Abstract
Deficiency of the naturally occurring anticoagulant proteins, such as antithrombin, protein C and protein S, and activated protein C resistance due to the factor V Leiden gene mutation is associated with inherited thrombophilia. So far, no direct comparison of the thrombotic risk associated with these genetic defects is available. In this study, we wish to compare the lifetime probability of developing thrombosis, the type of thrombotic symptoms, and the role of circumstantial triggering factors in 723 first- and second-degree relatives of 150 index patients with different thrombophilic defects. We found higher risks for thrombosis for subjects with antithrombin (risk ratio 8.1, 95% confidence interval [CI], 3.4 to 19.6), protein C (7.3, 95% CI, 2.9 to 18.4) or protein S deficiency (8.5, 95% CI, 3.5 to 20.8), and factor V Leiden (2.2, 95% CI, 1.1 to 4.7) than for individuals with normal coagulation. The risk of thrombosis for subjects with factor V Leiden was lower than that for those with all three other coagulation defects (0.3, 95% CI, 0.1 to 1.6), even when arterial and superficial vein thromboses were excluded and the analysis was restricted to deep vein thrombosis (0.3, 95% CI, 0.2 to 0.5). No association between coagulation defects and arterial thrombosis was found. The most frequent venous thrombotic manifestation was deep vein thrombosis with or without pulmonary embolism (90% in antithrombin, 88% in protein C, 100% in protein S deficiency, and 57% in factor V Leiden), but a relatively mild manifestation such as superficial vein thrombosis was common in factor V Leiden (43%). There was a predisposing factor at the time of venous thromboembolism in approximately 50% of cases for each of the four defects. In conclusion, factor V Leiden is associated with a relatively small risk of thrombosis, lower than that for antithrombin, protein C, or protein S deficiency. In addition, individuals with factor V Leiden develop less severe thrombotic manifestations, such as superficial vein thrombosis.
INHERITED THROMBOPHILIA is a genetically determined tendency to venous thromboembolism that develops in young patients (less than 45 years old) and tends to be recurrent. Among the inherited defects of the hemostatic mechanisms that cause a thrombophilic state, the most frequent is resistance to activated protein C.1 Caused by an Arg506Gln mutation on human factor V (factor V Leiden), this abnormality is present in up to 40% of the patients who develop venous thromboembolism and has a high frequency in the general population of European ancestry.2-4 Defects of the naturally occurring anticoagulant proteins antithrombin, protein C, and protein S account altogether for 5% to 10% of cases.5,6 The risk of thrombosis induced by each of these coagulation defects has been investigated by family studies2,7-10 or case-control studies11-14 that showed an increased thrombotic risk in carriers of the defect compared with noncarriers.
So far, only one family study has compared deficiencies of the naturally occurring anticoagulant proteins with respect to the lifelong probability of affected individuals developing thrombosis,15 but the most common defect, activated protein C resistance due to factor V Leiden, was not considered. Because knowledge about any difference in the thrombotic risk might have implications in management strategies, we performed a retrospective cohort family study of 723 affected and unaffected relatives of 150 index patients with inherited deficiency of antithrombin, protein C, protein S, or with factor V Leiden. Secondary goals of the study were to compare the types of thrombotic manifestations and to evaluate the role of circumstantial factors predisposing to venous thromboembolism (such as surgery, trauma and immobilization, pregnancy or puerperium and oral contraceptive intake) in triggering such events.
MATERIALS AND METHODS
Patient selection criteria.
Families were identified through index patients (probands) who between 1980 and 1995 attended two Italian Thrombosis Centers in Milan and Rome, specialized in the management of coagulation disorders. The selection criteria for inclusion of families in the study were the presence of an inherited coagulation defect predisposing to thrombosis (antithrombin, protein C, protein S deficiency, or factor V Leiden) in at least two family members and the availability of laboratory results for the diagnosis of the four defects in each family member.
Index patients were asked to bring to the two centers as many as possible first- and second-degree relatives. Their personal and family histories of thrombosis were collected with a validated structured questionnaire.16 17 None of them had any evidence of overt neoplastic or other systemic diseases. The date of occurrence and the site of any episode of venous or arterial thrombosis, the presence of other factors predisposing to venous thromboembolism in the month preceding the event (such as surgery, trauma, prolonged immobilization, pregnancy or puerperium, and oral contraceptive intake) were collected. A surgical procedure was defined as one in which general anesthesia was performed; trauma included bone fractures or the application of casts, excluding those of the upper extremity; a period of prolonged immobilization was defined as one in which bed rest was complete for at least 2 weeks.
Symptomatic patients, ie, those who reported having had thrombosis in the past, were asked to bring with them the diagnostic documentation of thrombotic events and a copy of clinical records. The term venous thromboembolism was used for deep vein thrombosis whether or not complicated by pulmonary embolism; the term deep vein thrombosis was used for venous thrombotic episodes affecting the extremities, the portal-mesenteric circulation, or the cerebral circulation. The diagnosis of superficial vein thrombosis was based on the description of typical signs and symptoms, and in some cases it was confirmed by Doppler ultrasound. Other thrombotic events had to be confirmed by objective methods: venography or compression ultrasonography for deep vein thrombosis; (ventilation)/perfusion lung scan for pulmonary embolism; electrocardiography, abnormal myocardial enzymes, and chest pain for myocardial infarction; computed tomography or magnetic resonance imaging for ischemic strokes. The diagnosis was transient ischemic attacks when focal neurological signs appeared de novo and disappeared within 24 hours, whether or not abnormalities in computed tomography or magnetic resonance imaging were detected. Five patients who reported previous episodes of deep vein thrombosis of the lower extremities, but were unable to provide objective documentation, were included in the study because they had been on anticoagulant treatment and/or because a Doppler ultrasound performed at the time of our visit showed reflux in the deep veins indicative of a postphlebitic syndrome.
Laboratory tests.
Antithrombin heparin cofactor activity was measured by an amidolytic assay (Coamate AT; Chromogenix AB, Mölndal, Sweden); when antithrombin levels were low, the defect was further characterized by measuring antigen levels (immunoelectrophoresis, polyclonal antibody; Stago, Asnieres, France), and functional activity in the absence of heparin and by performing crossed immunoelectrophoresis with or without heparin.18 Protein C activity was assayed in Milan by a clotting assay (ProClot, Instrumentation Laboratory, Milan, Italy) and in Rome by an amidolytic assay (Coamatic Protein C; Chromogenix) after activation with a snake venom. When plasma levels of protein C activity were low, antigen levels were also measured by enzyme-linked immunosorbent assay (ELISA) using polyclonal antibodies (Dako A/S, Glostrup, Denmark). The diagnosis of protein S deficiency was based on finding low plasma levels of free protein S as measured by ELISA after precipitation of the C4b-binding protein-protein S complex with polyethyleneglycol 6000 (3.5% final concentration)19or using an ELISA commercial kit based on a specific monoclonal antibody (Asserachrom Free Protein S; Stago). Protein S activity was not measured, because no currently available functional assay was thought to have sufficient specificity for protein S. The Arg506Gln mutation in the factor V gene was searched for by specific DNA amplification and digestion, as previously published.20
Statistical analysis.
The t-test was used to compare mean age values. The Cochran-Cox approximation for unequal variances was used when the variable was not normally distributed. The χ2 test was used to compare the prevalence of thrombotic manifestations. The absolute annual incidence of thrombosis was calculated for relatives with and without a thrombophilic defect by dividing the number of relatives with thrombosis by the total number of patient year in each group. The thrombosis-free survival was estimated from birth to the age of the first thrombotic event by the Kaplan-Meier method curves and the different thrombophilic defects were compared by the Wilcoxon test, which places more weight on early events, and the log-rank test, which places more weight on later events. Relative risks and 95% confidence intervals (CI) for the different defects were calculated with the PHREG procedures of the SAS package,21 which adjusts for other variables in the model. Variables included in the model were sex and family status, which is a code number that identifies all of the family members belonging to the same family.
RESULTS
Characteristics of index patients.
Index patients were 180 unrelated individuals who, after a first thrombotic episode, were referred consecutively to the Thrombosis Centers and diagnosed as having a coagulation defect. Thirty index patients were excluded because no relatives were available for study. Among the remaining 150 index patients, venous thromboembolism was the most frequent symptom (77% of cases), followed by superficial vein thrombosis (16%), and arterial thrombosis (7%). Twenty-five patients (17%) had antithrombin deficiency (19 type I and 5 type II, 2 of them with a heparin-binding site defect), 23 (15%) had protein C deficiency (22 type I and 1 type II), 15 (10%) had protein S deficiency, and 81 (54%) had factor V Leiden. Six (4%) had double defects: 2 factor V Leiden and antithrombin deficiency (type I), 2 factor V Leiden and protein S, 1 factor V Leiden and protein C deficiency (type I), and 1 protein C (type I) and protein S deficiency. Six index patients with factor V Leiden and 2 with protein C deficiency were homozygotes.
Characteristics of relatives.
The entire cohort of first- and second-degree relatives of the 150 index patients consisted of 1,213 individuals, some dead, some alive at the time of the study. From 723 relatives, blood was obtained for coagulation testing, and they represent the actual study group. The average number of relatives per family was 8.1 (range, 1 to 12) considering the entire cohort and 4.8 (range, 1 to 20) considering the study group. For 569 of the 723 relatives, medical histories were obtained directly during a personal interview, using the same questionnaire administered to the index patients, while for the remaining 154 relatives, it was obtained by interviewing the index patients. Laboratory tests became available after the medical history was obtained and the laboratory staff was unaware of the clinical data. The response rate of living relatives was 72%. The coagulation status for 490 of the entire cohort of 1,213 relatives could not be investigated because they had died (n = 203) or refused to attend the Thrombosis Centers (n = 287). However, for 411 of them, both demographic data (sex and age) and information about the medical history could be obtained from the index patients. Among dead relatives, 31% died of unknown causes at a mean age of 66 years, 45% died of cancer at a mean age of 63 years, and 24% died of cardiovascular accident at a mean age of 53 years. In the noninvestigated relatives, the prevalence of thrombosis (20%) and sex and age distribution were similar to those of the relatives who could be investigated (16%, see below). For the remaining 79 relatives not investigated, only demographic information could be obtained.
Table 1 shows the main characteristics of the study group, which did not differ for relatives recruited in Milan or Rome. The vast majority of relatives had the same defect as the index patient of the family to whom they belonged. There were three relatives with protein S deficiency who had an index patient with factor V Leiden and one with factor V Leiden who had an index patient with antithrombin deficiency. A total of 327 relatives had no coagulation defects, 396 relatives carried at least one defect. Of these, 85 had antithrombin deficiency (62 type I and 23 type II, 10 of them with heparin-binding site defect), 64 protein C deficiency (62 type I and 2 type II), 41 protein S deficiency, 200 had factor V Leiden and six had double defects (2 protein C and protein S deficiencies, 2 protein C and antithrombin deficiencies, 1 factor V Leiden and antithrombin deficiency, 1 factor V Leiden and protein C deficiency). Homozygosity for factor V Leiden was detected in 6 of 200 relatives with this defect.
Characteristics of the Study Group of First- and Second-Degree Relatives Screened for Coagulation Defects
| . | Total . | Coagulation Defect . | |||||
|---|---|---|---|---|---|---|---|
| No Defect . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V Leiden . | Double Defects . | ||
| Relatives, no. | 723 | 327 (45%) | 85 (12%) | 64 (9%) | 41 (6%) | 200 (28%) | 6 (1%) |
| Men/women | 332/391 | 152/175 | 45/40 | 35/29 | 18/23 | 77/123 | 5/1 |
| Age (yr), mean ± SD | 40 ± 19 | 38 ± 18 | 40 ± 20 | 40 ± 21 | 44 ± 20 | 42 ± 20 | 45 ± 23 |
| Range | 1-94 | 1-90 | 2-84 | 1-91 | 6-79 | 6-94 | 6-68 |
| . | Total . | Coagulation Defect . | |||||
|---|---|---|---|---|---|---|---|
| No Defect . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V Leiden . | Double Defects . | ||
| Relatives, no. | 723 | 327 (45%) | 85 (12%) | 64 (9%) | 41 (6%) | 200 (28%) | 6 (1%) |
| Men/women | 332/391 | 152/175 | 45/40 | 35/29 | 18/23 | 77/123 | 5/1 |
| Age (yr), mean ± SD | 40 ± 19 | 38 ± 18 | 40 ± 20 | 40 ± 21 | 44 ± 20 | 42 ± 20 | 45 ± 23 |
| Range | 1-94 | 1-90 | 2-84 | 1-91 | 6-79 | 6-94 | 6-68 |
Type and age at development of thrombotic events.
A total of 116 (16%) of the 723 relatives had had at least one thrombotic episode (35% in antithrombin deficiency, 30% in protein C, 37% in protein S, 18% in factor V Leiden, 5% in those with no defect, and 33% in those with double defect) (Table 2). Table 2 also shows the types of the first thrombotic event in the relatives with different coagulation defects. Venous thromboembolism was the most frequent thrombotic manifestation. The sites of venous thrombosis were the lower extremities in all except 3 cases (1 with antithrombin deficiency had portal-mesenteric vein thrombosis, 1 with protein S deficiency had cerebral vein thrombosis, and 1 with factor V Leiden had upper extremity deep vein thrombosis). Deep vein thrombosis was accompanied by pulmonary embolism in 11% of cases (3 with antithrombin, 1 with protein C, 1 with protein S deficiency, 2 with factor V Leiden, and 1 with no defect). Superficial vein thrombosis accounted for 10% of the thrombotic episodes in antithrombin deficiency, for 13% in protein C deficiency, and was not seen in protein S deficiency. The prevalence of venous thromboembolism in factor V Leiden (57%) was lower than in antithrombin (90%, P = .007), protein C (88%, P = .05) or protein S deficiency (100%, P = .008), while that of superficial vein thrombosis was higher (43% compared with 10%,P = .03; 13%, P = .05 and 0%, P = .02). Arterial thrombosis accounted for 3% to 7% of thrombotic episodes in factor V Leiden, protein C or protein S deficiency did not occur in antithrombin deficiency and was present in 2% of relatives with no defects (P = .9, .8, and .8, respectively). Two protein C–deficient and 1 protein S–deficient relatives, and 2 normal relatives had acute myocardial infarction; 1 relative with protein C, 2 with protein S deficiency, and 2 normal relatives had stroke; the 5 activated protein C-resistant relatives with arterial thrombosis had transient ischemic attacks.
Type of the First Thrombotic Event and Age at the Time of Development for the 116 Symptomatic Relatives
| . | Total . | Coagulation Defect . | |||||
|---|---|---|---|---|---|---|---|
| No Defect . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V Leiden . | Double Defects . | ||
| Symptomatic relatives/total relatives (%) | 116/723 (16%) | 15/327 (5%) | 30/85 (35%) | 19/64 (30%) | 15/41 (37%) | 35/200 (18%) | 2/6 (33%) |
| Symptomatic men/women | 45/71 | 5/10 | 16/14 | 5/14 | 10/5 | 7/28 | 2/0 |
| Relatives with venous thrombosis/total relatives (%) | 99/723 (14%) | 9/327 (3%) | 30/85 (35%) | 16/64 (25%) | 12/41 (29%) | 30/200 (15%) | 2/6 (33%) |
| Venous thromboembolism | 74/99 (75%) | 3/9 (33%) | 27/30 (90%) | 14/16 (88%) | 12/12 (100%) | 17/30 (57%) | 1/2 (50%) |
| Superficial vein thrombosis | 25/99 (25%) | 6/9 (67%) | 3/30 (10%) | 2/16 (13%) | 0/12 | 13/30 (43%) | 1/2 (50%) |
| Relatives with arterial thrombosis/total relatives (%) | 17/723 (2%) | 6/327 (2%) | 0/85 | 3/64 (5%) | 3/41 (7%) | 5/200 (3%) | 0/6 |
| Age at thrombosis (yr), mean ± SD Range | 34 ± 14 10-80 | 40 ± 14* 21-62 | 35 ± 15 10-77 | 40 ± 9* 20-58 | 40 ± 15 20-67 | 37 ± 15 15-80 | 17 ± 2 15-19 |
| . | Total . | Coagulation Defect . | |||||
|---|---|---|---|---|---|---|---|
| No Defect . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V Leiden . | Double Defects . | ||
| Symptomatic relatives/total relatives (%) | 116/723 (16%) | 15/327 (5%) | 30/85 (35%) | 19/64 (30%) | 15/41 (37%) | 35/200 (18%) | 2/6 (33%) |
| Symptomatic men/women | 45/71 | 5/10 | 16/14 | 5/14 | 10/5 | 7/28 | 2/0 |
| Relatives with venous thrombosis/total relatives (%) | 99/723 (14%) | 9/327 (3%) | 30/85 (35%) | 16/64 (25%) | 12/41 (29%) | 30/200 (15%) | 2/6 (33%) |
| Venous thromboembolism | 74/99 (75%) | 3/9 (33%) | 27/30 (90%) | 14/16 (88%) | 12/12 (100%) | 17/30 (57%) | 1/2 (50%) |
| Superficial vein thrombosis | 25/99 (25%) | 6/9 (67%) | 3/30 (10%) | 2/16 (13%) | 0/12 | 13/30 (43%) | 1/2 (50%) |
| Relatives with arterial thrombosis/total relatives (%) | 17/723 (2%) | 6/327 (2%) | 0/85 | 3/64 (5%) | 3/41 (7%) | 5/200 (3%) | 0/6 |
| Age at thrombosis (yr), mean ± SD Range | 34 ± 14 10-80 | 40 ± 14* 21-62 | 35 ± 15 10-77 | 40 ± 9* 20-58 | 40 ± 15 20-67 | 37 ± 15 15-80 | 17 ± 2 15-19 |
*P = .04 compared with double defects.
The mean age at the occurrence of arterial thrombosis (52 ± 13 years; range, 23 to 80) was higher than that at the occurrence of venous thrombosis (36 ± 14 years; range, 10 to 70, P = .0001). Table 2 also shows that the age at the time of the first thrombotic episode did not differ for relatives with and without defects, varying from 35 to 40 years. The only exception was represented by the patients with double defects, who were younger than those with protein C deficiency and normal relatives (P = .04). Of the two symptomatic relatives with combined defects, one with protein C plus antithrombin deficiency, had deep vein thrombosis and one with factor V Leiden plus protein C deficiency had superficial vein thrombosis.
Information about predisposing factors at the time of the first episode of thrombosis were obtained from all of the 97 relatives with venous thromboembolism. Thrombosis occurred spontaneously in 45 (46%), while predisposing factors were present in 52 (54%) of them. Forty-four of the 88 relatives with a coagulation defect (50%) had thrombosis in the presence of predisposing factors, whose distribution was not different in the four thrombophilic defects (not shown).
Lifetime risk of thrombosis.
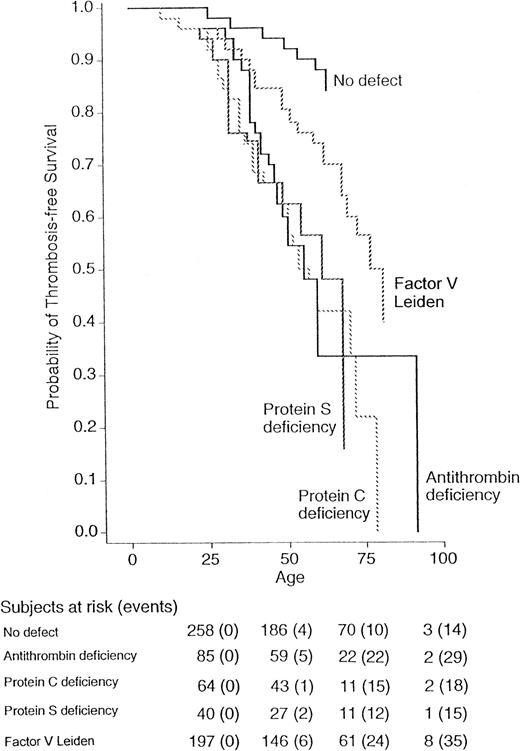
Figure 1 shows the probability for the first thrombotic episode in the four groups of relatives with coagulation defects and in normal relatives (relatives with double defects are not shown). A minority of relatives developed thrombosis during childhood, whereas the risk increased after the age of 20 years. Both Wilcoxon and log-rank tests show a significant difference in the thrombosis-free survival for all relatives (P = .001), even when subdivided into men (P = .008) and women (P = .004). The relative risk of thrombosis for relatives with factor V Leiden compared with that for relatives carrying all three other coagulation defects was 0.3 (95% CI, 0.1 to 1.6), and compared with that for normal relatives was 2.2 (95% CI, 1.1 to 4.7). The risk ratio in comparison to normal relatives was 8.1 (95% CI, 3.4 to 19.6) for antithrombin, 7.3 (95% CI, 2.9 to 18.4) for protein C, and 8.5 (95% CI, 3.5 to 20.8) for protein S deficiency. Because the thrombophilic defects evaluated in this study are established risk factors for venous thromboembolism, but are less well-established for arterial thrombosis and superficial vein thrombosis, the analysis was also done after excluding these thrombotic manifestations. The corresponding conditional risks are summarized in Table3. The results obtained after the exclusion of arterial thromboses did not change appreciably. When superficial vein thromboses were also excluded from the analysis, the risk of venous thromboembolism for factor V Leiden was 10.1 (95% CI 2.3 to 43.7) compared with normal relatives and 0.3 (95% CI 0.2 to 0.5) compared with relatives with all three other coagulation defects. Because the probability to develop thrombosis is higher in homozygous individuals than in heterozygous6,22 and is lower for heparin-binding defects of antithrombin,23 the analysis was repeated after exclusion of these relatives, and the results obtained were similar to those obtained for the whole series (data not shown). To assess the role of familial transmission of factors predisposing to thrombosis, we calculated the relative risk of thrombosis for relatives according to their belonging to a thrombophilic family. The risks of thrombosis was essentially the same for individuals belonging to an antithrombin-deficient family (risk ratio 1.2, 95% CI, 0.8 to 1.7), a protein C–deficient family (0.9, 95% CI, 0.6 to 1.5), or a protein S–deficient family (1.2, 95% CI, 0.7 to 1.9) as for those belonging to a factor V Leiden family. Table 3 also shows the absolute annual incidence of thrombosis in relatives with and without thrombophilic defects. The highest incidence was observed in the group with antithrombin deficiency (1.0%/yr) and the lowest incidence in the group of normal relatives (0.2%/yr).
Survival was measured from birth up to each age. The probabilities of relatives with normal laboratory tests or with antithrombin, protein C, protein S deficiencies, or factor V Leiden were calculated by Kaplan-Meier analysis. The total number of events/number of subjects at risk was 14 of 258 for relatives without defect, 29 of 85 for antithrombin, 18 of 64 for protein C, 15 of 40 for protein S deficiency, and 35 of 197 for factor V Leiden.
Survival was measured from birth up to each age. The probabilities of relatives with normal laboratory tests or with antithrombin, protein C, protein S deficiencies, or factor V Leiden were calculated by Kaplan-Meier analysis. The total number of events/number of subjects at risk was 14 of 258 for relatives without defect, 29 of 85 for antithrombin, 18 of 64 for protein C, 15 of 40 for protein S deficiency, and 35 of 197 for factor V Leiden.
Relative Risk and Annual Incidence for all Thromboses (venous and arterial), Venous Thrombosis (deep and superficial veins), and Venous Thromboembolism Only (deep vein thrombosis with or without pulmonary embolism) for Each Thrombophilic Defect
| . | Yes . | No . | Conditional Risk Ratio (95% CI) . | Incidence of Thrombosis (per 100 patient-yr) . |
|---|---|---|---|---|
| All thromboses | ||||
| No defect | 15 | 312 | 1 (Ref.) | 0.15 |
| Antithrombin deficiency | 30 | 55 | 8.1 (3.4-19.6) | 1.0 |
| Protein C deficiency | 19 | 45 | 7.3 (2.9-18.4) | 0.85 |
| Protein S deficiency | 15 | 26 | 8.5 (3.5-20.8) | 1.0 |
| Factor V Leiden | 35 | 165 | 2.2 (1.1-4.7) | 0.29 |
| Venous thrombosis | ||||
| No defect | 9 | 312 | 1 (Ref.) | 0.09 |
| Antithrombin deficiency | 30 | 55 | 8.1 (3.4-19.6) | 1.0 |
| Protein C deficiency | 16 | 45 | 7.4 (2.7-20.5) | 0.72 |
| Protein S deficiency | 12 | 26 | 10.4 (3.8-28.7) | 0.78 |
| Factor V Leiden | 30 | 165 | 4.6 (1.5-13.7) | 0.25 |
| Venous thromboembolism | ||||
| No defect | 3 | 312 | 1 (Ref.) | 0.03 |
| Antithrombin deficiency | 27 | 55 | 42.8 (10.2-180.3) | 0.93 |
| Protein C deficiency | 14 | 45 | 31.3 (7.0-138.8) | 0.63 |
| Protein S deficiency | 12 | 26 | 35.7 (7.9-160.1) | 0.78 |
| Factor V Leiden | 17 | 165 | 10.1 (2.3-43.7) | 0.14 |
| . | Yes . | No . | Conditional Risk Ratio (95% CI) . | Incidence of Thrombosis (per 100 patient-yr) . |
|---|---|---|---|---|
| All thromboses | ||||
| No defect | 15 | 312 | 1 (Ref.) | 0.15 |
| Antithrombin deficiency | 30 | 55 | 8.1 (3.4-19.6) | 1.0 |
| Protein C deficiency | 19 | 45 | 7.3 (2.9-18.4) | 0.85 |
| Protein S deficiency | 15 | 26 | 8.5 (3.5-20.8) | 1.0 |
| Factor V Leiden | 35 | 165 | 2.2 (1.1-4.7) | 0.29 |
| Venous thrombosis | ||||
| No defect | 9 | 312 | 1 (Ref.) | 0.09 |
| Antithrombin deficiency | 30 | 55 | 8.1 (3.4-19.6) | 1.0 |
| Protein C deficiency | 16 | 45 | 7.4 (2.7-20.5) | 0.72 |
| Protein S deficiency | 12 | 26 | 10.4 (3.8-28.7) | 0.78 |
| Factor V Leiden | 30 | 165 | 4.6 (1.5-13.7) | 0.25 |
| Venous thromboembolism | ||||
| No defect | 3 | 312 | 1 (Ref.) | 0.03 |
| Antithrombin deficiency | 27 | 55 | 42.8 (10.2-180.3) | 0.93 |
| Protein C deficiency | 14 | 45 | 31.3 (7.0-138.8) | 0.63 |
| Protein S deficiency | 12 | 26 | 35.7 (7.9-160.1) | 0.78 |
| Factor V Leiden | 17 | 165 | 10.1 (2.3-43.7) | 0.14 |
Conditional risk ratios are adjusted by sex and family status.
DISCUSSION
This study compared the risk for thrombosis of individuals with inherited thrombophilia due to factor V Leiden or to antithrombin, protein C, or protein S deficiency. We found that factor V Leiden is associated with a lower risk of thrombosis and with less severe thrombotic manifestations. The probability of developing thrombosis during the lifetime was 8.1 times higher for carriers of antithrombin deficiency, 7.3 for protein C deficiency, and 8.5 for protein S deficiency, in agreement with previous data.15 The thrombotic risk was also greater for factor V Leiden, the most frequent thrombophilic defect, but its magnitude was only 2.2 times higher than that for noncarriers. This lower thrombotic tendency in carriers of factor V Leiden than in individuals with deficiencies of the naturally occurring anticoagulant proteins is compatible with the biochemical mechanism of this thrombophilic defect (activated factor V is only partially resistant to inactivation by activated protein C).24 25
In theory, a milder thrombophilic defect should be characterized by the onset of thrombosis at an older age and by milder clinical manifestations. The mean age at the occurrence of the first thrombotic episode was similar for all of the patients with single defects, ranging from 35 to 40 years, and was also similar for men and women, and for heterozygotes and homozygotes with factor V Leiden. This is in agreement with a recent observation that in heterozygous relatives with factor V Leiden or protein C deficiency,26 differences in age at development of symptoms mainly depend on the way the patients are selected and not on the type of defect. Even so, the type of thrombotic manifestation was different for factor V Leiden, with the frequency of severe manifestations, such as deep vein thrombosis and pulmonary embolism, being lower and that of a relatively mild manifestation, such as superficial vein thrombosis higher than for antithrombin, protein C, or protein S deficiency. Our observations contrast with those reported by other investigators,27 who have found similar clinical manifestations in factor V Leiden and in protein C or protein S deficiency, despite later occurrence of the first thrombotic event in factor V Leiden. The larger number of patients tested in our study and the exclusion of index patients from the analysis probably explain the discrepancy in results.
This study included not only individuals who had venous thrombosis, but also those who had arterial thrombosis, because they are often referred to our centers for thrombophilia screening, despite the fact that the association between coagulation defects and arterial thrombosis is still debated.28 The small proportion of index patients who had arterial thrombosis (7%) might have led to an underestimation of the risk of thrombosis in their relatives. However, this is unlikely because the rate of venous thrombotic events among these relatives was very similar to that among relatives whose proband had venous thrombosis. The prevalence of arterial thrombosis was low and was similar in the four groups (3% to 7%). The mean age at the time of the first occurrence of arterial thrombosis in the relatives was higher (52 years) than that in relatives with venous episodes, indicating that carriers of thrombophilic defects are exposed to a lower risk of developing arterial thrombosis than venous thrombosis at a young age.
The risk of thrombosis was evaluated only for relatives of the index patients to avoid a selection bias that would have led to overestimating the risk. Complete data about both coagulation tests and clinical history were available for 723 relatives. Although for 21% of them the clinical history was obtained by interviewing the index patients, we are confident to have correctly evaluated the rate of occurrence of thrombosis, as we have previously reported a nearly 100% agreement between the information obtained from the probands and that obtained directly from relatives.17 The demographic characteristics of the 490 relatives with incomplete data (only clinical or demographic information) were similar to those for personally interviewed and screened relatives. The prevalence of thrombosis was also similar for the two groups (20% and 16%), suggesting that a selection bias due to lack of participation in the laboratory screening is unlikely. One possible limitation of this study may be a recall bias, as the design was retrospective. The small rate of nonobjectively confirmed thrombotic events (five) and our decision to consider thrombosis as certain only when anticoagulant treatment had been given or signs of the postphlebitic syndrome were objectively detected should have minimized this bias. Furthermore, it is unlikely that there were differences in reporting the events for the four thrombophilic conditions. In addition to having excluded index patients from the analysis, overestimation of the risk was also minimized by the second inclusion criterion, ie, each relative had to be tested for all four coagulation defects, because the risk for thrombosis is higher in carriers of double defects.29-32 Because the cosegregation of factor V Leiden with the other inherited defects is relatively rare in Italy,33 the relatives with double defects were too few to be included in the statistical analysis. One limitation of the study may be the relatively young age of relatives, which may have led to underestimation of their lifetime risk of thrombosis.
There is at present no published randomized study and hence no established guideline on primary prophylaxis for asymptomatic individuals with factor V Leiden exposed to predisposing factors for thrombosis.1,28 We found that the lifetime risk of thrombosis for this defect is lower than that for the other three thrombophilic defects (relative ratio 0.3) and thrombotic symptoms are less severe. However, antithrombotic prophylaxis with unfractionated heparin or low molecular weight heparin at standard dosages should be implemented at the time of exposure of these individuals to predisposing factors for thrombosis. These views are supported by our observation that there were concurrent predisposing factors in about half of the patients with factor V Leiden who developed venous thromboembolism. On the other hand, lifelong anticoagulant therapy of asymptomatic individuals with inherited thrombophilia is not justified in the absence of prospective trials demonstrating its efficacy. This view is supported by the risk of hemorrhage, which is higher than the annual incidence of thrombosis,34 by the cost of laboratory monitoring of anticoagulant therapy, and by the observation that mortality is not increased in individuals with antithrombin35 or protein C deficiency36 or factor V Leiden37 in comparison to the general population.
In conclusion, this family study gives the first direct answer to the question of the varying thrombotic risks of congenital coagulation defects associated with thrombophilia. Factor V Leiden carries a lower risk than antithrombin, protein C, or protein S deficiency, but the risk is three times that for the control group of individuals with normal coagulation. Although the age at the first thrombosis is similar for all four inherited thrombophilic defects, less severe symptoms such as superficial vein thrombosis are more frequent for factor V Leiden than for the remaining defects. Obviously, our findings apply only to families in which the hereditability of the coagulation defect has been demonstrated and not to unselected individuals.
Supported by a grant from Istituto Superiore di Sanità, Rome, (Progetto Sangue), by institutional grants of the IRCCS Maggiore Hospital, Milan, and by a grant from Rolo Banca 1473 Spa, Milan.
Address reprint requests to Ida Martinelli, MD, PhD, Hemophilia and Thrombosis Center, IRCCS Maggiore Hospital, Via Pace, 9, 20122 Milano, Italy; e-mail: martin@polic.cilea.it.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal