Abstract
To gain further insights in the pathogenesis of herpesvirus pneumonia in allogeneic bone marrow transplant recipients, transplanted mice (B10.BR → CBA) with graft-versus-host disease (GVHD) and control mice (transplanted mice without GVHD and normal CBA mice) were infected intranasally with herpes simplex virus type 1 (HSV-1). When compared with infected control mice, infected allogeneic transplant recipients with GVHD showed increased periluminal mononuclear cell infiltrates. However, infected allogeneic transplant recipients with GVHD showed lower virus content in the lung tissue than infected control mice. High concentrations of transforming growth factor-beta 1 (TGF-β1) were detected in the bronchoalveolar lavage (BAL) fluid of mock-infected allogeneic transplant recipients with GVHD, which increased slightly after infection. Anti–TGF-β treatment of allogeneic transplant recipients with GVHD significantly decreased the histological evidence of pneumonitis at day 4 after HSV-1 infection. We conclude that allogeneic transplant recipients with GVHD have (1) increased pneumonia, (2) highly elevated levels of TGF-β1 in the BAL fluid, and (3) reduced pulmonary virus content after HSV-1 infection. Our data suggest that the newly recognized dysregulation of cytokine (TGF-β1) production may be more important than the viral load for the increased severity of HSV-1 pneumonia in allogeneic transplant recipients with GVHD.
ALLOGENEIC BONE MARROW transplantation (BMT) has become an important treatment for a number of hematologic, malignant, and genetic disorders. Late infectious complications are the leading cause of nonrelapse death, and they are often associated with the presence of graft-versus-host disease (GVHD).1,2 A recent study showed that viruses, particularly herpesviruses including cytomegalovirus (CMV) and herpes simplex virus type 1 (HSV-1), caused 37% of late infections after allogeneic BMT.3 The lung was one of the most common single organ sites of life-threatening infections, accounting for 22.8% of the localized infections.3
Our new model of HSV-1 pneumonia4 allows a direct comparison of viral pneumonia in normal and immunocompromised (transplanted) animals. The HSV-1 system may be more useful for clinically related studies than the murine cytomegalovirus (MCMV) model, because MCMV causes pneumonia only in immunosuppressed, but not normal mice.5,6 Antigen-specific humoral and cellular mechanisms and innate immunity may protect against HSV infection.7,8 Antibody-mediated protection in vivo may involve neutralization, antibody-dependent cellular cytoxicity, and Fc receptor-dependent mechanisms.8 Cellular protective mechanisms against HSV include T-cell–mediated cytokine release by CD4+ T cells and cytolysis of infected cells by CD8+ T cells.8,9 Mediators of innate immunity include macrophages and natural killer cells.7,10 For example, macrophages stimulated by interferon-γ (IFN-γ) inhibit the replication of HSV-1 by the generation of nitric oxide (NO).11 12
In contrast to the protective responses described above, immune responses during viral infections can also be detrimental to the host. For example, T cells mediate the tissue injury in herpetic stromal keratitis.13 Furthermore, the inflammatory response associated with NO production can increase pathology; we showed recently that inhibition of NO production decreased the cellular influx and pathology in HSV-1 pneumonia.4
In this study, we wished to model late posttransplant pulmonary infection in BMT recipients, in particular with regard to the presence or absence of GVHD. Specifically, we wanted to address the following questions. First, do allogeneic BMT recipients with GVHD develop more severe pneumonitis than allogeneic BMT recipients without GVHD after pulmonary HSV-1 infection? Second, is there a correlation between the severity of HSV-1–induced pneumonitis and the pulmonary viral titer? Finally, are there differences in the pulmonary immune response after HSV-1 infection with regard to inflammatory cell influx and cytokines between allogeneic BMT recipients with and without GVHD?
Previous murine studies of posttransplant viral infections were performed in the early period after BMT rather than after engraftment.14 Because the current clinical problem is infection after engraftment, mice were infected intranasally with HSV-1 at 12 weeks after BMT across minor histocompatibility differences.15 The number of T cells injected into recipient mice was chosen to produce a mild GVHD in the experimental group (Allogeneic GVHD) to determine the effects of mild GVHD on HSV-1–induced pneumonia. Recipients injected with T-cell–depleted bone marrow only (Allogeneic No GVHD), as well as normal CBA mice, were used as controls.
Our data show that, when compared with control mice, allogeneic transplant recipients with GVHD had more severe pneumonitis, reduced pulmonary influx of inflammatory cells, and antibody production after intranasal HSV-1 infection as well as highly elevated levels of transforming growth factor-beta 1 (TGF-β1) in the bronchoalveolar lavage (BAL) fluid. Unexpectedly, they also showed reduced pulmonary virus content. Treatment with anti–TGF-β monoclonal antibody (MoAb) decreased the histological evidence of pneumonitis. Therefore, dysregulated cytokine production may be more important than the cytopathic effects of the virus in the development of HSV-1 pneumonia in allogeneic transplant recipients with GVHD.
MATERIALS AND METHODS
Mice and BMT.
Female CBA/J (H2k) and B10.BR (H2k) mice were purchased from The Jackson Laboratories (Bar Harbor, ME), and BMT was performed between the ages of 10 to 14 weeks. Bone marrow cells were obtained from the femurs and tibias of donor B10.BR mice, and transplanted into B10.BR recipients as syngeneic transplant control. After T-cell depletion by treatment with anti-Thy1.2 and complement (Accurate Chemicals & Scientific Corp, Westbury, NY), 5 × 106 bone marrow cells alone or mixed with 4 × 105 nylon wool nonadherent donor splenic T cells were resuspended in Leibovitz's L-15 medium (Life Technologies, Grand Island, NY) and transplanted into CBA recipients via tail vein infusion (0.5 mL total volume per mouse). Before transplantation, recipient mice received 11 Gy of total body irradiation (137Cs source) delivered in two fractions separated by 3 hours to reduce gastrointestinal toxicity. Mice were subsequently housed in sterilized micro-isolator cages and received normal chow and autoclaved hyperchlorinated water for the first 2 weeks after BMT and filtered water thereafter.
Assessment of GVHD.
The severity of GVHD was assessed by following the weights of mice after BMT. Failure to gain weight has been found to be a reliable indicator of systemic GVHD in this murine model.16 Using the above described transplant parameters, a mild GVHD was induced in allogeneic recipients transplanted with donor bone marrow and nylon wool nonadherent donor splenic T cells. Approximately 80% of the allogeneic transplanted mice with GVHD were available for infection at 12 weeks after BMT. At this time point after BMT, the mice with GVHD showed a 20% weight loss due to GVHD as compared with the control mice. The mortality related to the BMT in this model occurred within the first 6 to 8 weeks after BMT; beyond 8 weeks after BMT, little further mortality was observed.
Infection of mice.
Mice were infected intranasally with 2.5 × 105plaque-forming units of HSV-1, strain KOS 1.1,17 kindly provided by Drs R. Finberg and J. Brubaker (Dana-Farber Cancer Institute, Boston, MA), as described.4This viral dose caused pneumonitis without mortality. The virus stocks were prepared as clarified lysates of infected Vero cells. Briefly, Vero cells were infected at a multiplicity of infection of 0.1. After 72 hours, virus stocks were prepared, after removing the supernatants, by two rounds of freezing and thawing of the infected cell pellets in culture medium. The clarified lysates were aliquoted and stored at −80°C until used. Uninfected control stocks were prepared from Vero cells in the same manner. The Vero cells and culture media were screened and shown to be free of mycoplasma and endotoxin contamination.
Tissue sampling and flow cytometry.
Virus titration and histology.
Determination of HSV-1–specific antibodies.
HSV-1–specific IgG1 and IgG2a antibodies in BAL samples of mice 14 days after infection were determined as described,4 using a specific enzyme-linked immunosorbent assay (ELISA).18 In all assays, samples and standards were run at least in duplicate. The quantitation of HSV-1–specific antibodies was performed by the method of Zollinger and Boslego.19
Cytokine determinations.
Levels of IFN-γ, IL-2, IL-4, IL-10, IL-5, tumor necrosis factor–α (TNF-α), and TGF-β in BAL fluids were determined by ELISA. Except for TGF-β, all measurements were performed using recombinant cytokine standards, and “pairs” of capture and peroxidase-conjugated secondary antibodies, all purchased from Pharmingen (San Diego, CA), were used according to the protocol supplied by Pharmingen. The plates were developed using avidin-peroxidase and 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate (Sigma Chemical Co, St Louis, MO), and read at 405 nm using an ELISA reader. The lower detection limit for all assays was 100 pg/mL. In some cases, BAL fluids were tested after approximately 5-fold concentration by using Centricon-10 concentrators (Amicon, Beverly, MA). Total TGF-β1 in BAL fluids was assayed using a previously described capture ELISA.20 Briefly, 96-well microtiterplates were coated overnight at 4°C with polyclonal chicken-anti-TGF-β1 antibody (R & D Systems, Minneapolis, MN), and then blocked for 2 hours at room temperature with 10% BSA Diluent/Blocking Solution Concentrate (Kirkegaard & Perry Laboratories Inc, Gaithersburg, MD). After washing with phosphate-buffered saline containing 0.05% Tween 20, 50 μL of samples, or recombinant human TGF-β1 (R & D Systems) as standard were added to duplicate wells, followed by overnight incubation at 4°C. After washing, plates were incubated with a mouse anti–TGF-β1, -β2, -β3 MoAb (Genzyme, Cambridge, MA) for 45 minutes at room temperature. After washing, plates were incubated with peroxidase-labeled goat anti-mouse IgG (Kirkegaard & Perry) for 30 minutes at room temperature, washed again, developed using 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate (Sigma), and read at 405 nm. This ELISA has been shown to be 10-fold more sensitive in measuring TGF-β than the bioassay using mink lung epithelial cells.20
Treatment of mice with anti–TGF-β MoAb.
Mouse anti–TGF-β1, -β2, -β3 MoAb (Genzyme) was administered at a dose of 100 μg per injection intraperitoneally (IP) in a volume of 0.2 mL at days −1, 1, and 3.21 22 Control mice were treated with the same amount of mouse IgG (Sigma). Mice were infected intranasally at day 0, and lung samples were prepared for histological examination at day 4 after infection.
Statistical analyses.
The Mann-Whitney test and the Student's t-test (unpaired) were used for data analysis where appropriate. Group differences in the viral titer per lung over time after infection (see Fig 3B) were analyzed by the Kruskal-Wallis test — a nonparametric analysis of variance (ANOVA).
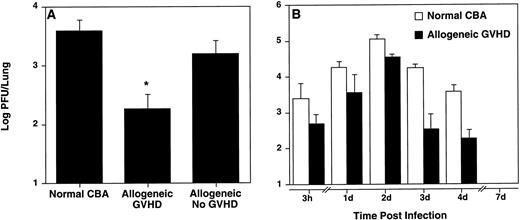
Allogeneic transplant recipients with GVHD showed less pulmonary virus content. Viral titers in the lungs were determined by plaque assay at day 4 (A) and at the indicated time points (B) after intranasal HSV-1 infection. Data represent means ± SE of individual mice, derived from at least two separate experiments. Numbers of mice in (A) were: 7 (normal CBA), 5 (allogeneic GVHD), and 3 (allogeneic No GVHD). Numbers of mice in (B) were: 3, 3, 3, 3, 7, and 6 (normal CBA; 3 hours, Days 1, 2, 3, 4, and 7, respectively); and 4, 5, 3, 5, 5, and 3 (allogeneic GVHD; 3 hours, Days 1, 2, 3, 4, and 7, respectively). The asterisk in (A) indicates a significant difference from both normal CBA and allogeneic No GVHD (P < .04; Student'st-test). The difference between normal CBA and allogeneic GVHD (B) was significant (P = .0037 using the Kruskal-Wallis test, a nonparametric ANOVA).
Allogeneic transplant recipients with GVHD showed less pulmonary virus content. Viral titers in the lungs were determined by plaque assay at day 4 (A) and at the indicated time points (B) after intranasal HSV-1 infection. Data represent means ± SE of individual mice, derived from at least two separate experiments. Numbers of mice in (A) were: 7 (normal CBA), 5 (allogeneic GVHD), and 3 (allogeneic No GVHD). Numbers of mice in (B) were: 3, 3, 3, 3, 7, and 6 (normal CBA; 3 hours, Days 1, 2, 3, 4, and 7, respectively); and 4, 5, 3, 5, 5, and 3 (allogeneic GVHD; 3 hours, Days 1, 2, 3, 4, and 7, respectively). The asterisk in (A) indicates a significant difference from both normal CBA and allogeneic No GVHD (P < .04; Student'st-test). The difference between normal CBA and allogeneic GVHD (B) was significant (P = .0037 using the Kruskal-Wallis test, a nonparametric ANOVA).
RESULTS
Increased periluminal mononuclear cell infiltrates in allogeneic transplant recipients with GVHD.
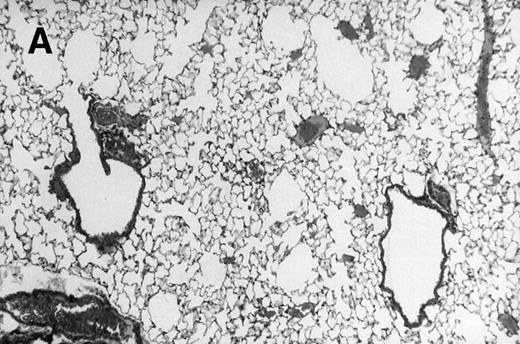
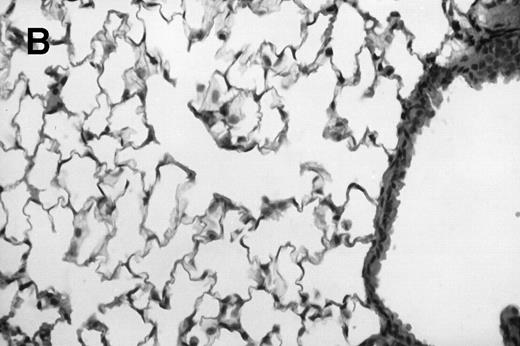
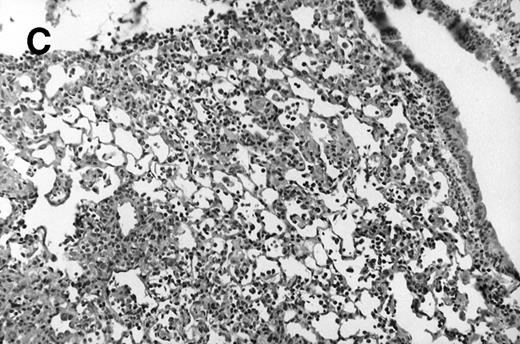
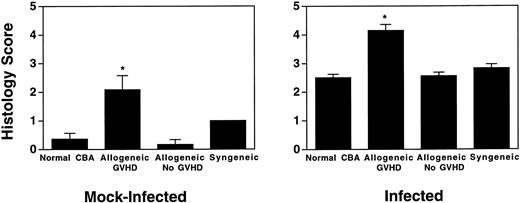
Allogeneic transplanted mice (B10.BR → CBA) with GVHD and, as controls, allogeneic transplanted mice without GVHD and syngeneic transplanted mice (B10.BR → B10.BR) were infected intranasally with HSV-1 at 12 weeks after transplantation. As an additional control, age-matched normal CBA mice were infected as well. Histological examination of lungs was performed in mock-infected mice and in mice at day 7 after infection. Allogeneic transplant recipients with GVHD showed increased pathology (Fig 1). Scores reflecting the periluminal histopathologic changes were significantly higher (P = .05; Student's t-test) in allogeneic transplant recipients with GVHD, when compared with control mice (Fig 2). The periluminal histopathologic scores were also higher in allogeneic transplant recipients with GVHD, when compared with normal CBA mice, at days 4, 10, and 14 after infection (data not shown). No significant differences were observed in the scores reflecting the parenchymal histopathologic changes (data not shown). Because the results in syngeneic transplanted mice were very similar to those in allogeneic transplanted mice without GVHD, the latter, which are the most appropriate control for procedural effects and for the effect of GVHD, and normal CBA mice, were used as controls in further experiments.
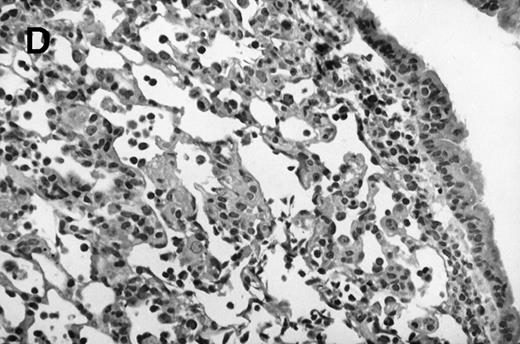
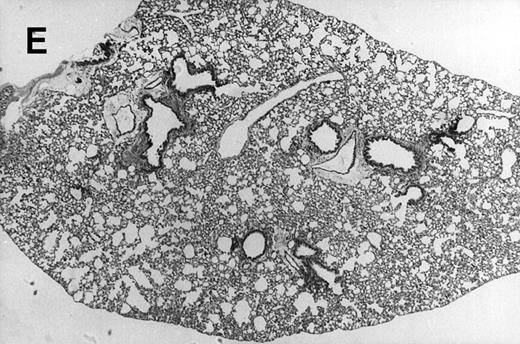
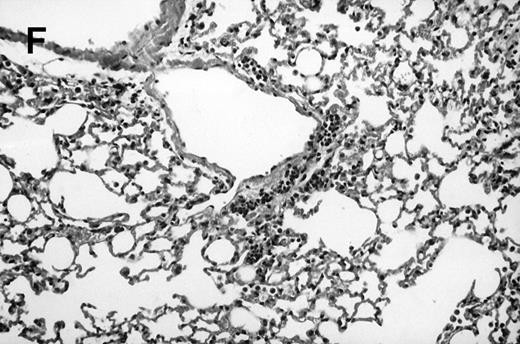
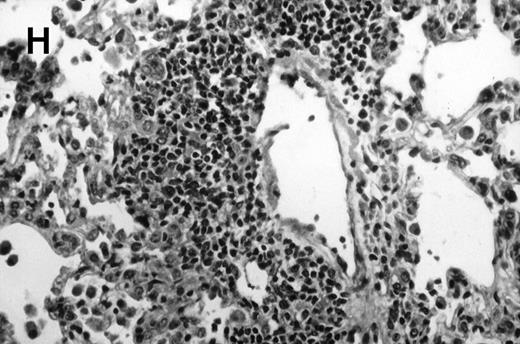
Allogeneic transplant recipients with GVHD showed increased pathology. Photomicrographs of lung sections stained with hematoxylin and eosin show evidence of increased pathology in infected allogeneic GVHD mice. (A) Normal CBA, mock-infected, original magnification × 25; (B) normal CBA, mock-infected, original magnification × 100; (C) normal CBA, infected, original magnification × 40; (D) normal CBA, infected, original magnification × 100; (E) allogeneic GVHD, mock-infected, original magnification × 10; (F) allogeneic GVHD, mock-infected, original magnification × 75; (G) allogeneic GVHD, infected, original magnification × 10; (H) allogeneic GVHD, infected, original magnification × 100.
Allogeneic transplant recipients with GVHD showed increased pathology. Photomicrographs of lung sections stained with hematoxylin and eosin show evidence of increased pathology in infected allogeneic GVHD mice. (A) Normal CBA, mock-infected, original magnification × 25; (B) normal CBA, mock-infected, original magnification × 100; (C) normal CBA, infected, original magnification × 40; (D) normal CBA, infected, original magnification × 100; (E) allogeneic GVHD, mock-infected, original magnification × 10; (F) allogeneic GVHD, mock-infected, original magnification × 75; (G) allogeneic GVHD, infected, original magnification × 10; (H) allogeneic GVHD, infected, original magnification × 100.
Allogeneic transplant recipients with GVHD showed increase of periluminal mononuclear infiltrates. The pathology scores of mock-infected mice and mice 7 days after intranasal HSV-1 infection were determined. Data represent means ± SE of individual mice, derived from at least two separate experiments. Numbers of mock-infected mice were: 11 (normal CBA), 9 (allogeneic GVHD), 6 (allogeneic No GVHD), and 2 (syngeneic). Numbers of infected mice were: 17 (normal CBA), 13 (allogeneic GVHD), 8 (allogeneic No GVHD), and 4 (syngeneic). The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P = .05; Student'st-test).
Allogeneic transplant recipients with GVHD showed increase of periluminal mononuclear infiltrates. The pathology scores of mock-infected mice and mice 7 days after intranasal HSV-1 infection were determined. Data represent means ± SE of individual mice, derived from at least two separate experiments. Numbers of mock-infected mice were: 11 (normal CBA), 9 (allogeneic GVHD), 6 (allogeneic No GVHD), and 2 (syngeneic). Numbers of infected mice were: 17 (normal CBA), 13 (allogeneic GVHD), 8 (allogeneic No GVHD), and 4 (syngeneic). The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P = .05; Student'st-test).
Allogeneic transplant recipients with GVHD showed lower pulmonary virus burden.
Because infected allogeneic transplant recipients with GVHD displayed more severe pulmonary pathology when compared with allogeneic transplant recipients without GVHD, higher pulmonary viral titers were expected in mice with GVHD. However, somewhat surprisingly, allogeneic transplant recipients with GVHD had significantly (P < .04; Student's t-test) less pulmonary virus content than both control groups at day 4 after infection (Fig 3A). Kinetic studies comparing allogeneic transplant recipients with GVHD and normal CBA mice showed that allogeneic transplant recipients with GVHD had lower pulmonary virus content than normal CBA mice at all time points (Fig 3B). The difference in viral titer per lung over time after infection between normal CBA mice and allogeneic transplant recipients with GVHD was significant (P = .0037 using nonparametric ANOVA). The virus was cleared from the lungs in all groups of mice at day 7 after infection.
Reduced influx of lymphocytes to the lungs in allogeneic transplant recipients with GVHD.
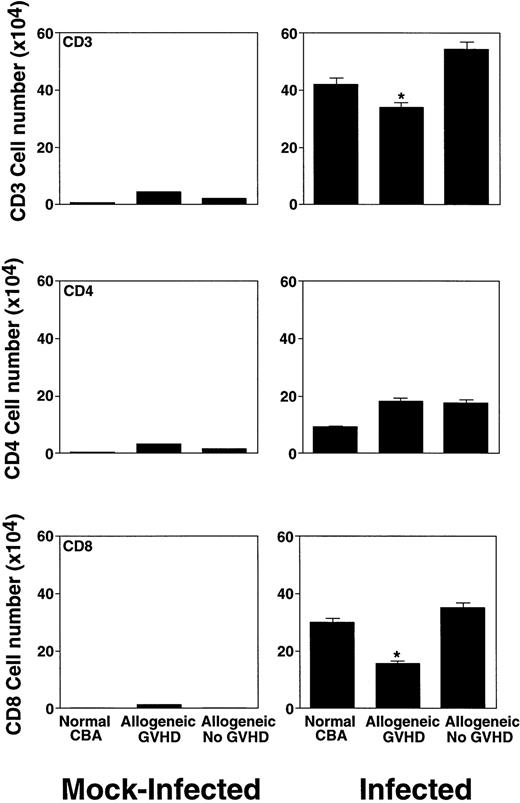
Intranasal infection with HSV-1 led to a pronounced increase in the BAL lymphocyte number at day 7 after infection (Fig 4). In all groups of both mock-infected and infected mice, we observed a dominance of CD3+ T cells. The vast majority of the cells were α/β TCR+; only very low numbers of γ/δ TCR+cells were detected (data not shown). In mock-infected mice, we found higher numbers of CD4 than CD8+ cells. After infection, the CD4/CD8 ratio in infected control mice changed to 1:3. However, in allogeneic transplant recipients with GVHD, the corresponding ratio was only 1:1 (Fig 4).
Characterization of the lymphocyte subsets in the BAL fluid of mock-infected mice and mice 7 days after intranasal HSV-1 infection. The number of lymphocytes was calculated from the percentage determined by fluorescence-activated cell sorter (FACS) analysis. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mock-infected mice were: 12 (normal CBA), 12 (allogeneic GVHD), and 6 (allogeneic No GVHD). Numbers of infected mice were: 17 (normal CBA), 13 (allogeneic GVHD), and 8 (allogeneic No GVHD). The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P < .02; Student's t-test).
Characterization of the lymphocyte subsets in the BAL fluid of mock-infected mice and mice 7 days after intranasal HSV-1 infection. The number of lymphocytes was calculated from the percentage determined by fluorescence-activated cell sorter (FACS) analysis. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mock-infected mice were: 12 (normal CBA), 12 (allogeneic GVHD), and 6 (allogeneic No GVHD). Numbers of infected mice were: 17 (normal CBA), 13 (allogeneic GVHD), and 8 (allogeneic No GVHD). The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P < .02; Student's t-test).
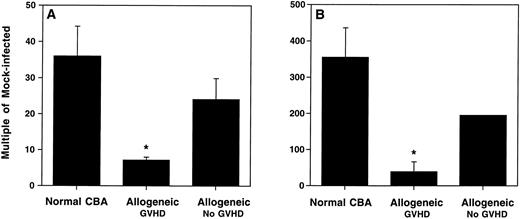
This result suggested a selective deficit in the influx of CD8+ T cells to the lungs of allogeneic transplant recipients with GVHD. When expressed as a multiple of cells in infected versus mock-infected mice, the increase in the total lymphocyte number in the control mice was between 24- to 36-fold (Fig 5A), and in the CD8+T-cell number, between 195- to 355-fold (Fig 5B). However, allogeneic transplant recipients with GVHD showed only a 7-fold increase of total lymphocytes (Fig 5A), and only a 39-fold increase of CD8+ T cells (Fig 5B) (P < .04; Student's t-test).
Allogeneic transplant recipients with GVHD showed a reduced pulmonary influx of lymphocytes. BAL cell numbers of mock-infected mice and mice 7 days after intranasal HSV-1 infection were determined. (A) Total lymphocytes recovered by BAL. (B) CD8+ T cells recovered by BAL. Numbers are expressed as the multiple of the cells recovered in infected versus mock-infected mice. The numbers were calculated from the percentages determined by FACS analysis. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were as shown in Fig 4. The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P < .04; Student's t-test). The error bar for the allogeneic no GVHD group in (B) falls within the symbol.
Allogeneic transplant recipients with GVHD showed a reduced pulmonary influx of lymphocytes. BAL cell numbers of mock-infected mice and mice 7 days after intranasal HSV-1 infection were determined. (A) Total lymphocytes recovered by BAL. (B) CD8+ T cells recovered by BAL. Numbers are expressed as the multiple of the cells recovered in infected versus mock-infected mice. The numbers were calculated from the percentages determined by FACS analysis. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were as shown in Fig 4. The asterisks indicate a significant difference from both normal CBA and allogeneic No GVHD (P < .04; Student's t-test). The error bar for the allogeneic no GVHD group in (B) falls within the symbol.
Reduced production of HSV-1–specific antibodies in allogeneic transplant recipients with GVHD.
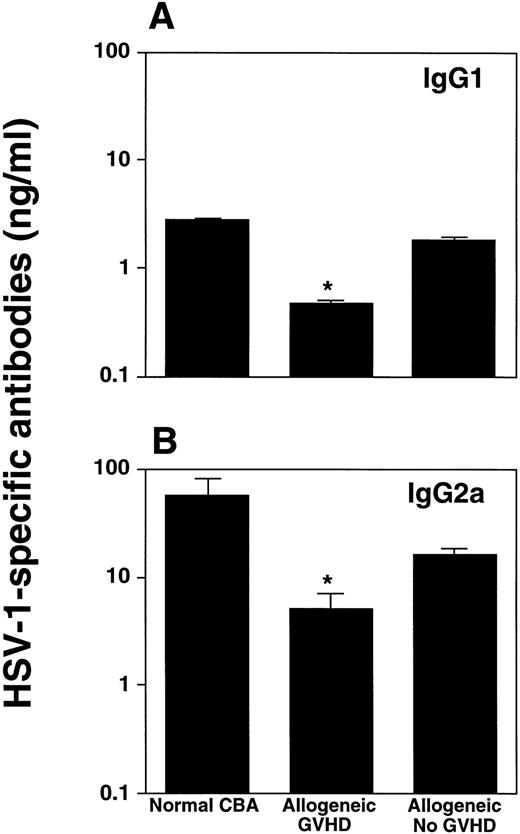
Because a humoral immune response may also be important in HSV-1 infection,8 we determined HSV-1–specific IgG1 and IgG2a, the latter isotype being predominantly induced during viral infections,23 in BAL fluids of infected mice. Allogeneic transplant recipients with GVHD produced significantly less antibodies of the IgG2a subclass, when compared with infected control mice (P < .04; Student's t-test) (Fig 6).
Allogeneic transplant recipients with GVHD showed reduced HSV-1–specific antibody production. HSV-1–specific IgG1 (A) and IgG2a (B) antibodies in the BAL fluid were determined by ELISA 14 days after intranasal HSV-1 infection. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were: 5 (normal CBA), 4 (allogeneic GVHD), and 10 (allogeneic no GVHD). The asterisks indicate a significant difference from both normal CBA and allogeneic no GVHD (P < .04; Student's t-test) for IgG2a, and a significant difference from normal CBA (P < .02; Student's t-test) for IgG1.
Allogeneic transplant recipients with GVHD showed reduced HSV-1–specific antibody production. HSV-1–specific IgG1 (A) and IgG2a (B) antibodies in the BAL fluid were determined by ELISA 14 days after intranasal HSV-1 infection. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were: 5 (normal CBA), 4 (allogeneic GVHD), and 10 (allogeneic no GVHD). The asterisks indicate a significant difference from both normal CBA and allogeneic no GVHD (P < .04; Student's t-test) for IgG2a, and a significant difference from normal CBA (P < .02; Student's t-test) for IgG1.
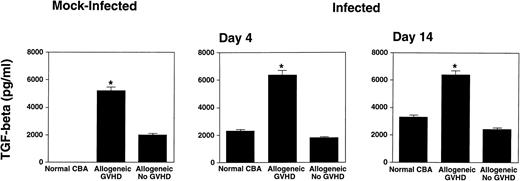
Increased levels of TGF-β1 in allogeneic transplant recipients with GVHD.
Because cytokines are involved in the pathogenesis of viral lung infections24 as well as in the pathophysiology of GVHD,25 we determined cytokine levels in BAL fluid of mock-infected mice, and at an early (day 4) as well as late (day 14) time point after infection. TGF-β1 and TNF-α were measured, because these cytokines have been shown to be induced after pulmonary radiation injury26 as well as during pulmonary GVHD.27Interestingly, both mock-infected as well as infected allogeneic transplant recipients with GVHD showed markedly elevated levels of BAL-fluid TGF-β1, when compared with control mice (Fig 7). Mice transplanted with higher T-cell numbers, and therefore increased GVHD, showed further elevated levels of TGF-β1 in the BAL fluid (data not shown). Elevated levels of TGF-β1 were not detected in the sera (data not shown). Additional cytokines assayed were IFN-γ, IL-2, IL-4, IL-5, TNF-α, and IL-10. Only IFN-γ was readily detectable in BAL fluids of normal CBA mice at days 4 and 7 after infection. None of the other cytokines could be detected in BAL fluids using ELISA, even after concentration of the samples (data not shown). However, cytokine levels in BAL fluid represent the excess after consumption or degradation,24and the cytokine levels may be below the detection limit of ELISA.
Allogeneic transplant recipients with GVHD showed increased levels of TGF-β1 in the BAL fluid. Total TGF-β1 was determined by ELISA. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were: Day 0: 8, 9, and 7; Day 4: 6, 4, and 6; Day 14: 6, 4, and 10; for normal CBA, allogeneic GVHD, and allogeneic no GVHD, respectively. BAL fluids of mock-infected mice were collected at several time points after mock infection. Because we observed no differences in the TGF-β1 levels at different time points after mock infection, the data are presented together. The asterisks indicate a significant difference from both normal CBA and allogeneic no GVHD (P < .05; Student's t-test).
Allogeneic transplant recipients with GVHD showed increased levels of TGF-β1 in the BAL fluid. Total TGF-β1 was determined by ELISA. Data represent means ± SE of individual or pooled BAL samples, derived from at least two separate experiments. Numbers of mice were: Day 0: 8, 9, and 7; Day 4: 6, 4, and 6; Day 14: 6, 4, and 10; for normal CBA, allogeneic GVHD, and allogeneic no GVHD, respectively. BAL fluids of mock-infected mice were collected at several time points after mock infection. Because we observed no differences in the TGF-β1 levels at different time points after mock infection, the data are presented together. The asterisks indicate a significant difference from both normal CBA and allogeneic no GVHD (P < .05; Student's t-test).
Treatment with anti–TGF-β MoAb decreased the histological evidence of pneumonitis in infected allogeneic transplant recipients with GVHD.
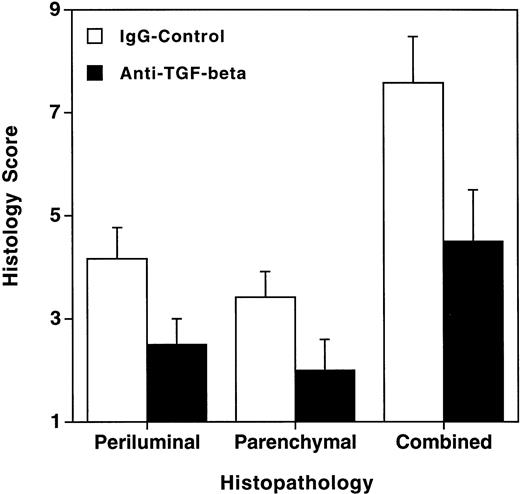
Because highly elevated levels of TGF-β1 were measured in the BAL fluid of allogeneic transplant recipients with GVHD, we hypothesized that TGF-β1 might be involved in the pathogenesis of HSV-1–induced pneumonia in allogeneic transplant recipients with GVHD. To test this hypothesis, infected allogeneic transplant recipients with GVHD were treated with anti–TGF-β MoAb, or as a control, with mouse IgG. Treatment with anti–TGF-β MoAb significantly decreased the histological evidence of pneumonitis (Fig8). We did not observe changes in the pulmonary viral titer or in the number and composition of the cells recovered by BAL after treatment with anti–TGF-β MoAb (data not shown).
Treatment with anti–TGF-β MoAb decreased the histological evidence of pneumonitis in infected allogeneic transplant recipients with GVHD. Animals were treated with 100 μg anti–TGF-β or control IgG at days −1, 1, and 3. Mice were infected intranasally at day 0, and histology was performed 4 days after infection. Data represent means ± SE of six individual mice per group, derived from three separate experiments. The difference between anti–TGF-β—treated animals and IgG control–treated animals was significant (P = .0336; Mann-Whitney test).
Treatment with anti–TGF-β MoAb decreased the histological evidence of pneumonitis in infected allogeneic transplant recipients with GVHD. Animals were treated with 100 μg anti–TGF-β or control IgG at days −1, 1, and 3. Mice were infected intranasally at day 0, and histology was performed 4 days after infection. Data represent means ± SE of six individual mice per group, derived from three separate experiments. The difference between anti–TGF-β—treated animals and IgG control–treated animals was significant (P = .0336; Mann-Whitney test).
DISCUSSION
In this study, intranasal HSV-1 infection of murine BMT recipients was used to gain insights in the pathogenesis of HSV-1–induced pneumonitis in allogeneic transplant recipients. The key findings were that (1) allogeneic transplant recipients with GVHD showed more severe pneumonitis, when compared with allogeneic transplant recipients without GVHD or normal CBA mice; (2) allogeneic transplant recipients with GVHD showed less pulmonary virus than the control mice; and (3) allogeneic transplant recipients with GVHD showed highly elevated levels of TGF-β1 in the BAL fluid. Treatment with anti–TGF-β significantly decreased the histological evidence of pneumonitis.
Our studies were designed to model pneumonia in a clinical BMT unit. Previous studies of viral infection after BMT predominantly used the Parent into F1 model, combined with virus inoculation in the immediate peritransplant period.28-30 These Parent into F1 models did not involve irradiation.28-30 Because patients receive total body irradiation, accompanied by the induction of cytokines25 capable of affecting the immune response to a subsequent infection, a murine BMT system involving irradiation is closer to the clinical setting. In this study, mice were infected at 12 weeks after transplantation, which is more appropriate for the clinical problem of late infection.
The results clearly showed that the increased pneumonitis was due to GVHD, because allogeneic transplant recipients without GVHD, which also received irradiation, showed less pneumonitis than mice with GVHD. Viral inoculation after allogeneic BMT resulted in an exacerbation of both viral infection and GVHD.28,31-34 However, mice with the more severe pneumonitis (allogeneic GVHD) had lower pulmonary virus content, when compared with control mice (allogeneic no GVHD, normal CBA). Therefore, the data suggested that the histopathology, rather than the viral titer, is the more important endpoint. We showed in our previous study that the histopathology correlated with lung compliance, a parameter of in vivo global lung function.4
Previously published data suggested that pulmonary pathology and viral titer may not be correlated. Shanley et al14 have found higher viral titers in the lungs of allogeneic transplanted mice, when compared with syngeneic recipients, after infection with murine CMV. In that study, mice were infected subcutaneously early (14 days) after BMT, and viral titers in the lung were determined 14 days after infection. The difference between the two studies might be due to the different time point of infection after BMT (14 days v 12 weeks), to the different virus and route of infection used, or to differences in the immune response to MCMV as opposed to HSV-1. The presence or absence of lung disease was not investigated in that study. However, Grundy et al30 showed that pulmonary disease did not correlate with the level of MCMV replication in the lung after IP infection of mice with GVHD in the Parent into F1 model. The lack of correlation between the extent of pneumonitis and pulmonary viral titers in MCMV lung infection is in agreement with our earlier work4 and with the results of our current study in HSV-1–induced pneumonitis.
The result that allogeneic transplant recipients with GVHD showed more severe pneumonitis in the presence of less virus suggested that alterations in the immune response of allogeneic transplant recipients with GVHD to HSV-1 infection may be more important for the development of pneumonitis than direct viral cytocidal effects. In particular, we hypothesized that an impaired cytokine production may be involved. Inappropriate production of cytokines may play an important role in the induction and progression of GVHD.25
Indeed, the second major finding was that allogeneic transplant recipients with GVHD had highly elevated BAL fluid TGF-β1 levels. TGF-β is a pleiotropic cytokine and can exert both immunosuppressive and proinflammatory effects.35 For example, TGF-β inhibits lymphocyte proliferation,35 suppresses human immunodeficiency virus expression and replication in infected cells,36 induces apoptosis,37 downregulates NO production,38 and is involved in fibrotic disorders of the lung by its ability to upregulate collagen expression.35Thus, TGF-β might be responsible for the decreased BAL lymphocyte number by interfering with lymphocyte proliferation and/or influx. By its immunosuppressive effects, TGF-β may enhance susceptibility to lung infections.3 TGF-β may account for the lower pulmonary virus content observed in allogeneic transplant recipients with GVHD by inhibiting proliferation of lung epithelial cells, a HSV-1 replication site,39,40 or by causing apoptosis of epithelial cells. TGF-β may be involved in the apoptosis of epithelial cells which is induced during GVHD.25,41Furthermore, TGF-β is involved in the late fibroproliferative phase of the idiopathic pneumonia syndrome, predominantly in patients with chronic GVHD.42 We did not observe changes in the pulmonary viral titer and in the BAL cell number after treatment with anti–TGF-β MoAb, possibly due to the particular dose of antibody and time point studied in our experiments.
This is the first report of a role of TGF-β1 in the pathogenesis of HSV-1–induced pneumonia in allogeneic transplant recipients with GVHD. Interestingly, TGF-β was shown to be involved in intestinal GVHD.43 The BAL fluid TGF-β1 levels increased slightly after i.n. infection in allogeneic transplant recipients with GVHD. In contrast, TGF-β1 was induced after intranasal infection of normal mice, in agreement with the report of the generation of TGF-β after IP lymphocytic choriomeningitis virus infection of mice.44 45
If the immune response is more important than the viral load, curative therapy for posttransplant viral infections may include the modulation of immune functions. The specific inhibition of mediators of the immune response, which may be produced in excess in response to a given infection and during GVHD, might be an effective treatment for posttransplant infection. Consistent with this hypothesis, we could show that anti–TGF-β treatment decreased the histological evidence of pneumonitis.
TGF-β downregulates NO,38 which is produced during GVHD46 and HSV-1–induced pneumonitis.4Consequently, whereas elevated levels of nitrite/nitrate in BAL fluids of infected normal mice were detected after intranasal HSV-1 infection, no increase was observed in allogeneic transplant recipients with GVHD. This absence of nitrite/nitrate elevation may be due to the high TGF-β1 concentrations in the BAL fluid of these animals. Thus, inhibition of NO production suppressed histological evidence of HSV-1–induced pneumonia in normal mice, but not in allogeneic transplant recipients with GVHD.4 Because TGF-β downregulates NO production, inhibition of TGF-β may lead to an enhanced generation of NO. Therefore, in allogeneic transplant recipients with GVHD, a combined treatment with inhibitors of both TGF-β and NO may be more effective than anti–TGF-β alone.
In conclusion, we showed that allogeneic transplant recipients with GVHD developed more severe pneumonitis after intranasal HSV-1 infection than control mice without GVHD, and that in allogeneic transplant recipients with GVHD an enhanced TGF-β1 production may be more important for the development of pneumonitis than the pulmonary viral load. Consistent with this hypothesis, we could show that anti–TGF-β treatment decreased the histological evidence of pneumonitis.
ACKNOWLEDGMENT
We thank Dr Steven Burakoff for helpful discussions, and Linda Callahan for the expert technical support. We thank Drs V. Kuchroo and M. Prabhu Das for the help in establishing the TGF-β1 ELISA.
Supported by grants from the National Institutes of Health, the Mallinckrodt Foundation, and the Aid for Cancer Research. H.A. is supported by a grant from the Deutsche Forschungsgemeinschaft (DFG). I.J.R. is a Claudia Adams Barr Investigator.
Address reprint requests to Ilonna J. Rimm, MD, PhD, Genetics Institute, 87 CambridgePark Dr, Cambridge, MA 02140; e-mail:irimm@genetics.com.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal