To the Editor:
Attached to and supporting the inner leaflet of the erythrocyte membrane is a two-dimensional network of spectrin filaments that crosslink actin. Spectrin heterodimers consist of an α and β monomer closely associated in an antiparallel fashion. Spectrin is divided into five α (I-V) and four β (I-IV) spectrin structural domains by tryptic digestion.1 The primary structures of both chains are dominated by tandemly repeated 106-amino acid homologous repeat motifs2 that fold into triple helical bundles. Spectrin attaches to the lipid bilayer through an association with the integral band 3 protein via ankyrin, which binds repeats 15 and 16 of β spectrin. Spectrin dimers self-associate into tetramers in a head-to-head fashion via reciprocal interactions of the α spectrin repeat α′ with repeat 17 of β spectrin.1
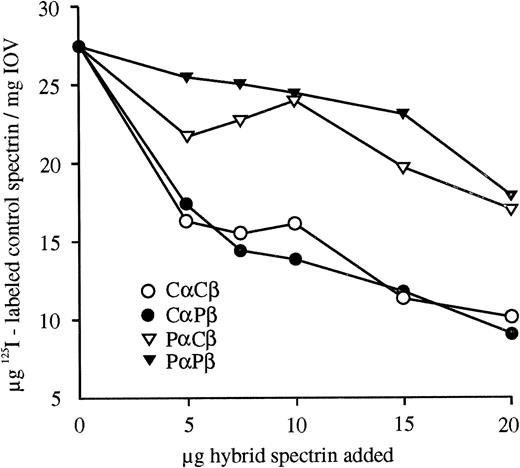
Hereditary elliptocytosis (HE) is a disorder characterized by elliptocytes on peripheral blood smears and is most commonly caused by a spectrin dimer self-association defect.3 Two probands from a white South African kindred with severe HE, characterized by partial spectrin deficiency in the membrane, 25% spectrin dimers,4 and severely decreased spectrin-ankyrin binding,5 were further investigated to identify the underlying spectrin mutation. The close association of spectrin subunits in the heterodimer allows a defect in one chain to manifest itself as an alteration observed in the second chain. To identify the defective proband spectrin subunit, reconstituted hybrid spectrin dimers prepared from control (C) and proband (P) monomers6were assayed. The hybrid spectrin-ankyrin binding assays in Fig1 show the effect of increasing amounts of hybrid spectrin dimer competitor on the amount of control125I-labeled spectrin dimers bound to spectrin depleted inside out vesicles. CαCβ and CαPβ were better able to compete with the labeled control spectrin for free ankyrin binding sites than PαCβ and PαPβ. Thus, a proband α spectrin defect reduces the ankyrin binding of the adjacent β spectrin. Quantitation of hybrid spectrin dimer self-association by densitometric scanning of nondenaturing gels indicated that the proband α spectrin also reduced dimer self-association, whereas proband β spectrin had no effect.
Competitive hybrid spectrin-ankyrin binding assays. Hybrid spectrins, formed from control (Cα and Cβ) and proband (Pα and Pβ) spectrin monomers, were bound to spectrin-depleted inside-out vesicles (IOV) in the presence of a constant amount of125I-labeled control spectrin dimer. The amount of bound control spectrin was plotted versus the amount of hybrid spectrin competitor added. All binding data are shown as the mean of duplicates that had ranges less than + or −8.5%. Proband α spectrin reduced the ankyrin binding of hybrid spectrins.
Competitive hybrid spectrin-ankyrin binding assays. Hybrid spectrins, formed from control (Cα and Cβ) and proband (Pα and Pβ) spectrin monomers, were bound to spectrin-depleted inside-out vesicles (IOV) in the presence of a constant amount of125I-labeled control spectrin dimer. The amount of bound control spectrin was plotted versus the amount of hybrid spectrin competitor added. All binding data are shown as the mean of duplicates that had ranges less than + or −8.5%. Proband α spectrin reduced the ankyrin binding of hybrid spectrins.
Structural analysis of proband spectrin using tryptic digestion showed that the αII domain was altered: on peptide maps there was an acidic shift of the pI of the 46-kD peptide and the 35-kD and 30-kD peptides were absent. These data indicated a defect between amino acids 916-981. Reticulocyte mRNA and genomic DNA analysis indicated that the probands were homozygous for a T → G transversion −13 bp from the α spectrin intron 19/exon 20 boundary named Spectrin Johannesburg7,8 or Spectrin St Claude.9 This mutation creates a 3′ acceptor splice site resulting in the expression of equal quantities of two abnormal messages. One message contains an in-frame 12-bp intron 19 insertion that introduces a translation stop codon and produces a truncated protein not incorporated into the membrane. In the second message exon 20 is excised by the spliceosome due to recognition of the stop codon. This mutant α spectrin lacks amino acids 935-965, which delete the B helix of the α9 repeat within the αII domain. This mutation perturbs the conformation of the spectrin heterodimer, which reduces dimer self-association and impairs the binding of β spectrin to ankyrin via long-range interactions. The ankyrin binding and dimer-dimer contact sites of β spectrin are in contiguous repeats 15-17 and, therefore, a single disruptive influence could affect both functions. Approximate models of the relative positions of the spectrin triple helical bundles of each monomer in the heterodimer place repeat α9 opposite either β1210 or β14.11 This is in close proximity to repeats β15-17. The altered α9 conformation may thus disrupt ankyrin binding and dimer self-association by transmission of a steric effect along α spectrin and subsequently to β15-17. The disruptive effect of the mutant repeat α9 may also be propagated further to the N-terminal α′ repeat and, hence, influence the α spectrin dimer self-association site.
The kindred is of Afrikaans origin and the parents are apparently unrelated. Because the probands are homozygotes, both parents are obligate heterozygotes. The prevalence of the Spectrin St Claude allele was investigated in unrelated white South African individuals. Two mutant alleles out of 134 were detected, which contrasts with white subjects of French origin where the allele was not detected.9
The partial spectrin deficiency in the probands' erythrocyte membranes, which is a result of the spectrin-ankyrin binding defect, destabilizes the lipid bilayer and causes spherocytes. The reduced membrane spectrin content in concert with the mild dimer self-association defect further weakens the membrane skeleton and allows deformation of the erythrocytes into elliptocytes and poikilocytes. Our studies illustrate how a single point mutation in the α spectrin gene impairs functions of both the α and β spectrin proteins, resulting in qualitative and quantitative membrane abnormalities. These have profound effects on red blood cell morphology and survival, manifesting as severe hemolytic anemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal