% v 94%, P = .07 by Fisher’s test). Patients who experienced ATRA syndrome had a Kaplan Meier estimate of relapse at 2 years of 32% ± 10% as compared with 15% ± 3% in patients who had no ATRA syndrome (relative risk [RR]: 1.97; 95% CI: 0.98 to 3.96; P = .05 by the log rank test). Kaplan Meier estimate of EFS and survival at 2 years were 63% ± 8% and 68% ± 7% in patients who had ATRA syndrome as compared with 77% ± 2% and 80% ± 2% in patients who had no ATRA syndrome: for EFS, RR = 1.97 (95% CI: 1.25 to 3.11),P = .003 by the log rank test (Fig1); for survival, RR = 1.78 (95% CI: 1.05 to 3.01), P = .03 by the log rank test.
EFS according to occurrence (yes) or not (no) of ATRA syndrome. N, number of patients.
EFS according to occurrence (yes) or not (no) of ATRA syndrome. N, number of patients.
Using a stepwise Cox model with ATRA syndrome and all prognostic baseline covariates, we found that ATRA syndrome remained predictive for poorer EFS (P = .004; RR = 1.99; 95% CI: 1.12 to 3.23) along with older age and higher circulating blast counts, for poorer survival (P = .02; RR = 1.9; 95%CI: 1.12 to 3.23) along with older age and higher circulating blast counts, but not for relapse (P = .33; RR = 1.43; 95% CI: 0.7 to 2.95) when considering male gender and higher circulating blast counts.
Of the 55 patients who achieved CR, 17 subsequently received ATRA for maintenance, but no recurrence of ATRA syndrome was seen in any case.
Predictive factors of ATRA syndrome.
As seen in Table 1, no predictive factor of ATRA syndrome could be found. There was a nonsignificant trend for higher incidence of ATRA syndrome in patients randomized to ATRA→CT as compared with those randomized to ATRA + CT (P = .17). No difference was observed according to initial WBC count or PML/RARα isoform (bcr1, bcr2, bcr3). CD13expression was not assessed in a sufficient number of patients to evaluate its prognostic value on the occurrence of ATRA syndrome.
Prognostic Factors of ATRA Syndrome in 413 Cases of Newly Diagnosed APL Included in APL 93 Trial
| . | Absence of ATRA Syndrome . | ATRA Syndrome . | P Value . |
|---|---|---|---|
| No. of patients | 349 | 64 | |
| M/F | 169/176 (0.95) | 36/28 (1.3) | .34 |
| Mean age | 46 | 44 | .33 |
| WBC (109/L) | |||
| Mean | 10.9 | 11.9 | .7 |
| <5.0 | 213 (61%) | 34 (53%) | .26 |
| 5.0 to 10.0 | 42 (12%) | 13 (20%) | 1.0 |
| 10.0 to 20.0 | 31 (9%) | 5 (8%) | ND |
| >20.0 | 63 (18%) | 12 (19%) | .85 |
| Mean platelet count (109/L) | 44 | 40 | .27 |
| Mean circulating blasts (109/L) | 7.7 | 8.0 | .09 |
| Mean fibrinogen level (g/L) | 1.9 | 1.7 | .47 |
| Microgranular variant APL | 16% | 19% | .57 |
| PML/RARα isoform (124 patients) | |||
| bcr1 | 80 (64%) | 12 (57%) | .3 |
| bcr2 | 2 (2%) | ||
| bcr3 | 42 (34%) | 9 (43%) | |
| Induction group | |||
| ATRA → CT | 89 (82%) | 20 (18%) | |
| ATRA + CT | 88 (89%) | 11 (11%) | P = .17 |
| High WBC group | 134 (82%) | 29 (18%) | |
| Elderly group | 38 (90%) | 4 (10%) |
| . | Absence of ATRA Syndrome . | ATRA Syndrome . | P Value . |
|---|---|---|---|
| No. of patients | 349 | 64 | |
| M/F | 169/176 (0.95) | 36/28 (1.3) | .34 |
| Mean age | 46 | 44 | .33 |
| WBC (109/L) | |||
| Mean | 10.9 | 11.9 | .7 |
| <5.0 | 213 (61%) | 34 (53%) | .26 |
| 5.0 to 10.0 | 42 (12%) | 13 (20%) | 1.0 |
| 10.0 to 20.0 | 31 (9%) | 5 (8%) | ND |
| >20.0 | 63 (18%) | 12 (19%) | .85 |
| Mean platelet count (109/L) | 44 | 40 | .27 |
| Mean circulating blasts (109/L) | 7.7 | 8.0 | .09 |
| Mean fibrinogen level (g/L) | 1.9 | 1.7 | .47 |
| Microgranular variant APL | 16% | 19% | .57 |
| PML/RARα isoform (124 patients) | |||
| bcr1 | 80 (64%) | 12 (57%) | .3 |
| bcr2 | 2 (2%) | ||
| bcr3 | 42 (34%) | 9 (43%) | |
| Induction group | |||
| ATRA → CT | 89 (82%) | 20 (18%) | |
| ATRA + CT | 88 (89%) | 11 (11%) | P = .17 |
| High WBC group | 134 (82%) | 29 (18%) | |
| Elderly group | 38 (90%) | 4 (10%) |
Abbreviation: ND, not done.
On the other hand, severe cases of ATRA tended to be more frequent in the high WBC group: 8 of the 13 cases of ATRA syndrome requiring mechanical ventilation (P = .17) and 8 of the 12 cases of pericarditis (P = .07) occurred in that group.
DISCUSSION
The pathophysiology of ATRA syndrome is still poorly understood, but the proposed mechanisms could involve changes in the cytokine secretion and adhesive qualities of APL cells during ATRA-induced differentiation. Some clinical manifestations of the ATRA syndrome (fever, hypotension, effusions) indeed suggest a role for cytokines, and the release of several cytokines, including interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor (TNF) α, by APL cells undergoing differentiation with ATRA has been demonstrated.26-28 Organ infiltration by APL cells found in postmortem studies in ATRA syndrome suggests that ATRA induces APL cells to acquise leukemic cell-endothelial cell adhesion followed by extravascular extravasation.22 In vitro, ATRA also leads to aggregation of APL cells, which appears to be mediated by induction by ATRA of the expression of high-affinity β2 integrins (especially leukocyte function-associated antigen-1 [LFA-1] and their counterstructure on the cell surface (in particular intercellular adhesion molecule [ICAM]-2). Methyl prednisolone rapidly inhibits APL cell aggregation in a dose-dependent manner, consistent with its prompt clinical effectiveness in vivo.29
In this multicenter trial that included 413 patients with newly diagnosed APL, the incidence of ATRA syndrome was 15%. In previously published reports, this incidence ranged from 6% to 27%30-33 (Table 2). Reasons for variable incidences among series possibly include differences in prophylactic approaches. A higher incidence in recent series could also be due to better recognition of the syndrome since its precise description by Frankel et al.22 Because diagnosis of ATRA syndrome remains purely clinical, it may be in some cases difficult to distinguish from other complications of the disease, particularly sepsis.
Incidence and Outcome of ATRA Syndrome in APL Treated With ATRA
| Authors . | No. of Patients . | Prophylaxis of ATRA Syndrome . | No. of ATRA Syndrome (%) . | Treatment of ATRA Syndrome . | Outcome of ATRA Syndrome . | Overall Incidence of Fatal ATRA Syndrome (%) . | |
|---|---|---|---|---|---|---|---|
| CR (%) . | Death (%) . | ||||||
| Fenaux15 (APL 91 trial) | 54 | Chemotherapy added if —WBC >5,000/μL at diagnosis —WBC >6,000, 10,000, 15,000/μL by days 5, 10 and 15 of ATRA, respectively | 6 (11) | Symptomatic | 100 | 0 | 0 |
| Kanamaru18 | 109 | Chemotherapy added if —WBC >3,000/μL at diagnosis —Circulating blasts >1,000/μL during treatment | 7 (6) | DXM: 3 cases | ND | 14 | 1 |
| Vahdat30 | 78 | No prophylaxis | 21 (27) | DXM: leukaphereses chemotherapy | 72 | 28 | 7 |
| Wiley31 | 22 | —if WBC <1,000/μL initially: oral prednisone —if WBC >1,000/μL —if WBC >1,000/μL initially: chemotherapy | 2 (9) | ½ | ½ | 4 | |
| Avvisatti32 | 20 | Chemotherapy in all patients | 2 (10) | DXM | ½ | ½ | 5 |
| Tallmann33 | 172 | Hydroxyurea if —WBC >1,000/μL at diagnosis —WBC >30,000/μL with ATRA | 47 (27) | DXM | ND | 7 | 1 |
| Cortes36 | 17 | Chemotherapy added if circulating blasts + promyelocytes >10,000/μL at diagnosis or increase with ATRA | 4 (24) | Chemotherapy leukaphereses | ¾ | ¼ | 5 |
| Present study (APL93 Trial) | 413 | Same as APL 91 trial | 64 (15) | DXM | 89 | 8 | 1 |
| Authors . | No. of Patients . | Prophylaxis of ATRA Syndrome . | No. of ATRA Syndrome (%) . | Treatment of ATRA Syndrome . | Outcome of ATRA Syndrome . | Overall Incidence of Fatal ATRA Syndrome (%) . | |
|---|---|---|---|---|---|---|---|
| CR (%) . | Death (%) . | ||||||
| Fenaux15 (APL 91 trial) | 54 | Chemotherapy added if —WBC >5,000/μL at diagnosis —WBC >6,000, 10,000, 15,000/μL by days 5, 10 and 15 of ATRA, respectively | 6 (11) | Symptomatic | 100 | 0 | 0 |
| Kanamaru18 | 109 | Chemotherapy added if —WBC >3,000/μL at diagnosis —Circulating blasts >1,000/μL during treatment | 7 (6) | DXM: 3 cases | ND | 14 | 1 |
| Vahdat30 | 78 | No prophylaxis | 21 (27) | DXM: leukaphereses chemotherapy | 72 | 28 | 7 |
| Wiley31 | 22 | —if WBC <1,000/μL initially: oral prednisone —if WBC >1,000/μL —if WBC >1,000/μL initially: chemotherapy | 2 (9) | ½ | ½ | 4 | |
| Avvisatti32 | 20 | Chemotherapy in all patients | 2 (10) | DXM | ½ | ½ | 5 |
| Tallmann33 | 172 | Hydroxyurea if —WBC >1,000/μL at diagnosis —WBC >30,000/μL with ATRA | 47 (27) | DXM | ND | 7 | 1 |
| Cortes36 | 17 | Chemotherapy added if circulating blasts + promyelocytes >10,000/μL at diagnosis or increase with ATRA | 4 (24) | Chemotherapy leukaphereses | ¾ | ¼ | 5 |
| Present study (APL93 Trial) | 413 | Same as APL 91 trial | 64 (15) | DXM | 89 | 8 | 1 |
Abbreviation: ND, not done.
Date of onset of signs of ATRA syndrome was variable in our experience, as in that of Frankel et al.22 Median time to occurrence of ATRA syndrome was 10 to 12 days in published series and 7 days in our experience. A few cases where a clinical picture resembling ATRA syndrome was observed before the onset of ATRA have been reported.34 Likewise, in two of our patients with high WBC counts at presentation, dyspnea, hypoxia, and pulmonary infiltrates were present before the onset of ATRA, although they worsened within hours of ATRA onset. Conversely, in 11% of the cases presented here, ATRA syndrome occurred upon recovery from the phase of aplasia secondary to the addition of CT (indicated because of high WBC counts at diagnosis or during ATRA treatment), a circumstance where ATRA syndrome had not been so far reported, to our knowledge.
The clinical picture we observed in the present series of cases of ATRA syndrome, the largest reported so far, was similar to that reported by Frankel et al,22 with a great variety in the intensity of signs, ranging from moderate pleural effusion discovered on chest x-ray film to major respiratory distress requiring mechanical ventilation.
In the present study, most of the patients who experienced ATRA syndrome had high WBC counts at diagnosis or had an increase in WBC counts with ATRA. However, in six of the 24 cases (25%) of ATRA syndrome that occurred in the ATRA→CT group and elderly group, ie, in patients who initially had low WBC counts and were started on ATRA alone, ATRA syndrome occurred at low WBC counts, below thresholds established by our group for starting CT. Those thresholds therefore applied to only 75% of the patients who developed ATRA syndrome in those two treatment groups (a correlation between evolution of WBC counts under ATRA and occurrence of ATRA syndrome could not be determined in other induction treatment groups, where CT was systematically started early). The WBC count at diagnosis was not a significant predictive factor of ATRA syndrome, and no other baseline predictor of ATRA syndrome could be found in our study. On the other hand, patients presenting with high WBC counts tended to have more severe forms of ATRA syndrome, more often associated with pericardial effusion and more often requiring mechanical ventilation. Vahdat et al30 also found no correlation between preteatment WBC counts and ATRA syndrome, but observed a correlation between the peak WBC count and the development of ATRA syndrome. Four of the 21 cases of ATRA syndrome they observed occurred at WBC counts below 10,000/μL,30 but in three of them, WBC were greater than 5,000/μL when symptoms occurred. These investigators found that the threshold used by our group for adding CT to ATRA could predict 64% of the cases of ATRA syndrome, a figure similar to our 75%. Vahdat et al30 also found that basal expression of CD13 on APL blasts was strongly associated with both development of the ATRA syndrome, as well as an elevated WBC count.
We found, in patients who experienced the ATRA syndrome, a trend for a higher risk of relapse by comparison to other patients with borderline significance. This finding had not been previously reported, to our knowledge. Thus, APL blasts, which when exposed to ATRA have biologic responses capable of triggering ATRA syndrome, may also be more resistant to the combination of ATRA and CT. Although this is speculative, cases developing ATRA syndrome may be associated with the induction of higher expression of adhesion molecules on APL cell surface by ATRA.29 This would give those cells a higher tendency to infiltrative disease, especially at extramedullary sites. This trend for a higher hazard of relapse, combined with the trend for lower CR rate, explained why patients who experienced ATRA syndrome had significantly lower EFS and survival than other patients. Furthermore, using a stepwise Cox model that also included prognostic baseline covariates, we found that ATRA syndrome remained predictive for EFS and survival.
Mortality of the ATRA syndrome ranged from 7% to 28% in the literature and was 8% in the current trial, ie, 1% of all treated patients. In the two other published series that involved more than 100 patients, the overall mortality from ATRA syndrome in APL was in the range of 1% (Table 2). Because of the severity of prognosis of the ATRA syndrome once full blown signs have developed, prophylaxis or at least early treatment at the first signs of the syndrome, are mandatory. Leukaphereses are incapable of sufficiently lowering WBC counts and preventing the ATRA syndrome in APL,22 and other approaches are required.
One approach, used mainly by European and Japanese groups,15-18 is to add anthracycline-AraC CT to ATRA from the onset of ATRA treatment in patients presenting with high WBC counts or during ATRA treatment if increases in the WBC are seen (Table2).21 The thresholds used by those groups for adding CT, as seen above, did not predict all cases of ATRA syndrome. However, the European and Japanese approach proved effective on a multicenter basis, as the ATRA syndrome seen in 64 of the 413 patients in the APL 93 trial and seven of 109 patients treated by the Japanese group,18was fatal in only six of those 522 patients. A disadvantage of this approach is that about two thirds of the patients treated with ATRA also received early CT. However, we found that in this case, the duration of neutropenia and thrombocytopenia was significantly shorter than when CT was administered alone, an effect that may be linked to the effect of ATRA on normal granulocytic proliferation and differentiation.15 Furthermore, intensive CT, if not administered early, would have to be aministered later on as consolidation treatment. Finally, first interim analysis of results of the present APL93 trial suggest a reduction in the incidence of relapse after ATRA + CT, as compared with ATRA→ CT.35 These findings support very early introduction of CT during induction treatment of APL. Along with these findings, the Italian GIMEMA group32 and the MD Anderson group36 are using a prophylactic approach to the ATRA syndrome where CT is systematically added to ATRA after 2 to 4 days of treatment.
Another approach, mainly used by the New York group and Australian group,19,30 31 is to prevent fatal cases of ATRA syndrome by administration of high dose intravenous corticosteroids (DXM at 10 mg intravenously twice daily for 3 or more days) as soon as the first symptoms occur. This treatment proved effective in the New York experience; although six of the 78 patients treated died of ATRA syndrome, only one death had occurred in the last 2½ years of the study. The Australian group, using the same approach, observed fatal toxicity from the ATRA syndrome in one of 19 newly diagnosed patients who received ATRA. This approach avoids the early use of CT and its side effects. However, it has mainly been used by a few experienced groups and has not been tested on a large multicenter basis.
The US intergroup study33 used a somewhat intermediate approach where single agent CT with hydroxyurea was administered before ATRA in cases presenting with WBC above 10,000/μL or was added to ATRA if WBC rose above 30,000/μL, intravenous DXM being started at the earliest clinical sign of ATRA syndrome. The incidence of ATRA syndrome (27%) was slightly higher than in European and Japanese studies, but the outcome was similar (overall incidence of death due to the ATRA syndrome of about 1%).
All of those approaches are obviously not exclusive. Our group recommends adding DXM to CT at the earliest sign of ATRA syndrome in cases presenting with leukopenia, but with rising WBC counts under ATRA. Finally, there is now a consensus over the fact that patients presenting with high WBC counts (eg, more than 15,000 to 20,000/μL) will very often develop severe ATRA syndrome with ATRA alone and should receive CT and intravenous DXM from the onset of treatment as prophylactic measures before any symptoms of ATRA syndrome develop.
In conclusion, the advent of ATRA has been a breakthrough in the treatment of APL. ATRA syndrome, its major side effect, is now well described. Prophylaxis of severe forms of ATRA syndrome by rapid addition of CT and/or early use of DXM has reduced the mortality of ATRA syndrome to about 1% of the APL patients treated with ATRA. On the other hand, occurrence of ATRA syndrome still appears to be associated with lower EFS and survival in APL.
APPENDIX
Dr P. Fenaux and Dr L. Degos served as cochairmen and Dr C. Chastang (Department of Biostatistics, Hopital St Louis, Paris) as biostatiscian. The following clinical departments participated in the APL 93 trial.
French APL group.
S. Castaigne, H. Dombret (Paris, St Louis), R. Zittoun (Paris, Hôtel Dieu), E. Archimbaud (Lyon), P. Travade (Clermont Ferrand), C. Gardin (Clichy), A. Guerci (Nancy), P. Fenaux (Lille), A.M. Stoppa (Marseille), F. Dreyfus (Paris, Cochin), F. Stamatoulas (Rouen), F. Rigal-Huguet (Toulouse), H. Guy (Dijon), J.J. Sotto (Grenoble), F. Maloisel (Strasbourg), J. Reiffers (Pessac), A. Gardembas (Angers), D. Bordessoule (Limoges), N. Fegueux (Montpellier), A. Buzyn (Paris, Necker), T. Lamy (Rennes), M. Hayat (Villejuif), E. Deconinck (Besançon), E. Guyotat (St Etienne), M. Martin (Annecy), E. Cony-Makhoul (Bordeaux), J.P. Abgrall (Brest), O. Reman (Caen), B. Desablens (Amiens), J.L. Harousseau (Nantes), Y. Bastion (Lyon), J.P. Pollet (Valenciennes), J. Pulik (Argenteuil), M. Lepeu (Avignon), M. Renoux (Bayonne), P. Morel (Lens), P. Henon (Mulhouse), N. Gratecos (Nice), P. Colombat (Tours), D. Machover (Villejuif), A. Dor (Antibes), P. Casassus (Bobigny), J. Donadio (Castelnou), B. Salles (Chalon), B. Legros (Clermont Ferrand), P. Audhuy (Colmar), A. Dutel (Compiègne), N. Philippe (Lyon), B. Benothman (Meaux), C. Christian (Metz), C. Margueritte (Montpellier), F. Witz (Nancy), A. Pesce (Nice), A. Baruchel (Paris, St Louis), L. Sutton (Paris, Pitié Salpétrière), C. Quetin (Pointe à Pitre), B. Pignon (Reims), E. Vilmer (Paris), E. Bourquard (St Brieuc), J.P. Marolleau (Paris, St Louis), P. Robert (Toulouse), B. Despax (Toulouse), G. Nedellec, P. Auzanneau (Paris), and M. Janvier (St Cloud).
Spanish AML group.
O. Rayon (Oviedo), M. Sanz (Valencia), J. San Miguel (Salamanca), J. Montagud (Valencia), E. Condé (Santander), P. Javier de la Serna (Madrid), G. Martin (Valencia), M. Perez Encinas (Santiago), J.P. Torres Carrete (Juan Canalejo), J. Zuazu (Barcelone), J. Odriozola (Madrid), E. Gomez-Sanz (Madrid), L. Palomera (Zaragoza), L. Villegas (Almeria), A. Deben (Juan Canalejo), and P. Besalduch (Palma de Mallorca).
Cooperative AML study group, Germany.
H. Link (Hannover), A. Ganser (Frankfurt), E. Wandt (Nurnberg), A. Breitenbach (Stuttgart), B. Brennscheidt (Freiburg), D. Herrmann (Ulm), H. Soucek (Dresden), and H. Strobel (Erlangen).
SAKK Swiss AML group.
K. Geiser (Berne), M. Fey (Berne), T. Egger (Berne), and E. Jacky.
Belgian group.
J.L. Michaux (Bruxelles), A. Bosly (Yvoir), E. Meeus (Anvers), and A. Boulet (Mons).
Dutch group.
P. Daenen (Groningen), and P. Muus (Nijmegen).
Address reprint requests to P. Fenaux MD, PhD, Service des Maladies du Sang, CHU, 1 Place de Verdun, 59037 Lille, France; e-mail:pfenaux.Lille@invivo.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal