Abstract
We have studied the in vitro biological activities and mechanisms of action of 1,25-dihydroxyvitamin D3 (1,25D3) and nine potent 1,25D3 analogs on proliferation and differentiation of myeloid leukemia cell lines (HL-60, retinoic acid-resistant HL-60 [RA-res HL-60], NB4 and Kasumi-1). The common novel structural motiff for almost all the analogs included removal of C-19 (19-nor); each also had unsaturation of the side chain. All the compounds were potent; for example, the concentration of analogs producing a 50% clonal inhibition (ED50) ranged between 1 × 10−9 to 4 × 10−11 mol/L when using the HL-60 cell line. The most active compound [1,25(OH)2-16,23E-diene-26-trifluoro-19-nor-cholecalciferol (Ro 25-9716)] had an ED50 of 4 × 10−11mol/L; in contrast, the 1,25D3 produced an ED50of 10−9 mol/L with the HL-60 target cells. Ro 25-9716 (10−9 mol/L, 3 days) was a strong inducer of myeloid differentiation because it caused 92% of the HL-60 cells to express CD11b and 75% of these cells to reduce nitroblue tetrazolium (NBT). This compound (10−8 mol/L, 4 days) also caused HL-60 cells to arrest in the G1 phase of the cell cycle (88% cells in G1v 48% of the untreated control cells). The p27kip-1, a cyclin-dependent kinase inhibitor which is important in blocking the cell cycle, was induced more quickly and potently by Ro 25-9716 (10−7 mol/L, 0 to 5 days) than by 1,25D3, suggesting a possible mechanism by which these analogs inhibit proliferation of leukemic growth. The NB4 promyelocytic leukemia cells cultured with the Ro 25-9716 were also inhibited in their clonal proliferation (ED50, 5 × 10−11mol/L) and their expression of CD11b was enhanced (80% positive [10−9 mol/L, 4 days] v 27% untreated NB4 cells). Moreover, the combination of Ro 25-9716 (10−9mol/L) and all-trans retinoic acid (ATRA, 10−7 mol/L) induced 92% of the NB4 cells to reduce NBT, whereas only 26% of the cells became NBT positive after a similar exposure to the combination of 1,25D3 and ATRA. Surprisingly, Ro 25-9716 also inhibited the clonal growth of poorly differentiated leukemia cell lines (RA-res HL-60 [ED50, 4 × 10−9 mol/L] and Kasumi-1 [ED50, 5 × 10−10 mol/L]). For HL-60 cells, Ro 25-9716 markedly decreased the percent of the cells in S phase of the cell cycle and increased the expression of the cyclin-dependent kinase inhibitor, p27kip-1. In summary, 19-nor vitamin D3 compounds strongly induced differentiation and inhibited clonal proliferation of various myeloid leukemia cell lines, suggesting a therapeutic niche for their use in myeloid leukemia.
CURRENTLY, CHEMOTHERAPY of cancer is based principally on agents that are toxic to the cells. Data suggest that induction of cellular differentiation may supplement the use of cytotoxic drugs in several forms of neoplasia, such as the successful use of retinoic acid in the treatment of acute promyelocytic leukemia (APL)1 or oral leukoplakia.2 Interest is also developing in the use of seco-steriod hormones (ie, derivatives of vitamin D3) for the chemoprevention and treatment of human malignancies.3-6 The physiologically active form of vitamin D3 is 1α,25 dihydroxyvitamin D3(1,25D3), and it can inhibit the growth in vitro of cancer cells from several different tissues including human myeloid leukemia,7,8 breast,9-11colon,12,13 squamous skin,14prostate,15,16 and glioma cells.17 Studies in vivo suggested that 1,25D3 is able to prolong the survival of mice injected with leukemic cells.18 A trial of oral administration of 1,25D3 to preleukemic patients was only partially effective, perhaps because the concentration of the seco-steriod required for observation of activity in vitro could not be achieved in vivo unless hypercalcemia developed.3Therefore, research activities have been directed at finding new 1,25D3 analogs with a more favorable therapeutic profile.
In the present study, we analyzed 1,25D3 analogs that had no carbon-19 (19-nor). The results indicated that the novel 19-nor vitamin D3 analog (Ro 25-9716) was an extremely potent compound which strongly inhibited both mature and immature myeloid leukemia cell lines in vitro. We also showed that HL-60 cells underwent myeloid differentiation and were arrested in G1 after their incubation with Ro 25-9716; this phenomenon was associated with the rapid and prominent accumulation of the p27kip-1cyclin-dependent kinase inhibitor (CDKI).
MATERIALS AND METHODS
Cell lines.
HL-60, retinoic acid-resistant HL-60 (RA-res HL-60), NB4, and Kasumi-1 cell lines were grown in RPMI 1640 medium (GIBCO Life Technologies, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (GIBCO) under standard culture conditions. The NB4 promyelocytic leukemia cell line was provided by Dr Lanotte (INSERM, Hospital Saint-Louis, Paris, France).19 RA-res HL-60, which is a subline of HL-60 cells, is resistant to both all-trans retinoic acid (ATRA) and 1,25D3 and has a mutation at codon 411 of the retinoic acid receptor α gene (kindly provided by Dr Gallagher, Albert Einstein Cancer Center, NY).20 Kasumi-1 cell line is an undifferentiated myeloid leukemia cell line that carries a t(8;21)(q22;q22) chromosomal abnormality.21
1,25D3 analogs.
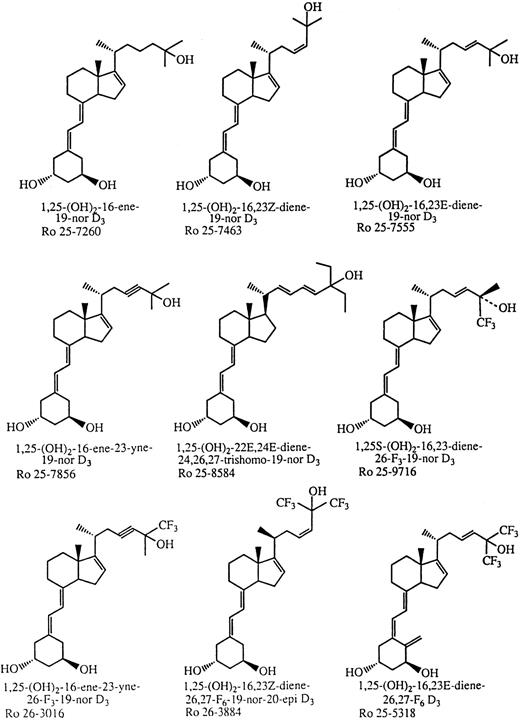
The parental compound [1a,25(OH)2D3] and its analogs were dissolved in absolute ethanol at 10-3 mol/L and stored at −20°C. The concentration of the analogs was determined by measurements of ultraviolet absorbance using their molar extinction coefficient at 264 nmol/L. Dilutions were made in the same tissue culture medium used for growing the leukemia cell lines. All manipulation with vitamin D analogs were performed in subdued light. The maximum concentration of ethanol used in this study had no influence on cell growth (data not shown). The simplified code names and structures of the 1,25D3 analogs are shown in Fig 1.
Structure and code name of the novel vitamin D3 analogs examined in this study.
Structure and code name of the novel vitamin D3 analogs examined in this study.
Cytotoxicity test.
HL-60 (105/mL) cells were incubated in liquid culture for 3 days with various concentrations (10-8 to 10-9mol/L) of 1,25D3 or Ro 25-9716. After the culture, cell viability was evaluated by staining with trypan blue. Inhibition of exponentially growing cells was estimated by counting the cell number after HL-60, RA-res HL-60, NB4, or Kasumi-1 cells (seeded at 5 × 104/mL) were treated with 10-7 mol/L of either 1,25D3 or Ro 25-9716 for 4 days.
Colony formation in soft agar.
Cells were seeded in a two-layer soft agar system as previously described.22 The lower layer consisted of 0.5% agar in which the test substances were mixed; upper layer was 0.3% agar (Difco Laboratories, Detroit, MI ) in which 1,000 to 5,000 cells were mixed per plate. All experimental points were performed in triplicate. All plating experiments were repeated at least twice. After 10 days of incubation at 37°C in a humidified atmosphere containing 5% CO2 in air, colonies (>40 cells) were counted using an inverted microscope.
Studies of induction of differentiation.
Differentiation of HL-60 and NB4 cells was assessed by their abilities to produce superoxide as measured by reduction of nitroblue tetrazolium (NBT),23 by morphology as detected on cytopsin preparations stained with Diff-Quick Stain Set (Baxter Healthcare Corp, Miami, FL), and by analysis of expression of CD11b surface marker by flow cytometry.
Pulse-exposure experiments.
HL-60 cells were incubated in liquid culture for various durations with 10-9 mol/L of either 1,25D3 or Ro 25-9716. After incubation, cells were carefully washed twice, once with phosphate-buffered saline and once with media before being counted and plated into 24-well plates for soft agar colony assay. Some HL-60 cells were plated in the liquid culture without compounds after two times wash-out; terminal differentiation of HL-60 cells was evaluated by NBT test at the third day of the culture.
Cell cycle analysis.
The cell cycle was analyzed by flow cytometry after 96 hours of incubation of cells (5 × 104 /mL) either with or without analogs (10-8 mol/L) as described.24Briefly, the cells were fixed in cold ethanol and incubated for 30 minutes at 4°C in the dark with a solution of 50 mg/mL propidium iodide, 1 mg /mL RNase (Sigma, Saint-Louis, MO), and 0.1% NP40 (Sigma). Analysis was performed immediately after staining using the CELLFIT program (Becton Dickinson, Mountain View, CA), whereby the S phase was calculated with an RFit model.
Expression of p27kip-1.
Western blot analysis was performed with polyclonal rabbit anti-p27 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and rabbit polyclonal antimyeloperoxidase (MPO) antibody, which recognizes the 55-kD MPO protein.25 Sodium dodecyl sulfate (SDS)-polyacrylamide gel electropheresis was performed as previously described.26 Briefly, proteins (40 μg) were size fractioned under denaturing conditions on 12.5% SDS-running gel and transferred to Immobilon polyvinylidine difuride membrane (Millipore, Bedford, MA). The protein was detected using the enhanced chemiluminescence system from Amersham (Arlington Heights, IL).
RESULTS
Effects of 1,25D3 analogs on clonal growth of leukemia cell lines.
All analogs of 1,25D3 were tested in a dose-response fashion (10-11 to 10-6 mol/L) and each was active in the clonal inhibition of HL-60 growth in soft agar. Dose-response curves were prepared (data not shown), and the concentration at which 50% of the colonies were inhibited (ED50) by each analog was calculated (Table 1). For all analogs, the ED50 ranged from 6 × 10-10 to 4 × 10-11 mol/L; by comparison, the parental 1,25D3achieved an ED50 of 1 × 10-9 mol/L. The most potent analog (Ro 25-9716, 1,25S-dihydroxy-16,23E-diene-26-trifluoro-19-nor-cholecalciferol) had an ED50 of 4 × 10-11 mol/L, which was 25-fold more potent than 1,25D3(Fig 2). Likewise, this analog (Ro 25-9716) was examined for its ability to inhibit clonal growth of the RA-res HL-60, NB4, and Kasumi-1 cell lines (Fig 2). The clonal growth of RA-res HL-60 was not inhibited by 1,25D3, but remarkably, Ro 25-9716 did suppress the clonal growth of RA-res HL-60 cells (ED50, 7 × 10-9 mol/L). The clonal growth of both NB4 and Kasumi-1 cells was inhibited by both 1,25D3and Ro 25-9716, but Ro 25-9716 was more active (ED50: NB4, 5 × 10-11 mol/L; Kasumi-1, 5 × 10-8mol/L) than 1,25D3 (ED50: NB4, 1 × 10-9 mol/L; Kasumi-1, 6 × 10-10 mol/L).
Effects of Vitamin D3 Analogs on Clonal Growth of HL-60 Cells
| Chemical Name . | Code Name . | ED50 (mol/L) . |
|---|---|---|
| 1,25-(OH)2-D3 | 1,25D3 | 1 × 10−9 |
| 1,25S-(OH)2-16,23-diene-26-F3-19nor D3 | Ro 25-9716 | 4 × 10−11 |
| 1,25-(OH)2-16-ene-23-yne-26-F3-19nor D3 | Ro 26-3016 | 6 × 10−11 |
| 1,25-(OH)2-16-ene-19-nor D3 | Ro 25-7290 | 3 × 10−10 |
| 1,25-(OH)2-16,23Z-diene-19-nor D3 | Ro 25-7463 | 4 × 10−10 |
| 1,25-(OH)2-22E,24E-diene-24,26,27-trishomo-19-nor D3 | Ro 25-8584 | 5 × 10−10 |
| 1,25-(OH)2-16-ene-23-yne-19-nor D3 | Ro 25-7856 | 5 × 10−10 |
| 1,25-(OH)2-16,23Z-diene-26,27-F6-19-nor-20-epi D3 | Ro 26-3884 | 5 × 10−10 |
| 1,25-(OH)2-16,23E-diene-19-nor D3 | Ro 25-7555 | 6 × 10−10 |
| 1,25-(OH)2-16,23E-diene-26,27-F6D3 | Ro 25-5818 | 6 × 10−10 |
| Chemical Name . | Code Name . | ED50 (mol/L) . |
|---|---|---|
| 1,25-(OH)2-D3 | 1,25D3 | 1 × 10−9 |
| 1,25S-(OH)2-16,23-diene-26-F3-19nor D3 | Ro 25-9716 | 4 × 10−11 |
| 1,25-(OH)2-16-ene-23-yne-26-F3-19nor D3 | Ro 26-3016 | 6 × 10−11 |
| 1,25-(OH)2-16-ene-19-nor D3 | Ro 25-7290 | 3 × 10−10 |
| 1,25-(OH)2-16,23Z-diene-19-nor D3 | Ro 25-7463 | 4 × 10−10 |
| 1,25-(OH)2-22E,24E-diene-24,26,27-trishomo-19-nor D3 | Ro 25-8584 | 5 × 10−10 |
| 1,25-(OH)2-16-ene-23-yne-19-nor D3 | Ro 25-7856 | 5 × 10−10 |
| 1,25-(OH)2-16,23Z-diene-26,27-F6-19-nor-20-epi D3 | Ro 26-3884 | 5 × 10−10 |
| 1,25-(OH)2-16,23E-diene-19-nor D3 | Ro 25-7555 | 6 × 10−10 |
| 1,25-(OH)2-16,23E-diene-26,27-F6D3 | Ro 25-5818 | 6 × 10−10 |
Each compound was tested at log intervals in dose-response studies (10−11 to 10−7 mol/L); means ± SD were determined and plotted on semilog paper. The dose that produced 50% inhibition of colony formation (ED50) was calculated. Each experimental point had three plates per experiment; the SD was always less than 10%.
Dose-response effects of 1,25S-(OH)2-16,23E-diene-26-F3-19-nor D3 (Ro 25-9716) and 1,25-(OH)2D3(1,25D3) on clonal proliferation of myeloid leukemia cell lines. Results are expressed as a percent of control plates containing no vitamin D3 compounds. Results are the mean of at least three independent experiments with triplicate dishes. (□), 1,25 D3; (⌾), Ro 25-9716.
Dose-response effects of 1,25S-(OH)2-16,23E-diene-26-F3-19-nor D3 (Ro 25-9716) and 1,25-(OH)2D3(1,25D3) on clonal proliferation of myeloid leukemia cell lines. Results are expressed as a percent of control plates containing no vitamin D3 compounds. Results are the mean of at least three independent experiments with triplicate dishes. (□), 1,25 D3; (⌾), Ro 25-9716.
Cytotoxicity test.
Viability of HL-60 cells was 98% in liquid culture without 1,25D3 analogs. Trypan blue–positive HL-60 cells increased to 28% after their exposure to Ro 25-9716 (10-8 mol/L, 3 days), as compared with 8% after culture with 1,25D3(10-8 mol/L, 3 days). Morphologically, the dead cells that appeared after exposure to Ro 25-9716 were apoptotic. In liquid culture, Ro 25-9716 (10-7 mol/L, 4 days) inhibited growth of the HL-60 cells, but 1,25D3 (10-7 M, 4 days) did not (cell counts were 64% or 99% of controls [no compounds], respectively). Cell counts of RA-res HL-60 (75% v 136%), NB4 (68% v 107%), and Kasumi-1 (38% v 54%) were also decreased with Ro 25-9716 as compared with 1,25D3(percentage of control cultures after treatment with Ro 25-9716 v1,25D3, respectively). Increase of apoptotic cells was not observed in these three cell lines after exposure to Ro 25-9716.
Pulse-exposure experiments.
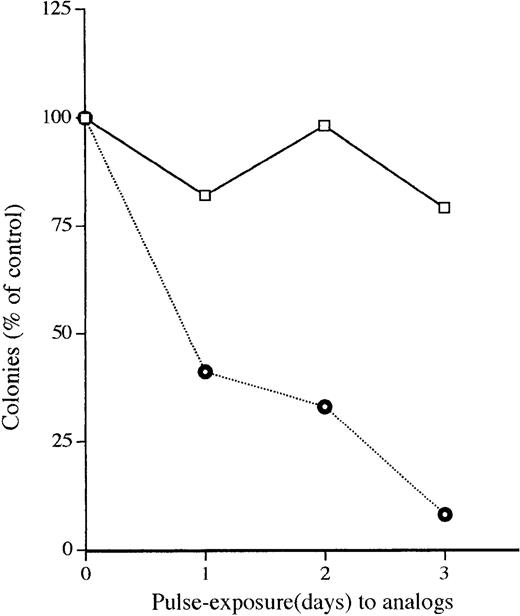
The HL-60 cells were cultured with vitamin D3 compound (10-9 mol/L) for different durations (1 to 4 days), throughly washed, counted (>99% of the population was viable), plated in soft-agar, and colonies were enumerated 10 days later. More than 50% of the HL-60 cells were inhibited by 1 day of exposure to analog Ro 25-9716 (10-9 mol/L), and a 3-day exposure inhibited more than 90% of the clonogenic cells, suggesting that this analog was capable of mediating an irreversible inhibition of growth of these cells (Fig 3). Notably, 1,25D3 (10-9 mol/L) inhibited only 21% of the clonogenic cells after 3 days of exposure. Terminal differentiation was observed in the cells exposed to Ro 25-9716 (10-9 mol/L, 4 days) (10% NBT-positive cells). On the other hand, less than 1% of NBT-positive HL-60 cells were observed after the cells were exposed to 1,25D3 (10-9 mol/L, 4 days).
Pulse-exposure of HL-60 cells to either 1,25D3 (□) or Ro-25-9716 (⌾). HL-60 cells were exposed for various durations to the vitamin D3 compounds (10−9 mol/L). The cells were then throughly washed three times, counted, plated (1,000 cells/well) into soft agar, and colonies were counted 14 days after plating. Each point represents the mean of three experiments with triplicate dishes per point.
Pulse-exposure of HL-60 cells to either 1,25D3 (□) or Ro-25-9716 (⌾). HL-60 cells were exposed for various durations to the vitamin D3 compounds (10−9 mol/L). The cells were then throughly washed three times, counted, plated (1,000 cells/well) into soft agar, and colonies were counted 14 days after plating. Each point represents the mean of three experiments with triplicate dishes per point.
Effect of 1,25D3 analogs on differentiation of leukemia cell lines.
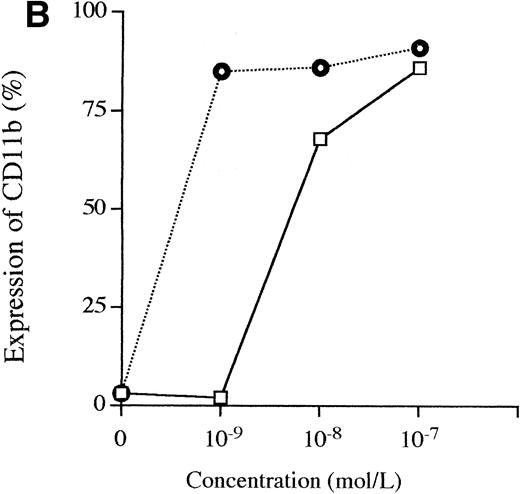
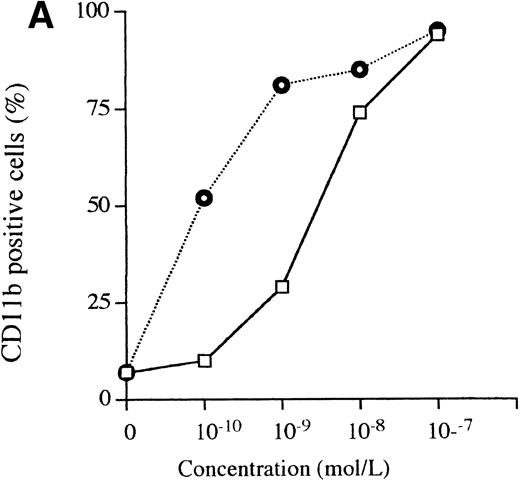
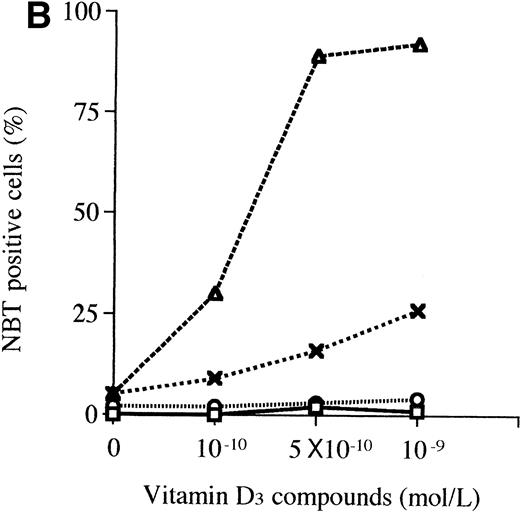
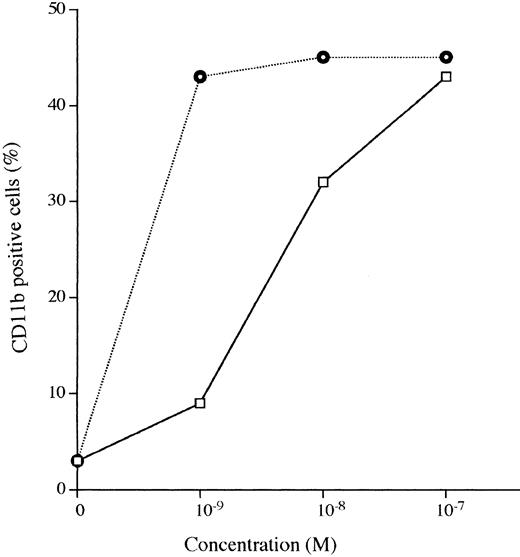
A 3-day exposure of HL-60 cells to either 1,25D3 or Ro 25-9716 (10-9 mol/L) resulted in 10% and 83% NBT-positive cells, respectively (Fig 4A). With the same culture conditions, 2% and 92% of the HL-60 cells expressed CD11b protein in the presence of either 1,25D3 or Ro 25-9716, respectively (Fig 4B). Also, with the same culture conditions, Ro 25-9716 at 10-10 mol/L induced 50% of NB4 cells to express CD11b, whereas approximately 40-fold more 1,25D3 was required to induce a similar degree of differentiation (Fig 5A). ATRA alone (10-7mol/L × 3 days) induced less than 5% of NB4 cells to differentiate as measured by NBT when Ro 25-9716 was combined with ATRA; it markedly enhanced differentiation (ED50, 2 × 10-10). Although the Kasumi-1 cells showed no morphological differentiation when cultured with either 1,25D3 or Ro 25-9716 (10-7 mol/L, 3 days) (data not shown), 9% and 43% of these cells became CD11b positive after their incubation with either 1,25D3 or Ro 25-9716 (10-9 mol/L, 3 days), respectively (Fig 6). The RA-res HL-60 neither differentiated morphologically nor expressed CD11b surface markers after culture with either 1,25D3 or Ro 25-9716 (10-7 mol/L, data not shown).
Comparison of the differentiation-inducing activities of 1,25D3 and R0-25-9716. (A) NBT reduction activities. HL-60 cells were cultured with various concentrations (10−11 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716 for 3 days and differentiation was determined by NBT reduction. (▧), 1,25D3; (▪), Ro 25-9716. (B) Expression of CD11b antigens on HL-60 cells. Cells were treated for 3 days with different concentrations (10−9 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716, and then analyzed by fluorescence-activated cell sorting (FACS) for expression of CD11b. (□), 1,25D3; (⌾), Ro 25-9716.
Comparison of the differentiation-inducing activities of 1,25D3 and R0-25-9716. (A) NBT reduction activities. HL-60 cells were cultured with various concentrations (10−11 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716 for 3 days and differentiation was determined by NBT reduction. (▧), 1,25D3; (▪), Ro 25-9716. (B) Expression of CD11b antigens on HL-60 cells. Cells were treated for 3 days with different concentrations (10−9 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716, and then analyzed by fluorescence-activated cell sorting (FACS) for expression of CD11b. (□), 1,25D3; (⌾), Ro 25-9716.
Induction of differentiation of NB4 cells. (A) Expression of CD11b antigens on NB4 cells. NB4 cells were treated for 3 days with several concentrations (10−10 to 10−7mol/L) of either 1,25D3 or Ro-25 9716, and then analyzed by FACS. (□), 1,25D3; (⌾), Ro 25-9716. (B) Combination of ATRA plus either 1,25D3 or Ro 25-9716 on differentiation of NB4 cells. NB4 cells were treated with 1,25D3 alone (10−9 to 10−10 mol/L, □), Ro 25-9716 alone (10−9 to 10−10 mol/L, ⌾), the combination of either ATRA (10−7 mol/L) plus 1,25D3 (10−9 to 10−10 mol/L, X), or ATRA (10−7 mol/L) plus Ro 25-9716 (10−9 to 10−10 mol/L, □), and differentiation was determined by NBT reduction. Results represent the mean of three experiments with triplicate dishes.
Induction of differentiation of NB4 cells. (A) Expression of CD11b antigens on NB4 cells. NB4 cells were treated for 3 days with several concentrations (10−10 to 10−7mol/L) of either 1,25D3 or Ro-25 9716, and then analyzed by FACS. (□), 1,25D3; (⌾), Ro 25-9716. (B) Combination of ATRA plus either 1,25D3 or Ro 25-9716 on differentiation of NB4 cells. NB4 cells were treated with 1,25D3 alone (10−9 to 10−10 mol/L, □), Ro 25-9716 alone (10−9 to 10−10 mol/L, ⌾), the combination of either ATRA (10−7 mol/L) plus 1,25D3 (10−9 to 10−10 mol/L, X), or ATRA (10−7 mol/L) plus Ro 25-9716 (10−9 to 10−10 mol/L, □), and differentiation was determined by NBT reduction. Results represent the mean of three experiments with triplicate dishes.
Expression of CD11b antigens on Kasumi-1 myeloid leukemia cells. Kasumi-1 cells were cultured for 3 days with various concentrations (10−9 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716, and then analyzed by FACS for expression of CD11b. (□), 1,25D3; (⌾), Ro 25-9716.
Expression of CD11b antigens on Kasumi-1 myeloid leukemia cells. Kasumi-1 cells were cultured for 3 days with various concentrations (10−9 to 10−7 mol/L) of either 1,25D3 or Ro 25-9716, and then analyzed by FACS for expression of CD11b. (□), 1,25D3; (⌾), Ro 25-9716.
Cell cycle analysis.
Effect of analog Ro 25-9716 on the cell cycle of the leukemia cell lines was examined. The HL-60 cells had a significant increased number of cells (88%) in the G1 phase of the cell cycle after their exposure to Ro 25-9716 (10-9 mol/L, 96 hours) as compared with wild-type HL-60 cells (48% in G1, Fig 7). The NB4 cells also had an increased number of cell (63%) in G1 after their exposure to Ro 25-9716 (10-7 mol/L, 96 hours) as compared with wild-type NB4 cells (47%). The Kasumi-1 cells had a small percentage increase (76%) in G1 and a small decrease (16%) in S phase after their exposure to Ro 25-9716 (10-9 mol/L, 96 hours), as compared with wild-type Kasumi-1 cells (71% in G1 and 21% in S, data not shown). In contrast, the RA-res HL-60 cells showed no increase in the number of cells in the G1 phase after incubation with either 1,25D3 or Ro 25-9716 (10-6 mol/L, 96 hours, data not shown).
Cell cycle analysis of HL-60 cells cultured with vitamin D3 compounds. The HL-60 cells were cultured without vitamin D3 compounds (A) or with either 1,25D3 (B) or Ro 25-9716 (C) at 10−8 mol/L for 4 days before cell cycle analysis.
Cell cycle analysis of HL-60 cells cultured with vitamin D3 compounds. The HL-60 cells were cultured without vitamin D3 compounds (A) or with either 1,25D3 (B) or Ro 25-9716 (C) at 10−8 mol/L for 4 days before cell cycle analysis.
Induction of expression of p27kip-1.
The HL-60 cells did not express easily detectable p27kip-1before exposure to the vitamin D3 compounds. However, these cells contained low levels of p27kip-1 after 1-day incubation with Ro 25-9716 (10-7 mol/L), and these levels markedly increased on day 2 of culture. In contrast, the expression of p27kip-1 did not increase until the third day of incubation with 1,25D3 (10-7 mol/L), and even at days 4 and 5 of culture, the intensity of expression was generally weak (Fig 8). The other three cell lines (RA-res HL-60, NB4, and Kasumi-1) weakly expressed p27 protein before exposure to the vitamin D3 compounds, and none of these cell lines had an increased level of p27 protein after exposure to vitamin D3 compounds (data not shown).
Expression of p27kip-1 in HL-60 cells as measured by Western blot. Cells were either untreated (lane 1) or treated with either Ro 25-9716 (10−7 mol/L, [A]) or 1,25D3 (10−7 mol/L, [B]) for 1 day (lane 2), 2 days (lane 3), 3 days (lane 4), 4 days (lane 5), or 5 days (lane 6). Lysates from the cells were analyzed for expression of p27kip-1 or MPO using Western blot analysis.
Expression of p27kip-1 in HL-60 cells as measured by Western blot. Cells were either untreated (lane 1) or treated with either Ro 25-9716 (10−7 mol/L, [A]) or 1,25D3 (10−7 mol/L, [B]) for 1 day (lane 2), 2 days (lane 3), 3 days (lane 4), 4 days (lane 5), or 5 days (lane 6). Lysates from the cells were analyzed for expression of p27kip-1 or MPO using Western blot analysis.
DISCUSSION
The 1,25D3 and its analogs inhibited the proliferation of various cancer subtypes in vitro and in vivo.7-18Previously, our data showed that 1,25D3 analogs that had the removal of their C-19 moiety (19-nor 1,25D3 analogs) were extremely active against prostate cancer cell lines that were resistant to other vitamin D3 analogs.27Moreover, the 19-nor analog with the code name LH [1,25-(OH)2-16-ene-23-yne-26,27-F6-19-nor-D3] was the most potent inhibitor of clonal proliferation of breast and prostate cancer cell lines.28 Therefore, we synthesized additional novel 19-nor 1,25D3 analogs and examined their biological effects on myeloid leukemia cell lines. This study showed that all nine novel analogs of 1,25D3 had a remarkable ability to inhibit the clonal growth of HL-60 cells. The most potent analog (ED50, 4 × 10-10 mol/L) was Ro-25-9716 (1,25S-dihydroxy-16,23E-diene-26-trifluoro-19-nor cholecalciferol) and therefore, we focused on the scope of activity of this analog compared with 1,25D3 on various myeloid leukemia cell lines.
The Ro 25-9716 was active against all four myeloid leukemia cell lines (HL-60, RA-res HL-60, NB4, and Kasumi-1). This compound strongly inhibited clonal proliferation and induced differentiation of HL-60 cells; its effect in some respects may be more potent than that of the 20-epi-1,25D3 analog known as KH1060, which we have previously reported to be one of the most potent vitamin D3inhibitors of clonal growth of HL-60 cells.29Pulse-exposure studies showed that 50% inhibition of clonal growth of HL-60 cells occurred with Ro 25-9716 (10-9 mol/L, 24 hours), whereas previously, our experiments showed that pulse-exposure of these cells for 60 hours was necessary for a 50% inhibition of clonal growth by KH1060 (10-8 mol/L).29Moreover, KH1060 (10-8 mol/L, 60 hours) induced 40% of HL-60 cells to become NBT positive,29 whereas Ro 25-9716 (10-9 mol/L, 72 hours) induced 82% NBT positive cells. Therefore, even though conditions were not completely identical, these data suggest that Ro 25-9716 may be a more potent inducer of differentiation than is KH1060.
The NB4 cells are APL cells expressing the PML/RARα fusion gene that is pathognomonic of this disease.19 Treatment of APL is with ATRA. Exposure of NB4 cells to either Ro 25-9716 or 1,25D3 induced these cells to express the myeloid differentiation marker, CD11b. Furthermore, the dose-response curves for induction of this cell surface marker by these compounds were similar to those observed using the HL-60 cells. Induction of CD11b expression reflects an early step of the differentiation of the promyelocytic leukemic NB4 cells.30 Recently, we have found that the combination of analog KH1060 and 9-cis retinoic acid synergistically induced differentiation of NB4 cells and fresh APL cells.31 Our results here showed that neither ATRA (10-7 mol/L) nor Ro 25-9716 (10-7 mol/L) alone induced more than 5% of the cells to become NBT positive. However, the combination of ATRA (10-7 mol/L) and Ro 25-9716 (10-9 mol/L) induced exuberant differentiation of NB4 cells (NBT positive, 92%, 3 days), and this combination was a more potent inducer of differentiation than was the combination of ATRA and 1,25D3. The combination of Ro 25-9716 vitamin D3 analog with ATRA is a good candidate for differentiation therapy of APL.
The Kasumi-1 cell line has an 8;21 chromosomal translocation, which is the most common recurrent chromosomal abnormality in French-American-British M2 type leukemias.21This cell line is an interesting model to investigate novel agents for this subtype of acute myeloid leukemia. Our results showed that vitamin D3 analogs (especially Ro 25-9716) may offer a therapeutic approach to the t(8;21) leukemias because Ro 25-9716 strongly suppressed clonal growth of the Kasumi-1 cells and induced expression of CD11b on their cell surfaces. Although we could detect neither morphological differentiation (data not shown) nor NBT reduction of these cells, further testing of these vitamin D3 compounds on samples from patients with t(8;21) leukemia may provide further support for their use.
The HL-60 cells were arrested in the G1 stage of the cell cycle after their culture with Ro 25-9716 (10-8 mol/L, 96 hours). The size of this G1-arrested population induced by Ro 25-9716 (G1 phase, 88%) was much greater than that produced by exposure to 1,25D3 (G1,42%). Recently, Wang et al32 suggested that the CDKI p27kip-1 is a strong candidate for the cell cycle regulator that blocks the entry into S phase in 1,25D3-treated HL-60 cells. Therefore, we speculated that an increased expression of p27kip-1 protein would be associated with the prominent G1 arrest that occurred after the cells were incubated with Ro 25-9716. Western blot analysis showed that the p27kip-1protein was expressed more rapidly and strongly after exposure of HL-60 cells to Ro 25-9716 compared with 1,25D3; this induction of expression of p27kip-1 was correlated with the strong G1 arrest induced by Ro 25-9716. A recent study has shown that overexpression of p27kip-1 could induce apoptosis in human carcinoma cell lines, melanoma cell line, lung fibroblasts, and rat fibroblast cell line.33 This study suggested that expression of p27kip-1 is associated with not only regulation of cell cycle but also apoptotic death of mammalian cells. Therefore, it is reasonable that expression of p27kip-1 was enhanced by Ro 25-9716 only in the HL-60 cell line that showed apoptotic death of the cells after treatment with Ro 25-9716. Our results support this hypothesis that the p27kip-1 protein may be one of the principle mediators of the antiproliferative activity of the vitamin D3 compounds by both blocking entry of the leukemic cells into the S phase and inducing apoptosis of these cells. However, this cannot be the complete explanation, because Ro 25-9716 inhibited the growth of NB4, RA-res HL-60, and Kasumi-1 cells but it did not cause an increased expression of p27kip-1 in these cells.
Of particular interest, the clonal growth of the RA-res HL-60 cells was inhibited by Ro 25-9716 but not by 1,25D3. This suggests that this novel analog has not only a quantitatively enhanced antileukemic activity compared with 1,25D3, but it also has a qualitative difference in activity. Perhaps the vitamin D receptors, when bound to Ro 25-9716, interact with a novel set of genes that regulate cellular proliferation. Further studies, such as the identification of transcriptional factors regulated differentially in either these or similarly resistant HL-60 cells34 will help to elucidate the mechanism leading to an expanded scope of activities of Ro 25-9716.
In summary, we have synthesized and identified a group of 19-nor 1,25D3 analogs (especially Ro 25-9716) with potent effects on proliferation and differentiation of myeloid leukemia cell lines in vitro. These interesting analogs will be studied for their ability to control myeloid leukemias in vivo.35
ACKNOWLEDGMENT
We thank Patricia Lin, from the Flow Cytometry Core Facility, for generous technical assistance and Marge Goldberg and Kim Burgin for excellent secretarial help.
Supported in part by grants from the National Institutes of Health, the US Army, and the Concern Foundation, and by the Parker Hughes Trust.
H.P.K. is a member of the Jonsson Comprehensive Cancer Center and holds the Mark Goodson Chair in Oncology Research.
Address reprint requests to H. Phillip Koeffler, MD, PhD, Department of Medicine, Division of Hematology/Oncology, Cedars-Sinai Medical Center/UCLA School of Medicine, 8700 Beverly Blvd, B208, Los Angeles, CA 90048.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 8. Expression of p27kip-1 in HL-60 cells as measured by Western blot. Cells were either untreated (lane 1) or treated with either Ro 25-9716 (10−7 mol/L, [A]) or 1,25D3 (10−7 mol/L, [B]) for 1 day (lane 2), 2 days (lane 3), 3 days (lane 4), 4 days (lane 5), or 5 days (lane 6). Lysates from the cells were analyzed for expression of p27kip-1 or MPO using Western blot analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2441/3/m_blod41932008aw.jpeg?Expires=1763478769&Signature=zcB6XIXxokp~hobPJIsOA9NwoTay05ewBJ2x296Cd~K3pmdt5oZHksL~iXDw9DbsGvxfFJzmuk~uERzDTMv0CVXtw9CrLj6mXi-jhbPImtZHMHCCo-eNL3fG8d~BsYVdVZhazzQTA90SOWOHbxOVImPplyvxGX940m55GiaY5nRPNvH4lUyodYB9s0mFAbb2NdDd8RKwnuQFPldBEljmaf0x0NatU5b9~azYx1Qe8NfUWfhSMsLBWZcXTCKC0CV1yFm0KxLQXU1Q~qlkxGIYPMhk8SyWx2yCZaIC1x0yQJhxveC1cQO79eqkSTQVhSwukInw7hY0ZTu3MdAByzh~hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Expression of p27kip-1 in HL-60 cells as measured by Western blot. Cells were either untreated (lane 1) or treated with either Ro 25-9716 (10−7 mol/L, [A]) or 1,25D3 (10−7 mol/L, [B]) for 1 day (lane 2), 2 days (lane 3), 3 days (lane 4), 4 days (lane 5), or 5 days (lane 6). Lysates from the cells were analyzed for expression of p27kip-1 or MPO using Western blot analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2441/3/m_blod41932008bw.jpeg?Expires=1763478769&Signature=WXON~BPizXJQjfcOw0fwZ8poJZrzEJ~sTbF4joFiEX7bRzABaSG2yzbLQY3IdaCdDXPlrWpjIU85r97StvhlUvR7B6w1-Vyws4rpux139pdNY8m7dWlpfNLVgG-N1R-1LIunksPfnYftj~~2OnS~GAVE6xzkNhbkUg6x8vWsKq-Ey2XklAyHZSOMC6ExpHYbwQe6YKnvBN2F-q8Voq1YOm1jLD51ESHCPdslCXILG6bdV0Kf9mdNx4if7GCoWMydcUAqnMrz8~HDDlAux3gF0dyZhDMtZeuLm4pltecY11bx2oFtzXesMKOfXXNmyjgA~D-IJ3Nrf4cBMQSpGw2BCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal