Abstract
Ninety-five patients undergoing an allogeneic bone marrow transplant (BMT) and developing acute graft-versus-host disease (aGvHD) were randomized to receive low-dose intravenous 6-methylprednisolone (6MPred; 2 mg/kg /d; n = 47) or high-dose 6MPred (10 mg/kg/d; n = 48) for 5 days, with subsequent tapering doses. On day 5 patients not responding or progressing on low-dose 6MPred could be switched to high-dose 6MPred. All patients, aged 1 to 55 years, were recipients of unmanipulated BMT from HLA identical sibling donors. Patients were stratified at randomization for age (</≥ 20 years), disease (acute leukemia, chronic myeloid leukemia [CML], nonneoplastic disease), disease status (early/advanced), and GvHD prophylaxis (cyclosporin/cyclosporin + methotrexate). Primary endpoints were response to treatment and evolution of aGvHD to grade III-IV. Secondary endpoints were cytomegalovirus (CMV) infections, transplant-related mortality (TRM), and relapse. The median interval between BMT and treatment was 12 days (6 to 43). Results in the two groups (2 v10 mg/kg) were as follows: response of aGvHD 68% versus 71% (P= .9), evolution to aGvHD grade III-IV 17% versus 20% (P= .6), CMV infections 55% versus 60% (P = .7), 3-year actuarial TRM 28% versus 32% (P = .7), relapse 17% versus 7% (P = .1). The actuarial survival at 3 years was 63% versus 62% (P = .9) with a median follow up of 580 and 778 days. On day 5 of therapy, 26 patients assigned to low-dose (2 mg/kg) 6MPred were switched to a higher dose of 6MPred because of no response or progression. Their actuarial TRM was 46%, which is significantly higher than TRM of patients who responded on 2 mg/kg and continued with tapering doses (TRM = 16%, P = .007). In conclusion, early treatment of acute GvHD with 6MPred 10 mg/kg/d does not improve the response rate as compared with 2 mg/kg/d, nor does it prevent evolution to aGvHD grade III-IV. CMV infections, TRM, and survival were also comparable. A group of patients at high risk of TRM can be identified after 5 days of treatment with 6MPred 2 mg/kg and could be eligible for alternative forms of therapy.
ACUTE GRAFT-VERSUS HOST disease (aGvHD) is a major cause of morbidity and mortality in patients receiving allogeneic bone marrow transplants (BMT) despite postgraft GvHD prophylaxis with cyclosporin (CS) and/or methotrexate (MTX).1-5 Combination of these two agents and improved management of infections have resulted in a reduced risk of severe aGvHD with time.4 In one of the GITMO Centers (Genova San Martino), the proportion of patients with grade III-IV aGvHD has decreased from 31% before 1980 to 6% after 1990 (P = .001; unpublished observation, January 1998).
Once aGvHD has developed, the strategy of treatment is not well established. Some centers will start with low-dose 6 methylprednisolone (6MPred) and increase the dose as clinically indicated,6whereas others have explored the use of high-dose 6MPred up front, in the range of 10 to 20 mg/kg.7-10 Responses up to 43% have been reported,7 with small numbers of long-term survivors if treated for grade III-IV aGvHD.8 Response and survival after aGvHD depends on a number of variables, such as patient clinical performance, concurrent infections, gastrointestinal bleeding, and pneumonitis, which often complicate aGvHD.11-13 There is actually no prospective study looking at the effect of different doses of 6MPred at the onset of the disease.
We have therefore conducted a prospective multicenter randomized trial designed to test the effect of low-dose (2 mg/kg/d) versus high-dose (10 mg/kg/d) methylprednisolone on aGvHD given early at the onset of aGvHD and are now reporting the results of this trial.
MATERIALS AND METHODS
Patients
Ninety-five patients with hematological neoplastic and nonneoplastic disease were randomized between April 22, 1991, to February 9, 1996, and analyzed as of May 1, 1997. They were treated in 11 BMT centers in Italy: Divisione Ematologia, Ospedale San Martino Genova (n = 45); Clinica Pediatrica Milano (n = 13); Divisione Ematologia Ospedale Cervello Palermo (n = 12); Clinica Pediatrica Pavia (n = 8); Cattedra di Ematologia Universita' La Sapienza Roma (n = 5); Divisione Ematologia Ospedale le Molinette Torino (n = 4); Oncologia Pediatrica Osp Regina Margherita Torino (n = 2); Cattedra Ematologia Ospedale Careggi Firenze (n = 2); Cattedra Ematologia Policlinico San Matteo, Pavia (n = 2); Medicina IV, Ospedale Gaslini Genova (n = 1); and Cattedra Ematologia, Universita' Napoli (n = 1). The diagnoses were severe aplastic anemia (SAA; n = 5), myelodysplasia (MDS; n = 3), paroxysmal nocturnal hemoglobinuria (PNH; n = 1), myeloma (n = 1), acute myeloid leukemia (AML; n = 30), acute lymphoblastic leukemia (ALL; n = 27), and chronic myeloid leukemia (CML; n = 28). Forty-eight patients were considered to be in early phase of the disease (first remission or first chronic phase), and the others were classified as advanced. The median age of the patients was 28 years (range 1 to 55 years). Conditioning regimen consisted of cyclophosphamide (CY) and total body irradiation (TBI) in 35 patients and chemotherapy alone in 60; CY alone (n = 5), thiotepa + CY (THIO-CY; n = 18), busulfan + CY (BU-CY; n = 24), BU+CY+VP16 (n = 8), BU+CY+melphalan (n = 5; Table 1).
Clinical Data of 95 Patients Randomized
| N Patients . | Randomization Group . | ||
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | P Value . | |
| 47 Patients . | 48 Patients . | ||
| Age | |||
| Median | 26 | 28 | .6 |
| Range | 1-55 | 2-50 | |
| Donator's Sex | |||
| M | 27 | 32 | .3 |
| F | 20 | 16 | |
| Recipient's Sex | |||
| M | 29 | 24 | .2 |
| F | 18 | 24 | |
| Disease status | |||
| 1ST CR | 25 | 23 | .3 |
| >1STCR | 22 | 25 | |
| TBI | |||
| Yes | 17 | 18 | .2 |
| No | 30 | 30 | |
| GvHD Prophylaxis | |||
| CS | 26 | 24 | .6 |
| CS + MTX | 21 | 24 | |
| Interv days BMT-TX | |||
| Median | 12 | 12 | |
| Range | 7-41 | 6-43 | .4 |
| Follow-up days | |||
| Median | 580 | 778 | .9 |
| Range | 12-1417 | 20-1824 | |
| N Patients . | Randomization Group . | ||
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | P Value . | |
| 47 Patients . | 48 Patients . | ||
| Age | |||
| Median | 26 | 28 | .6 |
| Range | 1-55 | 2-50 | |
| Donator's Sex | |||
| M | 27 | 32 | .3 |
| F | 20 | 16 | |
| Recipient's Sex | |||
| M | 29 | 24 | .2 |
| F | 18 | 24 | |
| Disease status | |||
| 1ST CR | 25 | 23 | .3 |
| >1STCR | 22 | 25 | |
| TBI | |||
| Yes | 17 | 18 | .2 |
| No | 30 | 30 | |
| GvHD Prophylaxis | |||
| CS | 26 | 24 | .6 |
| CS + MTX | 21 | 24 | |
| Interv days BMT-TX | |||
| Median | 12 | 12 | |
| Range | 7-41 | 6-43 | .4 |
| Follow-up days | |||
| Median | 580 | 778 | .9 |
| Range | 12-1417 | 20-1824 | |
Abbreviations: 1st CR, first complete remission; Interv BMT-TX, interval in days between transplant and first day of treatment for GvHD.
GvHD Prophylaxis
Eligibility
Patients undergoing an allogeneic unmanipulated BMT from an HLA identical sibling were eligible for randomization.
Randomization
Randomization was central and was stratified according to patients age (</≥ 20 years), disease (neoplastic/ nonneoplastic), disease status (early/advanced), and GvHD prophylaxis (CS/CS+MTX). The two randomization arms were balanced for age, donor and recipient sex-match, GvHD prophylaxis, interval BMT therapy, diagnosis, and early versus advanced disease (Table 1).
Study Design
Step 1 (48 hours; test dose of 6MPred).
Patients diagnosed as having aGvHD grade I were started on 6MPred 0.5 mg/kg/d intravenously for 2 consecutive days.
Step 2 (days 1 through 5).
Patients not clearing GvHD or presenting with aGvHD grade ≥II were randomized to receive 2 mg/kg/d intravenously days 1 through 5 (group A) or 10 mg/kg/d intravenously on days 1 through 5 (group B).
Step 3 (day 6 and onward).
For patients showing improvement, the dose of 6MPred was halved on day 5. For patients showing progression or poor response, if they were in the 2 mg/kg arm, they were switched to 10 mg/kg; if they were in the 10 mg/kg arm, their dose was not reduced.
Programmed Dose of 6MPred
Arm 10 mg/kg.
The programmed dose was: day 1 through 5, 10 mg/kg/d; day 6 through 10, 5 mg/kg/d; day 11 through 15, 2.5 mg/kg/d; day 16 through 30, 1 mg/kg/d.
Arm 2 mg/kg.
The programmed dose was: day 1 through 5 , 2 mg/kg/d; day 6 through 10, 1 mg/kg/d; day 11 through 30, 1 mg/kg/d.
Intention to Treat
If not stated otherwise, results are presented as “intention to treat,” thus patients were analyzed within their group of randomization.
Aim of the Study
The aim of the study was to establish if intensive immunosuppressive treatment in the early phase of aGvHD would modify the evolution of the disease, with the final aim of reducing transplant mortality.
End Points of the Study
Primary end points were response to therapy and evolution of GvHD. The secondary end points were incidence of CMV infections, transplant mortality, and relapse.
Response to Treatment
This was identified as the reduction of clinical signs of skin, liver, and/or gut GvHD such that patients could comply with the programmed dose reduction (50%) of 6MPred every 5 days. Failure to reduce the dose of 6MPred or requirement for greater doses was considered as failure to respond.
Statistical Analysis
The Chi square and Fisher exact tests were used together with the Mann Whitney rank sum test . Survival analyses were run according to Kaplan and Meier,15 and the log-rank test was used for differences between curves. The number cruncher software (NCSS, version 5.0; JL Hintze, Kaysville, UT) was used to run the analyses.
RESULTS
Compliance
Patients were randomized and started therapy at a median interval from BMT of 12 days (range 7 to 41 days) in the 2 mg/kg arm and 12 days (range 6 to 43 days) in the 10 mg/kg arm (P = .8). The average daily dose administered in the first 5 days was 2 mg/kg/d in the group randomized to receive 2 mg/kg and 9 mg/kg/d in the 10 mg/kg arm (P < .00001); between day 6 and 10 it was 1.92 and 4.6 mg/kg/d, respectively (P < .00001); between day 11 and 20 it was 1.4 and 1.8 mg/kg/d (P = .2); between day 21 and 30 it was 0.96 and 0.97 mg/kg/d (P = .9); between day 31 and 60 it was 0.40 and 0.41 mg/kg/d (P = .8); and between day 61 and 90 it was 0.27 and 0.32 mg/kg/d (P = .6). Therefore, the dose of 6MPred administered differed very significantly only in the first 10 days of therapy. The compliance to the programmed dose was very good in the first 5 days and less so thereafter.
Side Effects
Table 2 outlines side effects in the two randomization groups. There was no statistical difference in terms of number of patients with hyperglycemia (P = .2) or hypertension (P = .3). We also classified patients with infections (Table 3). Again, we saw no major effect on the risk of developing bacterial, fungal, viral, or combined infections (P= .7). CMV infections, as identified by CMV antigenemia, occurred in a similar proportion of patients in the two groups (55% v60%, P = .7).
Adverse Effect: Hyperglycemia and Hypertension
| N Patients . | Randomization Group . | Total . | |
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | ||
| 47 Patients . | 48 Patients . | ||
| Hyperglicemia | |||
| No | 16 | 19 | 35 |
| Yes | 15 | 18 | 33 |
| Unknown | 17 | 10 | 27 |
| Hypertension | |||
| No | 26 | 27 | 53 |
| Yes | 5 | 10 | 15 |
| Unknown | 17 | 10 | 27 |
| N Patients . | Randomization Group . | Total . | |
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | ||
| 47 Patients . | 48 Patients . | ||
| Hyperglicemia | |||
| No | 16 | 19 | 35 |
| Yes | 15 | 18 | 33 |
| Unknown | 17 | 10 | 27 |
| Hypertension | |||
| No | 26 | 27 | 53 |
| Yes | 5 | 10 | 15 |
| Unknown | 17 | 10 | 27 |
Chi square P value: hyperglycemia, P = .2; hypertension, P = .3.
Infections
| N Patients . | Randomization Group . | . | |
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | ||
| 47 Patients . | 48 Patients . | ||
| No infections | 9 | 9 | 18 |
| Bacterial | 7 | 3 | 10 |
| Fungal | 1 | 1 | 2 |
| Viral | 21 | 24 | 45 |
| Bacterial + fungal | 0 | 2 | 2 |
| Bacterial + viral | 3 | 3 | 6 |
| Fungal + viral | 3 | 2 | 5 |
| Bacterial + fungal + viral | 3 | 4 | 7 |
| Total | 47 | 48 | 95 |
| N Patients . | Randomization Group . | . | |
|---|---|---|---|
| 6MPred 2 mg/kg . | 6MPred 10 mg/kg . | ||
| 47 Patients . | 48 Patients . | ||
| No infections | 9 | 9 | 18 |
| Bacterial | 7 | 3 | 10 |
| Fungal | 1 | 1 | 2 |
| Viral | 21 | 24 | 45 |
| Bacterial + fungal | 0 | 2 | 2 |
| Bacterial + viral | 3 | 3 | 6 |
| Fungal + viral | 3 | 2 | 5 |
| Bacterial + fungal + viral | 3 | 4 | 7 |
| Total | 47 | 48 | 95 |
Chi square P value = .7.
Response to Therapy
Overall, 66 patients were classified as responders (69%), 27 (28%) as nonresponders, and 2 were unclassified because of early death (3 and 4 days after treatment), one in each randomization group (Table 4). Death due to transplant-related complications occurred in 10 of 66 responders (15%) and in 17 of 27 nonresponders (63%; P < .00001). There was no difference in response between the two groups of patients (68% and 71% in the 2 mg/kgv 10 mg/kg group, P = .9; Table 4). The proportion of nonresponders/responders was also not different when patients were stratified for age (</≥ 20 years), GvHD prophylaxis (CS/CS+MTX), conditioning regimen (TBI yes or no), and phase of the disease (early/advanced; Table 4). The proportion of responders was comparable in patients treated early (≤day 12) or later after BMT (>day 12; 70% v 71%, P = .4). In addition., we could not find a correlation between the total dose of 6MPred administered in the first 10 days and response. The average cumulative dose of 6MPred given on days 1 through 5 was 25 mg/kg in nonresponders v 28 mg/kg in responders (P = .6); on days 6 through 10 it was 17 versus 15 mg/kg, respectively (P = .5).
Response Rate According to Randomization
| . | Randomization Group . | P Value . | |||
|---|---|---|---|---|---|
| 6Mpred 2 mg/kg . | 6Mpred 10 mg/kg . | ||||
| No . | Yes . | No . | Yes . | ||
| No. of patients responding* | 14 | 32 | 13 | 34 | .9 |
| Age | |||||
| ≤20 yr | 6 | 12 | 4 | 11 | |
| >20 yr | 8 | 20 | 9 | 23 | .9 |
| GvHD Prophylaxis | |||||
| CS | 11 | 14 | 7 | 16 | |
| CS + MTX | 3 | 18 | 6 | 18 | .3 |
| Conditioning | |||||
| No TBI | 8 | 25 | 8 | 25 | |
| TBI | 6 | 7 | 5 | 9 | .5 |
| Disease status | |||||
| 1ST CR | 6 | 19 | 5 | 18 | |
| >1ST CR | 8 | 13 | 8 | 16 | .8 |
| Interval (d) BMT-TX | |||||
| ≤12 | 8 | 19 | 7 | 17 | |
| >12 | 6 | 13 | 6 | 17 | .7 |
| . | Randomization Group . | P Value . | |||
|---|---|---|---|---|---|
| 6Mpred 2 mg/kg . | 6Mpred 10 mg/kg . | ||||
| No . | Yes . | No . | Yes . | ||
| No. of patients responding* | 14 | 32 | 13 | 34 | .9 |
| Age | |||||
| ≤20 yr | 6 | 12 | 4 | 11 | |
| >20 yr | 8 | 20 | 9 | 23 | .9 |
| GvHD Prophylaxis | |||||
| CS | 11 | 14 | 7 | 16 | |
| CS + MTX | 3 | 18 | 6 | 18 | .3 |
| Conditioning | |||||
| No TBI | 8 | 25 | 8 | 25 | |
| TBI | 6 | 7 | 5 | 9 | .5 |
| Disease status | |||||
| 1ST CR | 6 | 19 | 5 | 18 | |
| >1ST CR | 8 | 13 | 8 | 16 | .8 |
| Interval (d) BMT-TX | |||||
| ≤12 | 8 | 19 | 7 | 17 | |
| >12 | 6 | 13 | 6 | 17 | .7 |
*Two patients were considered not evaluable for response.
Abbreviation: 1st CR, first complete remission.
Evolution of GvHD
Eighteen patients (19%) progressed to aGvHD grade III-IV. The actuarial probability was 17% and 20% for patients randomized to receive 2 mg/kg or 10 mg/kg (P = .6). Patients treated early (≤day 12) had a 20% chance of progressing to aGvHD grade III-IV versus 17% for patients treated later (>day 12). The average cumulative dose of 6MPred given on days 1 through 5 and on days 6 through 10 was comparable in patients who did or did not progress to aGvHD grade III-IV.
Chronic GvHD (cGvHD).
There were 68 patients evaluable for cGvHD; 8,14,12 and 9,12,13 in the two treatment arms developed no, limited, or extensive cGvHD (P= .8).
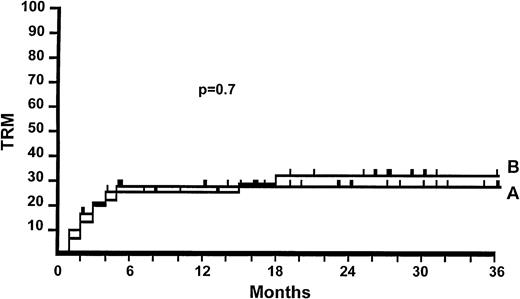
TRM.
The overall actuarial 3-year TRM was 28%, and it was not different in the two groups (28% v 32%, P = .7; Fig1, Table 5). It was not different in patients stratified for age (≤/>20 years), for GvHD prophylaxis (CS/CS+MTX), conditioning regimen (TBI or non TBI), or disease status at transplant (early/advanced). TRM was influenced by the maximum grade of aGvHD; it was 23% for 77 patients who experienced aGvHD grade I-II and 58% for 18 patients with aGvHD grade III-IV. The actuarial TRM was also influenced by response to treatment; it was 14% for responders and 66% for nonresponders (P < .0001).
Actuarial probability of TRM for patients randomized to receive 2 mg/kg of 6MPred (47 patients, 28%) (A), or 10 mg/kg (48 patients, 32%) (B). There is no significant difference (P = .7).
Actuarial probability of TRM for patients randomized to receive 2 mg/kg of 6MPred (47 patients, 28%) (A), or 10 mg/kg (48 patients, 32%) (B). There is no significant difference (P = .7).
Actuarial 3-Year TRM in Percentage
| . | Randomization Group . | ||
|---|---|---|---|
| 2 mg/kg (%) . | 10 mg/kg (%) . | P Value . | |
| All patients | 28 | 32 | .7 |
| Age | |||
| ≤20 yr | 32 | 32 | .5 |
| >20 yr | 26 | 28 | .9 |
| GvHD Prophylaxis | |||
| CS | 29 | 28 | .4 |
| CS + MTX | 15 | 24 | .1 |
| Conditioning | |||
| No TBI | 23 | 28 | .8 |
| TBI | 32 | 30 | .7 |
| Disease status | |||
| 1ST CR | 17 | 22 | .6 |
| >1STCR | 43 | 32 | .8 |
| . | Randomization Group . | ||
|---|---|---|---|
| 2 mg/kg (%) . | 10 mg/kg (%) . | P Value . | |
| All patients | 28 | 32 | .7 |
| Age | |||
| ≤20 yr | 32 | 32 | .5 |
| >20 yr | 26 | 28 | .9 |
| GvHD Prophylaxis | |||
| CS | 29 | 28 | .4 |
| CS + MTX | 15 | 24 | .1 |
| Conditioning | |||
| No TBI | 23 | 28 | .8 |
| TBI | 32 | 30 | .7 |
| Disease status | |||
| 1ST CR | 17 | 22 | .6 |
| >1STCR | 43 | 32 | .8 |
Abbreviation: 1st Cr, first complete remission.
Leukemia relapse.
The probability of relapse was not statistically different in the two groups of patients (17% v 7%, P = .1).
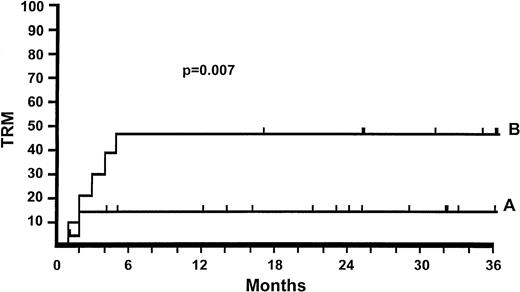
Day-5 responders.
On day 5 of therapy, 26 patients assigned to low-dose (2 mg/kg) 6MPred were switched to a larger dose of 6MPred because of no response or progression. Their actuarial TRM is 46%. Twenty patients who responded on 2 mg/kg and continued with tapering doses (n = 19) had a TRM of 16% (Fig 2). The difference in TRM between day-5 responders and nonresponders on 2 mg/kg of 6MPred is significant (P = .007). In the 10 mg/kg group, 36% of the patients reduced the dose on day 5 and therefore were considered responders; their TRM is 20%. The TRM of the remaining patients who did not reduce the dose on day 5 is 33% (P = .6).
Actuarial TRM for patients randomized to receive 2 mg/kg of 6MPred. Day-5 responders (A) are patients who continued with tapering doses of 6MPred on day 5 of therapy (n = 19; TRM = 16%). Day-5 nonresponders are patients who had the dose of 6MPred increased on day 5 because of nonresponse or progression of aGvHD (n = 25; TRM = 46%). The difference in TRM between day-5 responders and day-5 nonresponders is significant (P = .007).
Actuarial TRM for patients randomized to receive 2 mg/kg of 6MPred. Day-5 responders (A) are patients who continued with tapering doses of 6MPred on day 5 of therapy (n = 19; TRM = 16%). Day-5 nonresponders are patients who had the dose of 6MPred increased on day 5 because of nonresponse or progression of aGvHD (n = 25; TRM = 46%). The difference in TRM between day-5 responders and day-5 nonresponders is significant (P = .007).
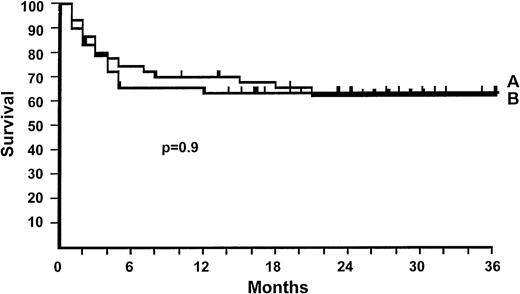
Survival and causes of death.
The actuarial survival of patients randomized to receive 2 mg/kg or 10 mg/kg is, respectively, 63% versus 62% at 3 years (P = .9; Fig 3), and the median follow up is, respectively, 580 and 778 days (P = .09). There were 17 and 20 deaths in the 2 mg/kg and 10 mg/kg groups, respectively. Causes of failure in the two groups were the following: leukemia recurrence (4 v 4), acute GvHD (3 v 5), infections (5 v 4), interstitial pneumonia (4 v 3), multiorgan failure (0 v 2), hepatitis (1 v 0), heart failure (0 v 1), hemorrhage (0v 1; P = 0.6).
Actuarial survival for patients randomized to receive 2 mg/kg of 6MPred (47 patients, 63%) (A), or 10 mg/kg (48 patients, 62%) (B). There is no significant difference (P = .9).
Actuarial survival for patients randomized to receive 2 mg/kg of 6MPred (47 patients, 63%) (A), or 10 mg/kg (48 patients, 62%) (B). There is no significant difference (P = .9).
DISCUSSION
We have shown in this prospective trial that early treatment of acute GvHD with 6MPred 10 mg/kg/d does not prevent progression of the disease to grade III-IV, and treatment with 6MPred 2 mg/kg/d for 5 days identifies two subsets of patients: responders, who have a good prognosis and a low TRM , and nonresponders with a greater risk of progressing to severe GvHD and of experiencing a high TRM.
As to the first point, GvHD will start in most patients with mild symptoms involving either skin, liver, gut, or combinations.11 Some will improve on low-dose of prednisolone, some will remain stable, and a fraction will deteriorate and develop severe GvHD.11-13 The question is, if we treat patients with intensive immunosuppression up front, can we alter the evolution of the disease?
The results of the present study would suggest that 2 mg/kg and 10 mg/kg of 6MPred have comparable effects. Despite an administered dose of 6MPred 5-fold greater in the first 5 days of therapy and 2.4-fold greater between days 6 and 10, the proportion of the patients progressing to grade III-IV acute GvHD was comparable in the two randomization groups. This occurred despite the fact that treatment was initiated very early, at a median interval of 12 days from transplant. This result may be interpreted in opposite ways. Either 6MPred at the dose of 10 mg/kg is not useful as initial therapy of aGvHD, or this dose is inadequate to prevent progression to life-threatening disease. Similarly, in patients with severe autoimmune disorders, one is confronted with the question whether a given drug is ineffective or the dose used is inadequate. Recent programs of myeloablative chemotherapy and autografting would suggest that more intensive treatment may be successful in patients who have failed one or more courses of immunosuppressive therapy.16
On the other hand, it may also be possible that GvHD is predetermined to some extent, and patients with a given level of T-cell activation eventually progress to experience severe life-threatening GvHD, irrespective of the dose of methylprednisolone given very early after BMT. If the model proposed by Ferrara is correct,17 it may be very difficult to switch off GvHD once the disease is fully established. This is true for two main reasons: (1) tissue disruption has taken place weeks before the diagnosis of aGvHD, especially during the conditioning regimen with high-dose cytotoxic agents and radiation, and (2) this is maintained via inflammatory cytokines that can be produced in large quantity by host macrophages, relatively insensitive to immunosuppressive or lymphocytotoxic drugs.17 To this regard it is interesting that lyposomal diphosphonates can abrogate monocyte/macrophages activity for 2 to 3 weeks in the experimental model.18 Whether this could reduce the severity of aGvHD in humans, with tolerable side effects, remains to be determined.
Other end points of the study, such as infections and transplant mortality, were also comparable in the two treatment arms and resulted in comparable survival at 2 years (P = .9). To test for patient compliance we analyzed the actual dose of 6MPred received on days 1 through 5, 6 through 10, 11 through 20, and 21 through 30 of therapy. Again, we could not find a correlation between dose and response, not only in the first 10 days, but also thereafter, because patients not responding or generally not doing well were kept on larger doses of 6MPred.
Patients randomized to receive 2 mg/kg/d received their prescribed dose on days 1 through 5. On day 5, patients not improving or progressing were allowed per protocol to receive an increased dose of 6MPred. Twenty-six patients were switched on day 5 to a higher dose of 6MPred, and these patients had a TRM of almost 50%. The remaining patients (responders) followed the protocol by tapering the dose of 6MPred on day 5, and their TRM was 16%. When comparing the outcome of day-5 responders to 2 mg/kg (5% TRM) with the outcome of day-5 responders to 10 mg/kg of 6MPred (20% TRM), it seems that the former is a subset of patients at better prognosis. Therefore, a relatively small dose of 6MPred (2 mg/kg) on days 1 through 5 appears to identify responders and nonresponders with low or high transplant mortality, and this may be clinically relevant. In fact, day-5 responders could proceed with tapering doses of 6MPred, whereas day-5 nonresponders would be eligible for alternative immunosuppressive treatment. Antithymocyte globulin,19-21 monoclonal antibodies,22-28cytotoxic therapy, and other experimental drugs29 30 are all candidate agents to be tested in prospective trials.
The early use of antithymocyte globulin as compared with intermediate-dose 6MPred is now being tested in a randomized trial.
Supported by Associazione Italiana Ricerca contro il Cancro (A.I.R.C.), Milano, and Associazione Ricerca Trapianto Midollo Osseo (A.RI.T.M.O.), Genova, Italy.
Address reprint requests to M.T. Van Lint, MD, Divisione Ematologia 2, Ospedale San Martino, Genova, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal