Abstract
CD40 is a member of the tumor necrosis factor receptor family and plays an important role in B-cell survival, growth, differentiation, and isotype switching. Recently, CD40 has been shown to associate with JAK3, a member of the family of Janus Kinases, which are nonreceptor protein kinases involved in intracellular signaling mediated by cytokines and growth factors. To investigate the role of JAK3 in CD40-mediated signaling, we studied the effect of CD40 stimulation on B-cell proliferation, IgE isotype switching, and upregulation of surface expression of CD23, ICAM-1, CD80, and LT-α in JAK3-deficient patients. Our studies show that stimulation of B cells with monoclonal antibody to CD40 in the presence of interleukin-4 (IL-4) or IL-13 resulted in similar responses in JAK3-deficient patients and normal controls. This suggests that JAK3 is not essential for CD40-mediated B-cell proliferation, isotype switching, and upregulation of CD23, ICAM-1, CD80, and LT-α surface expression.
CD40 IS A B-CELL receptor and a member of the TNF-R superfamily of surface molecules.1 Signaling through CD40 promotes B-cell growth, survival, and differentiation; rescues B cells from apoptosis induced by Fas (CD95) or by surface IgM crosslinking; induces homotypic B cell adhesion; upregulates B-cell expression of B7 (CD80), ICAM-1, CD23, and LT-α1-3; and most importantly induces Ig class switching.4 Interaction between CD40 and its ligand (CD40L), which is expressed on activated CD4+ T lymphocytes, plays an important role in immune functions. This is illustrated by the observations that humans with X-linked hyper-IgM syndrome (HIGMX-1) who have a defect in CD40L, are unable to undergo isotype switching in vitro and in vivo.5Furthermore, mice with targeted disruption of CD40L6 or CD407 8 fail to undergo isotype switching and to form germinal centers in response to T-cell–dependent antigens.
Four intracellular proteins that belong to the family of tumor necrosis factor (TNF) receptor–associated factors (TRAF), TRAF-2, TRAF-3, TRAF-5, and TRAF-6, have been found to associate with the intracytoplasmic (IC) domain of CD40.9-12 TRAF-2, TRAF-3, and TRAF-5 share a binding site that is distinct from the TRAF-6 binding site. TRAF-2, TRAF-5, and TRAF-6, but not TRAF-3, mediate activation of NF-κB.10,12,13 Thus far the function of TRAF-3 is not known. Engagement of CD40 activates protein tyrosine kinases (PTKs) and serine/threonine kinases,14 and PTKs play an important role in CD40-mediated aggregation, LT-α expression, and Ig class switching.15,16 Recently, we have reported that the tyrosine kinase JAK3 is constitutively associated with the intracellular domain of CD40. This interaction requires a proline-rich sequence in the membrane proximal region of CD40. Moreover, engagement of CD40 induces tyrosine phosphorylation and activation of JAK3.17
The roles of TRAF proteins and of JAK3 in CD40-mediated B-cell activation remain to be established. A dominant negative TRAF-3 mutant, which has the potential to displace the binding of TRAF-2, TRAF-3, and TRAF-5 to CD40, was shown to inhibit CD40-mediated expression of CD23 but not of ICAM-1.9 Although mice deficient for TRAF-3 and JAK3 are available,18-21 B cells do not develop in these mice, precluding assessment of the role of TRAF-3 and JAK3 in CD40-mediated functions.
Mutations in JAK3 have been found in a number of cases of autosomal severe combined immune deficiency (SCID) with a phenotype similar to that of X-SCID.22-24 This phenotype consists of absent to very low numbers of circulating T cells and relatively normal to high numbers of circulating B cells.22 The similarity between the two phenotypes is thought to reflect the fact that JAK3 is an immediate downstream effector of the interleukin-2 receptor γ (IL-2Rγ) common chain (γc), which is deficient in X-SCID and is physically associated with γc.25 Bone marrow transplantation (BMT) in patients with JAK3 deficiency results in donor-derived reconstitution of T cells. However, B cells remain of recipient origin. We have taken advantage of this observation and of the availability of B cells from a JAK3-deficient patient before BMT to examine the role of JAK3 in CD40-mediated B-cell activation. The results obtained show that JAK3 is not essential for CD40-mediated induction of B-cell proliferation, isotype switching, and upregulation of CD23, ICAM-1, CD80, and LT-α cell-surface expression.
MATERIALS AND METHODS
Patients.
Five patients in whom JAK3 deficiency was diagnosed were studied. Studies were performed on peripheral blood mononuclear cell (PBMC) either freshly prepared or obtained from frozen aliquots with viability exceeding 80% as assessed by trypan blue. The characteristics of these patients are shown in Table 1. Patient no. 1 was studied pre-BMT; the remaining four patients were studied post-BMT. PBMC from normal donors were used as controls.
Characteristics of JAK3-Deficient Patients
| Patient . | Gender . | Time of Study . | BMT Donor . | B Cell Origin . |
|---|---|---|---|---|
| 1 | Female | Pre-BMT | — | Patient |
| 2 | Male | Post-BMT | Mother | Recipient: 100% of B cells are XY |
| 3 | Female | Post-BMT | Mother | Uncertain |
| 4 | Male | Post-BMT | Mother | Recipient: 100% of B cells are XY |
| 5 | Female | Post-BMT | Father | Recipient: 100% of B cells are XX, also ascertained by genotypic analysis at the microsatellite markers, D1S80 & DQa |
| Patient . | Gender . | Time of Study . | BMT Donor . | B Cell Origin . |
|---|---|---|---|---|
| 1 | Female | Pre-BMT | — | Patient |
| 2 | Male | Post-BMT | Mother | Recipient: 100% of B cells are XY |
| 3 | Female | Post-BMT | Mother | Uncertain |
| 4 | Male | Post-BMT | Mother | Recipient: 100% of B cells are XY |
| 5 | Female | Post-BMT | Father | Recipient: 100% of B cells are XX, also ascertained by genotypic analysis at the microsatellite markers, D1S80 & DQa |
Cell preparations and cultures.
PBMC were isolated from heparinized blood obtained from JAK3-deficient patients or normal nonatopic donors by density gradient centrifugation on Ficoll Hypaque (Pharmacia, Piscataway, NJ). Cells were then washed and resuspended in RPMI 1640 containing 10% fetal calf serum (FCS; Hyclone Sterile Systems, Inc, Logan, UT), 2 mmol/L L-glutamine, 50 μg/mL streptomycin, and 100 U/mL penicillin (complete medium). In some experiments T cells were depleted by using Dynabeads conjugated to CD3 (Dynal, Inc, Lake Success, NY ) according to the manufacturer's instructions and the resulting B-cell–enriched populations that contained less than 1% T cells were studied.
For proliferation studies, cells were cultured in duplicate at 1 × 106 cells/mL in the presence or absence of IL-4 (100 U/mL; DNAX Research Institute, Palo Alto, CA), IL-13 (100 ng/mL; R&D Systems, Minneapolis, MN), anti-CD40 monoclonal antibody (MoAb) 626.1 (5 μg/mL; a kind gift of Dr S.M. Fu, University of Virginia, Charlottesville), and IL-4 or IL-13 plus anti-CD40 MoAb. After 96 hours, incorporation of [3H] thymidine pulsed for the last 18 hours of culture was assessed. For IgE production, cells were cultured at 1.5 × 106 cells/mL in complete medium in the presence or absence of IL-4 or IL-13 plus anti-CD40 MoAb. After 12 to 14 days supernatants were obtained and assessed for their IgE content by radioimmunoassay (RIA) with a sensitivity of 150 pg/mL.26
Nested polymerase chain reaction (PCR) amplification of Sμ/Sε switch fragments.
Nested PCR runs were performed on high-molecular-weight DNA isolated from stimulated PBMC cultures using two sets of nested primers located 5′ of Sμ and 3′ of Sε as described.27
Fluorescence-activated cell sorting (FACS) analysis.
PBMC from patients or controls were cultured for 24 hours with medium or with soluble mouse-CD8:CD40L obtained as previously described7 plus rat anti-mouse CD8 MoAb (PharMingen, San Diego, CA). The cells were then procured, washed in phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) and 0.01% sodium azide, and resuspended in 100 μL of this buffer supplemented with 10% normal mouse serum to block nonspecific binding. They were then stained for 30 minutes on ice with either anti–CD23-phycoerythrin (PE) MoAb or anti–CD80-PE MoAb plus anti–CD19-fluorescein isothiocyanate (FITC) MoAb, or with anti–ICAM-1-FITC MoAb plus anti–CD19-PE (Becton Dickinson, San Jose, CA). For LT-α expression, an IgG1 biotinylated anti–LT-α MoAb (kindly provided by Dr Jeffrey Browning, Biogen, Cambridge, MA) was added to cells preincubated with human IgG (50 μg/mL). After 40 minutes on ice, cells were washed and stained with strepavidin-PE (Becton Dickinson) plus CD19-FITC MoAb. Appropriate isotype controls were used for each staining. The cells were then washed and a minimum of 10,000 total events were analyzed using a FACScalibur flow cytometer (Becton Dickinson).
RESULTS
Characteristics of patients with JAK3 deficiency.
Table 1 shows the characteristics of the five patients with JAK3 deficiency studied. Three of the patients were female and two were male. The diagnosis of JAK3 deficiency was ascertained by demonstrating absence of JAK3 protein in patients 1 through 4 by Western blotting (data not shown). JAK3 is detected by Western blot analysis in patient no. 5 but fails to undergo phosphorylation following treatment of B cells with IL-2 (data not shown) and the patient is homozygous for a point mutation (Arg577 Trp change) in JAK3. At diagnosis, all patients had virtual absence of circulating T cells (0% to 5%) and a high percentage of circulating B cells (45% to 94%). Blood was available before BMT on only one of the five patients (patient no. 1). In three of the four patients who were studied post-BMT (patients no. 2, 4, and 5), we had evidence that B cells remained exclusively of recipient origin. In the remaining patient (patient no. 3), the origin of the B cells could not be ascertained. However, we included this patient in our analysis because B cells are almost always of recipient origin after BMT without cytoreductive therapy (as is the case in this patient).28 29
PBMCs isolated from patient no. 1 pretransplant consisted of greater than 94% B cells and less than 1% T cells. Because only limited numbers of frozen PBMC were available on the four transplanted patients, we studied the responses of unfractionated PBMCs in these patients and were able to purify and study B cells from two of them.
JAK3-deficient B cells proliferate normally in response to anti-CD40 + IL-4 and to anti-CD40 + IL-13.
Ligation of CD40 by MoAb synergizes with the cytokines IL-4 and IL-13 to induce cell proliferation of purified B cells30 as well as PBMCs (data not shown). In contrast, IL-4, IL-13, and anti-CD40 MoAb each by itself caused negligible proliferation of both B cells and PBMCs (data not shown).
The receptor for IL-4 (IL-4R) in B cells is composed of two chains, the IL-4–binding IL-4Rα chain, which is shared with the IL-13R,31 and the γc chain, which is shared with IL-2R, IL-7R, IL-9R, and IL-15R.32 The IL-4Rα chain is associated with the tyrosine kinase JAK1 whereas the γc chain is associated with JAK3.33,34 The IL-13R is a heterodimer that consists of an IL-13 binding (IL-13Rα) chain and of the IL-4Rα chain.35 Although IL-13 activates STAT6 and induces Cε germline transcripts, it does not activate JAK3,36,37 and IL-13R signaling is intact in B cells from JAK3-deficient patients.38 For this reason, IL-13 was used, in addition to IL-4, to assess the role of JAK3 in CD40-mediated proliferation. Table 2A clearly shows that PBMCs from five JAK3-deficient patients studied proliferate vigorously in response to anti-CD40 + IL-13 as well as to anti-CD40 + IL-4. The strikingly high proliferative response of PBMCs from patient no. 1 most likely reflects the high percentage of circulating B cells of this patient before BMT (94%). Table 2B shows that purified B cells from the patients also proliferate normally to anti-CD40 + IL-4 and anti-CD40 + IL-13. This together with the results on the patient studied before transplant strongly suggest that the proliferative response of B cells to anti-CD40 + IL-4/IL-13 is independent of JAK3.
Proliferation of PBMC in Response to Anti-CD40 MoAb in the Presence of IL-4 or IL-13
| A Stimulus . | cpm of [3H]Thymidine Incorporated/Culture . | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | |||||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium | 2,100 | 1,580 | 1,551 | 3,251 | 1,200 | 1,338 | 1,124 | 357 |
| Anti-CD40 + IL-4 | 11,692 | 10,162 | 37,743 | 82,844 | 9,359 | ND | 23,852 | 10,180 |
| Anti-CD40 + IL-13 | 7,955 | 6,170 | 21,335 | 92,562 | 12,639 | 32,485 | 18,219 | 7,655 |
| A Stimulus . | cpm of [3H]Thymidine Incorporated/Culture . | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | |||||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium | 2,100 | 1,580 | 1,551 | 3,251 | 1,200 | 1,338 | 1,124 | 357 |
| Anti-CD40 + IL-4 | 11,692 | 10,162 | 37,743 | 82,844 | 9,359 | ND | 23,852 | 10,180 |
| Anti-CD40 + IL-13 | 7,955 | 6,170 | 21,335 | 92,562 | 12,639 | 32,485 | 18,219 | 7,655 |
| B Stimulus . | Controls . | Patients . | ||
|---|---|---|---|---|
| 1 . | 2 . | 2 . | 5 . | |
| Medium | 3,412 | 4,627 | 2,933 | 2,546 |
| Anti-CD40 + IL-4 | 22,269 | 54,459 | 20,917 | 33,878 |
| Anti-CD40 + IL-13 | 14,809 | 39,903 | 18,965 | 33,687 |
| B Stimulus . | Controls . | Patients . | ||
|---|---|---|---|---|
| 1 . | 2 . | 2 . | 5 . | |
| Medium | 3,412 | 4,627 | 2,933 | 2,546 |
| Anti-CD40 + IL-4 | 22,269 | 54,459 | 20,917 | 33,878 |
| Anti-CD40 + IL-13 | 14,809 | 39,903 | 18,965 | 33,687 |
PBMCs (panel A) or T-cell–depleted PBMCs (panel B) (1 × 106 cells/mL) were cultured for 96 hours with medium or with anti-CD40 MoAb (5 μg/mL) plus either IL-4 (100 U/mL) or IL-13 (100 ng/mL). [3H]thymidine incorporation was measured in the last 18 hours of culture. Results shown represent the mean of duplicate cultures.
Abbreviation: ND, not done.
JAK3-deficient B cells undergo normal isotype switching to IgE in response to anti-CD40 + IL-4 and to anti-CD40 + IL-13.
Induction of IgE synthesis by human B cells requires two signals.39 The first signal is delivered by the cytokines IL-4 or IL-13, and results in Cε germ line transcription. The second signal is delivered via ligation of the B-cell antigen CD40 by its ligand, CD40L, which is expressed on T cells upon activation and triggers deletional switch recombination. Table 3 shows that B cells from five JAK3-deficient patients studied synthesized IgE vigorously in response to stimulation with anti-CD40 + IL-13 and/or anti-CD40 + IL-4. The relatively modest responses observed in patient no. 2 (2 ng/mL for anti-CD40 + IL-13, 1 ng/mL with anti-CD40 + IL-4 vundetectable <150 pg/mL in unstimulated cells) are occasionally observed in normal subjects. Patient no. 5 displayed spontaneous IgE synthesis. This is consistent with the presence of elevated serum IgE in this patient (6,935 U/mL; normal, <120 U/mL).
IgE Synthesis by PBMCs Stimulated With Anti-CD40 MoAb in the Presence of IL-4 or IL-13
| Stimulus . | Net IgE Synthesis (ng/mL) . | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | |||||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | 31 |
| Anti-CD40 + IL-4 | 9.6 | 53 | 50 | 109 | 1 | ND | 137 | 52 |
| Anti-CD40 + IL-13 | 4.6 | 42 | 18 | ND | 2 | 22 | ND | 53 |
| Stimulus . | Net IgE Synthesis (ng/mL) . | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | |||||||
| 1 . | 2 . | 3 . | 1 . | 2 . | 3 . | 4 . | 5 . | |
| Medium | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | 31 |
| Anti-CD40 + IL-4 | 9.6 | 53 | 50 | 109 | 1 | ND | 137 | 52 |
| Anti-CD40 + IL-13 | 4.6 | 42 | 18 | ND | 2 | 22 | ND | 53 |
PBMCs (1.5 × 106 cells/mL) were cultured with medium or with anti-CD40 MoAb (5 μg/mL) plus either IL-4 (100 U/mL) or IL-13 (100 ng/mL). After 14 days the culture supernatants were procured and assessed for IgE by RIA and their net IgE content was calculated by subtracting the amount of IgE detected in the supernatants of cycloheximide-treated cultures. Results shown represent the mean of duplicate cultures.
Abbreviation: ND, not done.
In addition to the pretransplant patient whose PBMCs consisted of 94% B cells, we were able to obtain enough purified B cells from one of the other four patients. Purified B cells from this patient synthesized IgE in response to anti-CD40 + IL-4 or IL-13 (1.5 ng/mL for unstimulated, 8.7 ng/mL for anti-CD40 + IL-4, and 6.2 ng/mL for anti-CD40 + IL-13). These results strongly suggest that CD40-mediated isotype switching to IgE is independent of JAK3.
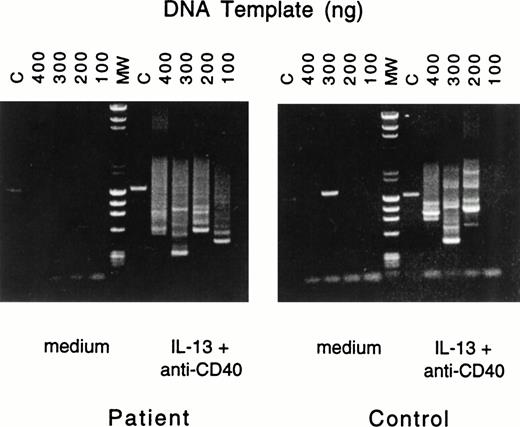
To ascertain that IgE synthesis in B cells from patients with JAK3 deficiency involved deletional switch recombination, we used a previously described nested PCR assay27 to show the presence of Sμ-Sε fragments. Because IL-4 signals both via the IL-4R, which activates JAK3, and via the IL-13R, while IL-13 signals via a single receptor (IL-13R), which is independent of JAK3, we examined the presence of Sμ-Sε fragments in B cells stimulated with anti-CD40 + IL-13. Figure 1 shows that multiple switch fragments were amplified from genomic DNA prepared from PBMC of normals as well as JAK3-deficient patients after stimulation with anti-CD40 + IL-13. No switch fragments were amplified from PBMC cultured in the absence of anti-CD40 + IL-13.
Nested PCR amplification of switch fragments. Serially diluted aliquots of DNA from PBMCs of patient no. 2 (left) or control (right), cultured with medium or with IL-13 plus anti-CD40 MoAb, were amplified by nested primer PCR. The quantities of serially diluted template DNA used in the first round of PCR are noted above the gel; in the second round of PCR 10% of the original PCR reaction mixture was used as DNA template. Final PCR products were analyzed by agarose gel electrophoresis. In the control lane (C), primers specific for the human IL-1β promoter yielded the expected 1.4-kb fragment. Lane MW represents the molecular-weight markers.
Nested PCR amplification of switch fragments. Serially diluted aliquots of DNA from PBMCs of patient no. 2 (left) or control (right), cultured with medium or with IL-13 plus anti-CD40 MoAb, were amplified by nested primer PCR. The quantities of serially diluted template DNA used in the first round of PCR are noted above the gel; in the second round of PCR 10% of the original PCR reaction mixture was used as DNA template. Final PCR products were analyzed by agarose gel electrophoresis. In the control lane (C), primers specific for the human IL-1β promoter yielded the expected 1.4-kb fragment. Lane MW represents the molecular-weight markers.
The observation that CD40-mediated in vitro isotype switching to IgE was normal in B cells from JAK3-deficient patients prompted us to examine serum Ig levels on the three BMT patients who were not receiving gamma globulin replacement therapy. Table 4 shows that serum IgG was present in all three patients and was within or close to the normal range in two of them. Serum IgA was also detected in all three patients, although it was low in two of them. Serum IgE was detectable in all three patients and was elevated in one of them. CD40L:CD40 interactions are essential for isotype switching in humans as evidenced by the virtual absence of IgG, IgA, and IgE in patients with CD40 ligand deficiency. Because all B cells from two out of three of the JAK3-deficient patients studied (patients 2 and 5) were clearly of recipient origin, these data suggest that JAK3-deficient B cells can undergo CD40 mediated isotype switching in vivo.
Serum Ig Levels in JAK3-Deficient Patients Post-BMT
| Patient . | Age at Evaluation Yr . | IgGIgAIgM . | IgE . | ||
|---|---|---|---|---|---|
| mg/dL . | IU/mL . | ||||
| 2 | 8.5 | 389 | 17 | 127 | 39 |
| (600-1,500) | (50-200) | (50-200) | (0-200) | ||
| 3 | 1.4 | 569 | 12 | 105 | 1 |
| (400-1,300) | (20-230) | (30-120) | (0-30) | ||
| 5 | 23.5 | 586 | 168 | 145 | 5,985 |
| (600-1,500) | (50-200) | (50-200) | (0-200) | ||
| Patient . | Age at Evaluation Yr . | IgGIgAIgM . | IgE . | ||
|---|---|---|---|---|---|
| mg/dL . | IU/mL . | ||||
| 2 | 8.5 | 389 | 17 | 127 | 39 |
| (600-1,500) | (50-200) | (50-200) | (0-200) | ||
| 3 | 1.4 | 569 | 12 | 105 | 1 |
| (400-1,300) | (20-230) | (30-120) | (0-30) | ||
| 5 | 23.5 | 586 | 168 | 145 | 5,985 |
| (600-1,500) | (50-200) | (50-200) | (0-200) | ||
Serum Ig levels were assessed in the three patients who were not receiving gamma globulin replacement. IgG, A, and M were measured by radial immunodiffusion, and IgE by fluoroenzymatic immuno assay. Normal values for each age are indicated in brackets parentheses.
JAK3-deficient B cells upregulate CD23, ICAM-1, CD80, and LT-α expression in response to CD40 ligation.
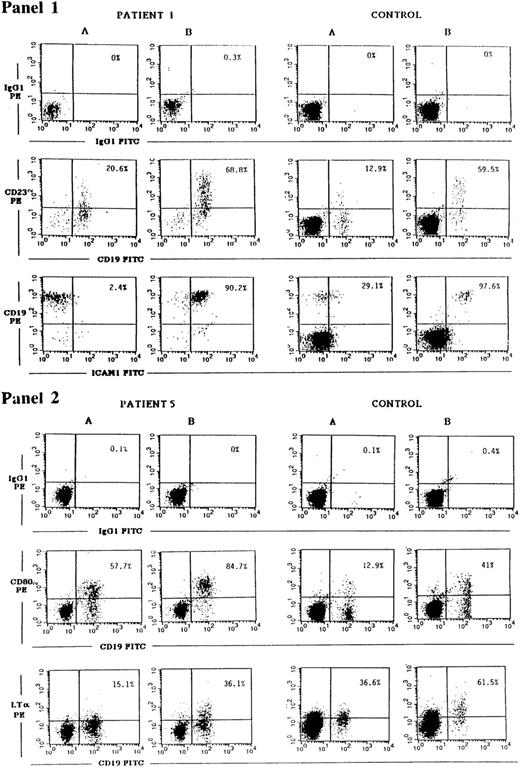
CD40 ligation has been shown to upregulate the expression of a number of B-cell surface molecules including CD23, ICAM-1, CD80, and LT-α.1-3 The transcription factor NF-κB, which is induced by CD40, plays a role in the induction of all the above genes. Both ICAM-1 and LT-α have in their promoters STAT binding consensus sequences,40,41 and STAT3 has been shown to be phosphorylated after CD40 ligation.17 For this reason we examined the induction of CD23, ICAM-1, CD80, and LT-α in JAK3-deficient B cells. CD40 on B cells from PBMCs of controls and of JAK3-deficient patients was ligated by incubation with soluble murine-CD40L:CD8 fusion protein and rat MoAb to murine CD8, to ensure optimal conditions of crosslinking. Twenty-four hours later, the cells were washed and surface expression of CD23, ICAM-1, CD80, and LT-α on CD19+ cells was assessed by FACS analysis. A representative experiment is shown in Fig 2 as dot plot, and Table 5 summarizes the results obtained on all the patients studied. They show that induction of CD23, ICAM-1, CD80, and LT-α expression on B cells after CD40 ligation was equivalent in JAK3-deficient patients and controls.
Upregulation of CD23, ICAM-1, CD80, and LT-α expression on JAK3-deficient B cells in response to CD40 ligation. PBMCs from patient no. 1 obtained pre-BMT or patient no. 5 (left panels), or from controls (right panels), were cultured for 24 hours with medium (A) or with soluble mouse CD40L:CD8 plus rat anti-mouse CD8 (B). Cells were then washed and stained with either CD19-FITC plus CD23-PE MoAbs, with CD19-PE plus ICAM-1-FITC MoAbs (panel 1), with CD19-FITC plus CD80-PE or biotinylated LT-α MoAbs followed by strepavidin-PE (panel 2), or with the appropriate isotype controls. Cells were then analyzed using a FACScalibur flow cytometer. Percentages shown in the upper right quadrant of each dot plot represent the percent of CD23+, ICAM-1+, CD80+, or LT-α+ B cells detected.
Upregulation of CD23, ICAM-1, CD80, and LT-α expression on JAK3-deficient B cells in response to CD40 ligation. PBMCs from patient no. 1 obtained pre-BMT or patient no. 5 (left panels), or from controls (right panels), were cultured for 24 hours with medium (A) or with soluble mouse CD40L:CD8 plus rat anti-mouse CD8 (B). Cells were then washed and stained with either CD19-FITC plus CD23-PE MoAbs, with CD19-PE plus ICAM-1-FITC MoAbs (panel 1), with CD19-FITC plus CD80-PE or biotinylated LT-α MoAbs followed by strepavidin-PE (panel 2), or with the appropriate isotype controls. Cells were then analyzed using a FACScalibur flow cytometer. Percentages shown in the upper right quadrant of each dot plot represent the percent of CD23+, ICAM-1+, CD80+, or LT-α+ B cells detected.
Summary of the Levels of Surface Expression of Molecules Studied on JAK3-Deficient B Cells in Response to CD40 Ligation
| % CD19+ Cells Expressing . | Patients . | Controls . | ||
|---|---|---|---|---|
| — . | CD40L:CD8 . | — . | CD40L:CD8 . | |
| CD23+ | 22 | 55 (n = 4) | 19 | 75 (n = 3) |
| ICAM-1+ | 19 | 80 (n = 4) | 15 | 87 (n = 3) |
| CD80+ | 29 | 55 (n = 2) | 20 | 54 (n = 3) |
| LT-α+ | 9 | 38 (n = 2) | 20 | 55 (n = 2) |
| % CD19+ Cells Expressing . | Patients . | Controls . | ||
|---|---|---|---|---|
| — . | CD40L:CD8 . | — . | CD40L:CD8 . | |
| CD23+ | 22 | 55 (n = 4) | 19 | 75 (n = 3) |
| ICAM-1+ | 19 | 80 (n = 4) | 15 | 87 (n = 3) |
| CD80+ | 29 | 55 (n = 2) | 20 | 54 (n = 3) |
| LT-α+ | 9 | 38 (n = 2) | 20 | 55 (n = 2) |
PBMCs from patients or normal controls were cultured for 24 hours with or without soluble mouse-CD40L:CD8 fusion protein + rat anti-mouse CD8. Cells were washed and stained as described in Materials and Methods. Results shown are the mean values of CD19+ cells expressing the different cell surface markers studied in response to CD40 ligation. n = number of donors studied.
DISCUSSION
The results of our studies on B cells from patients with JAK3 deficiency suggest that JAK3 does not play an essential role in CD40-mediated B-cell proliferation, isotype switching, or upregulation of CD23, ICAM-1, CD80, and LT-α surface expression.
PBMC as well as purified B cells from JAK3-deficient patients proliferated normally to anti-CD40 + IL-4 as well as to anti-CD40 + IL-13 (Table 2). Because JAK3 is associated both with the IC domain of the γc chain of the IL-4R as well as with the IC domain of CD40, these results suggest that JAK3 is not necessary for the delivery of mitogenic signals by these two receptors. In the case of IL-4, the lack of dependence on JAK3 for mitogenic signaling may simply reflect the capacity of IL-4 to bind to and to signal via the IL-13R which is not associated with JAK3.36-38 Alternatively, IL-4R signaling may be truly independent of JAK3. Recently, it has been shown that homodimerization of human IL-4Rα chains transfected in 32D cells (murine promyeloid cell line) or Ba/F3 cells (murine pro-B cell line) results in cellular proliferation.42,43 Homodimerization of the IL-4Rα chain by IL-4 could occur if (given its structural similarity to IL-2) IL-4, like IL-2, is able to form covalent dimers in vitro.44
None of the proteins known to associate with the IC domain of CD40, including all four CD40-associated TRAF proteins and JAK3, is unique to CD40. The singular capacity of CD40 to cause isotype switching could be caused by activation of a yet to be defined CD40-specific signaling pathway or to activation of a unique combination of already identified signaling pathways. For instance, the JAK3 signaling pathway and the TRAF signaling pathways may synergize to activate switch recombination. Our results rule out an essential role for JAK3 in CD40-mediated isotype switching. First, PBMCs from all five patients studied synthesized IgE in response to anti-CD40 + IL-4 and/or anti-CD40 + IL-13 (Table 3). Second, deletional switch recombination, with formation of Sμ/Sε switch junctions, was shown in CD40/IL-13–stimulated JAK3-deficient B cells (Fig 1). Third, serum IgG, IgA, and IgE were present in transplanted patients who were not receiving gamma globulin replacement and in whom all B cells were of recipient origin (patients 2 and 5). This argues for normal CD40-mediated in vivo isotype switching because isotype switching in vivo in humans is strictly dependent on CD40L-CD40 interactions as evidenced by the virtual absence of IgG and IgA antibody responses in patients with X-HIGM and CD40L deficiency.5
Ligation of CD40 resulted in normal upregulation of CD23, ICAM-1, CD80, and LT-α expression in JAK3-deficient B cells (Fig 2 and Table 5). These results suggest that JAK3 is not necessary for CD40-mediated upregulation of CD23, ICAM-1, CD80, and LT-α. In a previous study, we had found that ligation of chimeric CD8:CD40 molecules consisting of the extracellular domain of CD8 fused to the transmembrane and IC domains of CD40 causes upregulation of CD23, ICAM-1, and LT-α in the B-cell line BJAB, which is equivalent in degree to that obtained by ligating native CD40. Deletion of the proline-rich box in CD40IC, which is necessary for JAK3 association, abolished the capacity of the CD8:CD40 chimeras to upregulate CD23, ICAM-1, and LT-α expression.17 These results were compatible with the notion that JAK3 is necessary for CD40-mediated upregulation of surface expression of CD23, ICAM-1, and LT-α. A number of explanations may account for the discrepancy between the data obtained in JAK3-deficient B cells and in B cells transfected with mutant chimeric CD8:CD40 receptors. First, in the absence of JAK3, other JAKs expressed in B cells may bind to CD40 but could not bind to mutant CD40 with a deleted box1 region. However, we were unable to detect JAK1, JAK2, or TYK2 in CD40 immunoprecipitates from JAK3-deficient patients (data not shown). Second, deletion of the proline-rich region in CD40IC may disrupt the association of CD40 with proteins other than JAK3. These associations would be preserved in JAK3-deficient B cells. Moreover, CD40-mediated upregulation of CD23, ICAM-1, and LT-α in B-cell lines, but not in freshly isolated B cells, may be dependent on JAK3. Finally, unlike native CD40, CD8:CD40 chimeric receptors are expressed as dimers. These dimers may signal constitutively, possibly resulting in receptor desensitization. Upregulation of CD23, ICAM-1, and LT-α expression by crosslinking these dimeric receptors may require additional signals that include JAK3.
The results of the present study suggest that CD40 induction of proliferation, isotype switching, and upregulation of CD23, ICAM-1, CD80, and LT-α expression in resting B cells does not involve JAK3. The respective roles of other CD40-associated molecules in these events need to be investigated.
ACKNOWLEDGMENT
We thank Dr Hans Oettgen for reviewing the manuscript and Dr Silva Hanissian for valuable discussions.
Supported by National Institutes of Health Grants No. AI-31136 and AI-35714; Baxter, Olsten, and Alpha Therapeutics Corporations; CNRS (International Programs of Scientific Cooperation); and INSERM.
Address reprint requests to Haifa H. Jabara, Enders 8, Division of Immunology, 300 Longwood Ave, Children's Hospital, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal