Abstract
Immunization against the human platelet antigen (HPA)-1 alloantigen is the most common cause of severe fetal and neonatal thrombocytopenia. Fetal therapy has substantial risks and its indications need better definition. Of 24,417 consecutive pregnant women, 618 (2.5%) were HPA-1a negative of whom 385 entered an observational study. All were HLA-DRB3*0101 genotyped and screened for anti–HPA-1a. Their partners and neonates were HPA-1 genotyped and the latter were assessed by cord blood platelet counts and cerebral ultrasound scans. Anti–HPA-1a was detected in 46 of 387 pregnancies (12.0%; 95% CI 8.7%-15.2%). All but one were HLA-DRB3*0101 positive (odds ratio 140; 95% CI 19-1035;P< .00001). One baby died in utero, and of 26 HPA-1a–positive babies born to women with persistent antenatal antibodies, 9 were severely thrombocytopenic (8 with a count <10 × 109/L, 1 with a large porencephalic cyst), 10 were mildly thrombocytopenic, whereas 7 had normal platelet counts. Severe thrombocytopenia was significantly associated with a third trimester anti–HPA-1a titer ≥ 1:32 (P = .004), but was not observed in babies of women with either transient or postnatal-only antibodies. HPA-1a alloimmunization complicates 1 in 350 unselected pregnancies, resulting in severe thrombocytopenia in 1:1,200. HPA-1a and HLA-DRB3*0101 typing combined with anti–HPA-1a titration allows selection of the majority of pregnancies at risk of severe thrombocytopenia.
FETOMATERNAL ALLOIMMUNIZATION to paternal platelet-specific antigens on fetal platelets is the most common cause of severe thrombocytopenia in neonates.1 Although this condition is usually referred to as neonatal alloimmune thrombocytopenia (NAITP), intrauterine death or intracerebral hemorrhage can occur by 20 to 24 weeks of pregnancy.2 Most cases show disparity in the biallelic human platelet antigen (HPA)-1 system,3 with an HPA-1a (previously, PlA1 or Zwa) –negative woman and an HPA-1a–positive fetus.4 Approximately 2% to 2.5% of the white population is HPA-1a negative; however, the chance of HPA-1a alloimmunization is strongly associated with maternal HLA class-II DRB3*0101 (DR52a) type.5
Most severe cases of NAITP are diagnosed in the neonatal period, which justifies fetal blood sampling and antenatal therapy in subsequent pregnancies. Intravenous administration of high doses of immunoglobulin G to the mother6 and intrauterine transfusions of compatible platelets7 have been successfully used as antenatal therapies; however, they are associated with the possibility of nonresponse leading to ICH8 or fetal loss from hemorrhage9, respectively. Because both large-scale phenotyping suitable for pregnancy10 and genotyping of fetuses for HPA-1a11 are technically possible, some investigators have proposed antenatal screening for HPA-1a alloimmunization with interventional therapy to prevent ICH.12
Previous small-scale prospective studies have been undertaken,13-16 but the wide range of alloimmunization reported (0%-10%), combined with the small number of affected babies studied, prohibits meta-analysis of the outcome of these studies. For families with HPA-1a alloimmunization but with no previously affected children, knowledge of the likely outcome is still limited. No predictors of severe disease have been established that permit selection of cases for fetal blood sampling with a view to antenatal treatment. We therefore aimed to elucidate the natural history of HPA-1a alloimmunization in an observational study that examined the relationship between maternal antibodies to HPA-1a, cord platelet count, and infant morbidity in a cohort of HPA-1a–negative women identified by HPA-1a phenotyping of approximately 25,000 nonselected pregnant women.
METHODS
Patient selection and management.
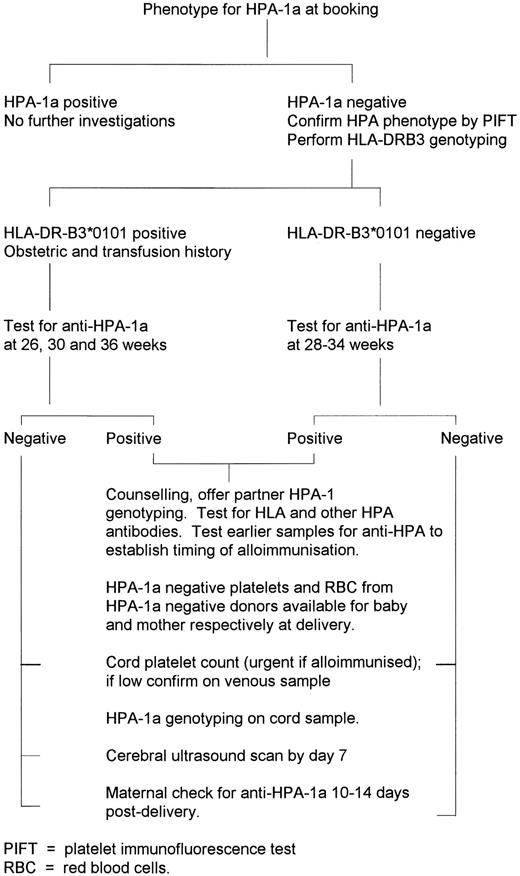
The initial study cohort was all pregnant women in the predominantly (>95%) white East Anglian region of England, from whom blood samples were received for testing in the regional program to prevent hemolytic disease of the newborn. This program receives samples from greater than 98% of all pregnancies in the region. The study received approval before commencement from the Ethics Committee of each of the nine participating hospitals, and was also approved by Professional Regional Advisory Committees in hematology, obstetrics, and pediatrics. A total of 24,417 consecutive EDTA anticoagulated blood samples received between September 1993 and September 1994 were typed for HPA-1a. Women found to be HPA-1a negative were sent an information leaflet and consent form. Subsequent investigations and management of HPA-1a–negative women who consented to join the study are summarized in Fig 1. No further investigations were permitted in women who did not enroll and give informed consent. A research midwife dedicated to the study (C.M.) was available to answer queries from study participants and hospital staff.
Women with detectable anti–HPA-1a were contacted for a full family history, and received written advice on avoidance of aspirin and vigorous exercise according to international guidelines.17An individualized pregnancy care/delivery plan was decided by the local obstetrician in consultation with the obstetrician in the study team (G.H.), and depended on previous obstetric history. Invasive procedures such as amniocentesis for fetal HPA-1 typing and fetal blood sampling were not part of the investigational protocol, but were available if requested (by kind arrangement with Prof Charles Rodeck, University College Hospital, London, UK).
Cord blood platelet counts were performed on the study cases and 200 normal babies as controls.
HPA-1a phenotyping assay.
The assay was a two-plate modification of antigen-capture design enzyme-linked immunosorbent assay (ELISA) assay.18 Briefly, a microtiter plate (Maxisorp; Nunc, Roskilde, Denmark) was precoated with goat antibodies to mouse immunoglobulin G (Jackson Immunoresearch Inc, W Baltimore, PA), and after washing, incubated with a mouse monoclonal antibody (MoAb) to glycoprotein (GP) IIb/IIIa (CD 41) [CLB-C17 culture supernatant; provided by Prof A.E.G. Kr. von dem Borne, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands (CLB)], then washed again. Platelet-rich plasma from each sample to be typed (200 μL, not adjusted for platelet count), was added to a second microtiter plate. After centrifugation and washing, the platelet pellets were solubilized in 200 μL of 1% Nonidet P-40 in Tris-buffered saline at 4°C for 1 hour, and 100 μL transferred to corresponding wells in the initial plate. After incubation and washing, 100 μL of a 1:100 dilution of human plasma containing a high titer IgG anti–HPA-1a but no reactivity against mouse immunoglobulin was added. After incubation and washing, alkaline phosphatase-conjugated goat antibodies to human immunoglobulin G (Jackson Immunoresearch) was added to each well and incubated for 1 hour, followed by four washes. Color was developed by adding p-nitrophenyl phosphate as substrate and absorbance was measured (Titertek Multiscan, version 1.4; Quest Biomedical, Knowle, UK). Each plate included a reagent blank and two wells each of control platelets of the HPA-1a1a, 1a1b, and 1b1b type. Cut-off values were established based on absorbance values obtained with the positive and negative controls. Evaluations of HPA-1a typing after the first 3 months showed that two HPA-1a–negative women had been typed HPA-1a positive and the cut-off value was redefined for the final 9 months to reduce the risk of false-positive HPA-1a phenotypes.
All women who enrolled for the study, and whose initial typing result was HPA-1a negative were retyped on a fresh EDTA anticoagulated sample using a polyclonal IgG anti–HPA-1a in the platelet immunofluorescence test19 read by flow cytometry (Becton Dickinson FACScan; Becton Dickinson UK Limited, Oxford, UK).
HPA-1 genotyping.
Genomic DNA from partners and babies of HPA-1a alloimmunized women was used for HPA-1 genotyping by polymerase chain reaction amplification of a 482–base-pair fragment of the GPIIIa gene, followed by restriction with MsP1 and subsequent fragment length polymorphism analysis by gel electrophoresis of the digested DNA.20 If cord blood samples were not received, amplification was performed by a modified method21 with samples of dried blood spots from cards taken for phenylketonuria screening.
Detection and titration of HPA antibodies.
Samples from HPA-1a–negative women were screened for antibodies against HPA-1a, HPA-3a, and HPA-3b by a standard MoAb immobilization of platelet glycoprotein assay (MAIPA),22 using the MoAb CLB-C17 to GP IIb/IIIa, and two reagent platelets homozygous for for HPA-1 and -3 antigens. If antibodies were found, the timing of immunization was assessed by retrospective testing of stored samples previously sent for HPA/HLA typing. Titration of anti–HPA-1a was performed in the MAIPA in duplicate, with twofold plasma dilutions in buffered phosphate-buffered saline (PBS) from undiluted to 1 in 512.
In cases in which neonatal thrombocytopenia was present, and the mother apparently negative for anti–HPA-1a, extra testing was performed for HPA antibodies. Maternal blood was retested for anti–HPA-1a in the MAIPA using a different antibody to capture GPIIIa (Y2.51, supplied by Dr D. Mason, Oxford, UK), and also in a solid-phase assay according to manufacturer's instructions (supplied by GTI, Brookfield, WI). Such samples were also examined for the presence of antibodies against the alloantigens of the HPA-2 and HPA-5 systems in the MAIPA assay using the MoAbs CLB-MB45 against GP Ib/IX (CD42b) and CLB-10G11 against GP Ia/IIa (CD49b) [both provided by Prof A.E.G. Kr. von dem Borne, CLB]. A four-cell platelet panel with homozygous expression of the HPA-1, -2, -3, and -5 alloantigens was used in these investigations.
HLA-DRB3*0101 genotyping.
The presence of the HLA-DRB3*0101 allele was determined by PCR amplification of a 271-base pair fragment of the DRB3 gene from genomic DNA, followed by membrane hybridization with a DRB3*0101 allele-specific digoxigenin-labeled oligonucleotide probe.23
Statistics.
Fisher's exact test was used to analyze the associations between HPA-1a alloimmunization and HLA-DRB3*0101 genotype, and titer of anti–HPA-1a antibodies and cord blood platelet count, and to compare the frequency of severe thrombocytopenia (<50 × 109/L) in babies of HPA-1a alloimmunized and nonalloimmunized women. For the first two associations calculations were made of odds ratios with confidence intervals, and positive and negative predictive values.24P < .05 was considered significant.
RESULTS
Study group.
Of 25,379 consecutive blood specimens, 962 (3.8%) could not be typed for HPA-1a because clotted blood samples were received. Of the remaining 24,417 women, 618 (2.5%) were typed as HPA-1a negative, of whom 457 (74%) consented to join the study. Seventy-two women were subsequently excluded for various reasons: confirmatory HPA-1a testing by PIFT showed them to be HPA-1a positive (20), incomplete or no blood samples were available (40), or they suffered a miscarriage (8), moved abroad (2), or requested removal from the study (2). Two further women were included, who were not originally typed as HPA-1a negative, but who had affected children and who were shown on retesting to be HPA-1a negative. The final study population thus comprised 387 women, of whom 55% were multiparous.
Maternal serology.
HPA-1a antibodies were detected in 46 women (12%), including 2 (SL and LR ) not originally typed as HPA-1a negative (Table 1). Antibodies were first detected at or before 20 weeks in 27 pregnancies, between 21 and 32 weeks in eight, between 33 weeks and term in three, and postnatally in seven. No primigravida had detectable antibodies to HPA-1a before 17 weeks. Antibodies present before 20 weeks became undetectable until the postnatal period in six pregnancies (transient antibodies); antibody titers increased with time in five pregnancies, were stable in 12, and decreased in 10. Neither antibody titer nor fluctuations therein was a reliable predictor of an HPA-1a–positive fetus. None of the eight women who suffered miscarriage had HPA-1a antibodies.
Maternal Serology and Cord Blood Platelet Counts in 46 HPA-1a Alloimmunized Women
| Unique Study Number . | Parity-150 . | Earliest Antibody Positive Sample . | Anti–HPA-1a Titer in Third Trimester . | HPA-1 Genotype/ Phenotype of Father . | Platelet Count at Birth (×109/L) . |
|---|---|---|---|---|---|
| Persistent Antenatal Antibodies With Severe Thrombocytopenia (n = 10) | |||||
| 274 | 4 + 0 | 7 | 32 | 1a1b | 37 |
| 57 | 0 + 1 | 8 | 128 | 1a1b | 6 |
| 622 | 1 + 0 | 10 | 64 | ND | 9 |
| 306-151 | 2 + 1 | 16 | 128 | ND | 17 |
| 479 | 2 + 0 | 17 | 64 | 1a1a | 8 |
| 152-152 | 0 + 1 | 16 | 64 | ND | |
| 0 + 2 | 21 | 16 | 1a1b | 5 | |
| 347-153 | 0 + 0 | 26 | 64 | 1a1a | 4 |
| 421 | 0 + 0 | 32 | 8 | 1a1a | 2 |
| LR-155 | 0 + 0 | ND | ND | 1a1a | 14 |
| SL-155 | 0 + 0 | ND | ND | 1a1a | ND |
| Persistent Antenatal Antibodies With Mild Thrombocytopenia (n = 10) | |||||
| 223 | 3 + 0 | 9 | 16 | 1a1b | 110 |
| 598 | 0 + 1 | 12 | 8 | ND | 60 |
| 639 | 1 + 0 | 12 | 2 | 1a1b | 107 |
| 60 | 1 + 4 | 14 | 1 | 1a1a | 117 |
| 424 | 1 + 0 | 17 | 1 | 1a1a | 52 |
| 620 | 0 + 0 | 17 | 2 | 1a1a | 132 |
| 64 | 1 + 0 | 19 | 4 | 1a1a | 94 |
| 330 | 2 + 0 | 21 | 32 | 1a1a | 97 |
| 54 | 0 + 0 | 26 | 1 | ND | 118 |
| 178 | 0 + 0 | 30 | 1 | ND | 140 |
| Persistent Antenatal Antibodies With Normal Platelet Counts (n = 7) | |||||
| 599 | 1 + 2 | 11 | 1 | 1a1a | 335 |
| 248 | 1 + 0 | 16 | 1 | 1a pos | 215 |
| 322 | 1 + 0 | 16 | 32 | 1a1a | 197 |
| 571 | 0 + 0 | 17 | 8 | 1a1a | 323 |
| 481 | 2 + 1 | 18 | 16 | 1a1b | 192 |
| 431 | 0 + 0 | 37 | 2 | 1a1b | 491 |
| 607 | 0 + 0 | 37 | 4 | ND | 228 |
| Transient Antenatal Antibodies (n = 6) | |||||
| 487 | 3 + 1 | 8 | 2 | 1a1b | ND |
| 614 | 1 + 0 | 8 | 2 | 1a1b | 267 |
| 605 | 1 + 0 | 9 | 1 | ND | 133 |
| 398 | 3 + 0 | 15 | 1 | ND | 261 |
| 206 | 2 + 1 | 16 | 1 | ND | 604 |
| 420 | 0 + 1 | 20 | 1 | ND | 352 |
| Postnatal Antibodies Only With HPA-1a–Positive Baby¶ (n = 7) | |||||
| 42-151 | 0 + 0 | PN | 1 | 1a1a | ND |
| 62 | 2 + 1 | PN | 8 | ND | 275 |
| 221 | 0 + 1 | PN | 2 | 1a1a | 153 |
| 267 | 1 + 0 | PN | 4 | 1a1b | 274 |
| 302 | 1 + 3 | PN | 4 | 1a1a | SA 15/40 |
| 325 | 0 + 0 | PN | 1 | ND | 146 |
| 412 | 1 + 2 | PN | 4 | 1a1a | 53 |
| Antenatal Antibodies With HPA-1a–Negative Baby (n = 6) | |||||
| 417 | 1 + 1 | 11 | 32 | 1a1b | 286 |
| 199# | 0 + 1 | 17 | 4 | 1a1b | 222 |
| 230-160 | 1 + 2 | 17 | 16 | 1b1b | 229 |
| 304 | 4 + 0 | 26 | 2 | 1a1b | 384 |
| 172 | 2 + 0 | 29 | 1 | 1a1b | 341 |
| 542 | 0 + 1 | 35 | 2 | ND | 285 |
| Unique Study Number . | Parity-150 . | Earliest Antibody Positive Sample . | Anti–HPA-1a Titer in Third Trimester . | HPA-1 Genotype/ Phenotype of Father . | Platelet Count at Birth (×109/L) . |
|---|---|---|---|---|---|
| Persistent Antenatal Antibodies With Severe Thrombocytopenia (n = 10) | |||||
| 274 | 4 + 0 | 7 | 32 | 1a1b | 37 |
| 57 | 0 + 1 | 8 | 128 | 1a1b | 6 |
| 622 | 1 + 0 | 10 | 64 | ND | 9 |
| 306-151 | 2 + 1 | 16 | 128 | ND | 17 |
| 479 | 2 + 0 | 17 | 64 | 1a1a | 8 |
| 152-152 | 0 + 1 | 16 | 64 | ND | |
| 0 + 2 | 21 | 16 | 1a1b | 5 | |
| 347-153 | 0 + 0 | 26 | 64 | 1a1a | 4 |
| 421 | 0 + 0 | 32 | 8 | 1a1a | 2 |
| LR-155 | 0 + 0 | ND | ND | 1a1a | 14 |
| SL-155 | 0 + 0 | ND | ND | 1a1a | ND |
| Persistent Antenatal Antibodies With Mild Thrombocytopenia (n = 10) | |||||
| 223 | 3 + 0 | 9 | 16 | 1a1b | 110 |
| 598 | 0 + 1 | 12 | 8 | ND | 60 |
| 639 | 1 + 0 | 12 | 2 | 1a1b | 107 |
| 60 | 1 + 4 | 14 | 1 | 1a1a | 117 |
| 424 | 1 + 0 | 17 | 1 | 1a1a | 52 |
| 620 | 0 + 0 | 17 | 2 | 1a1a | 132 |
| 64 | 1 + 0 | 19 | 4 | 1a1a | 94 |
| 330 | 2 + 0 | 21 | 32 | 1a1a | 97 |
| 54 | 0 + 0 | 26 | 1 | ND | 118 |
| 178 | 0 + 0 | 30 | 1 | ND | 140 |
| Persistent Antenatal Antibodies With Normal Platelet Counts (n = 7) | |||||
| 599 | 1 + 2 | 11 | 1 | 1a1a | 335 |
| 248 | 1 + 0 | 16 | 1 | 1a pos | 215 |
| 322 | 1 + 0 | 16 | 32 | 1a1a | 197 |
| 571 | 0 + 0 | 17 | 8 | 1a1a | 323 |
| 481 | 2 + 1 | 18 | 16 | 1a1b | 192 |
| 431 | 0 + 0 | 37 | 2 | 1a1b | 491 |
| 607 | 0 + 0 | 37 | 4 | ND | 228 |
| Transient Antenatal Antibodies (n = 6) | |||||
| 487 | 3 + 1 | 8 | 2 | 1a1b | ND |
| 614 | 1 + 0 | 8 | 2 | 1a1b | 267 |
| 605 | 1 + 0 | 9 | 1 | ND | 133 |
| 398 | 3 + 0 | 15 | 1 | ND | 261 |
| 206 | 2 + 1 | 16 | 1 | ND | 604 |
| 420 | 0 + 1 | 20 | 1 | ND | 352 |
| Postnatal Antibodies Only With HPA-1a–Positive Baby¶ (n = 7) | |||||
| 42-151 | 0 + 0 | PN | 1 | 1a1a | ND |
| 62 | 2 + 1 | PN | 8 | ND | 275 |
| 221 | 0 + 1 | PN | 2 | 1a1a | 153 |
| 267 | 1 + 0 | PN | 4 | 1a1b | 274 |
| 302 | 1 + 3 | PN | 4 | 1a1a | SA 15/40 |
| 325 | 0 + 0 | PN | 1 | ND | 146 |
| 412 | 1 + 2 | PN | 4 | 1a1a | 53 |
| Antenatal Antibodies With HPA-1a–Negative Baby (n = 6) | |||||
| 417 | 1 + 1 | 11 | 32 | 1a1b | 286 |
| 199# | 0 + 1 | 17 | 4 | 1a1b | 222 |
| 230-160 | 1 + 2 | 17 | 16 | 1b1b | 229 |
| 304 | 4 + 0 | 26 | 2 | 1a1b | 384 |
| 172 | 2 + 0 | 29 | 1 | 1a1b | 341 |
| 542 | 0 + 1 | 35 | 2 | ND | 285 |
Within each category, cases are listed according to timing of first detectable antibody.
Abbreviations: ND, not determined; SA, spontaneous abortion; PN, postnatal.
Parity: number of births after 24 weeks + number before 24 weeks.
Also produced anti–HPA-5b antenatally.
Two pregnancies during the study period.
Intracerebral hemorrhage in utero.
Not originally typed as HPA-1a negative.
¶Titre refers to postnatal sample.
#HLA-DRB3 *0101 negative.
New partner: previous child HPA-1a positive with severe thrombocytopenia.
Risk factors for alloimmunization.
The presence of antibodies to HPA-1a was associated with HLA-DRB3*0101 (Table 2), with a positive predictive value of 35% and a negative predictive value of 99.6%. The overall frequency of this allele (31.9%) was as expected for a largely white population.
Association of Maternal Antibodies to HPA-1a With the HLA DRB3*0101 Allele for the 385 Women in the Study Population
| Antibodies to HPA-1a . | HLA DRB3*0101 . | |
|---|---|---|
| Positive . | Negative . | |
| Positive | 43 (35%) | 1 (0.4%)* |
| Negative | 80 | 261 |
| Total | 123 | 262 |
| Antibodies to HPA-1a . | HLA DRB3*0101 . | |
|---|---|---|
| Positive . | Negative . | |
| Positive | 43 (35%) | 1 (0.4%)* |
| Negative | 80 | 261 |
| Total | 123 | 262 |
Odds ratio = 140 (95% confidence interval 19 to 1035).
P < .00001 (Fisher's exact test).
Eight out of 33 women (25.8%) with antenatal antibodies to HPA-1a and an HPA-1a–positive infant were primigravidas. In only 1 woman (USN 230) had neonatal alloimmune thrombocytopenia been diagnosed in her previous child. No other cases reported affected infants, or unexplained ICH/IUT. Other women may have been exposed to the HPA-1a antigen during previous pregnancy (29), spontaneous or therapeutic abortion (15), or blood transfusion (2), with some having more than one risk factor. One woman (USN 487, para 3 + 1) underwent amniocentesis at 16 weeks, but antibodies to HPA-1a were present in the first sample sent at 8 weeks.
Thrombocytopenia in infants of alloimmunized and apparently nonalloimmunized women.
Of 26 HPA-1a–positive infants born to women with persistent antenatal antibodies to HPA-1a, 9 were severely thrombocytopenic (34.6%), including 1 (LR) not originally typed as HPA-1a negative (Table 3). Ten infants were mildly thrombocytopenic (38.4%), and 7 had normal platelet counts. Severe thrombocytopenia was not observed in association with transient or postnatal antibodies, although cord samples were not received from 1 infant in each category. Thrombocytopenia (39-149 × 109/L) in cord samples was found in 12 of 237 infants of HPA-1a–negative women in whom no HPA-1a antibodies were detected in the initial MAIPA. Supplementary testing of sera from these 12 women using both MAIPA with an alternative GPIIb/IIIa capture antibody (MoAb Y2.51) and the solid-phase GTI assay likewise failed to detect HPA-1a antibodies. All 12 women were HLA-DRB3*0101 negative. In three cases, another risk factor for thrombocytopenia was present (one case each with antibodies to HPA-5b, extreme prematurity and respiratory distress syndrome in a set of twins, and a GPIIb/IIIa antibody reactive with all panel cells). No other risk factors were identified in the remaining nine cases, but in all nine, the platelet count was normal on venous sampling by 1 week.
Cord Blood Platelet Counts for HPA-1a–Positive Infants of Alloimmunized Women and All Infants of Nonalloimmunized Women and Normal Controls
| Cord Platelet Count (109/L) . | Alloimmunized . | Nonalloimmunized . | Normal Controls . | ||
|---|---|---|---|---|---|
| Persistent Antibodies . | Transient Antibodies* . | Postnatal Antibodies† . | |||
| >150 | 7 | 4 | 3 | 237 | 200 |
| 50-150 | 10 | 1 | 2 | 10 | 0 |
| <50 | 9‡ | 0 | 0 | 22-153 | 0 |
| Total | 26 | 5 | 5 | 249 | 200 |
| Cord Platelet Count (109/L) . | Alloimmunized . | Nonalloimmunized . | Normal Controls . | ||
|---|---|---|---|---|---|
| Persistent Antibodies . | Transient Antibodies* . | Postnatal Antibodies† . | |||
| >150 | 7 | 4 | 3 | 237 | 200 |
| 50-150 | 10 | 1 | 2 | 10 | 0 |
| <50 | 9‡ | 0 | 0 | 22-153 | 0 |
| Total | 26 | 5 | 5 | 249 | 200 |
*In one case, cord platelet count was not determined.
In two cases, cord platelet count was not determined.
P = .00001 Severe thrombocytopenia in persistently alloimmunized versus non-alloimmunized women (Fisher's exact test).
Platelet counts, 39 × 109/L and 41 × 109/L.
In 25 HPA-1a–positive infants tested, the cord blood platelet count was highly associated with the third trimester titer of antibodies to HPA-1a in the MAIPA assay (Table 4). A titer of 1 in 32 or greater had a positive-predictive value for severe thrombocytopenia of 75% and a negative-predictive value of 88%. Antibody titers taken earlier in the pregnancy were not a reliable indicator of severe thrombocytopenia. There was no clear relationship between antibody titer and parity. Although six of eight women with high third trimester antibody titers were multiparous, this was also true of 10 of 17 women with low titer antibodies. Similarly, severity did not clearly correlate with parity, with 11 of 17 mildly/unaffected and 5 of 10 severely affected cases being multiparous.
Relation of Titer of Anti–HPA-1a at Different Stages of Pregnancy to Cord Blood Platelet Count for Antenatally Alloimmunized Women With HPA-1a–Positive Infants
| Gestation (weeks) . | n . | Cord Platelet Count (109/L) . | Antibody Titer . | P . | |
|---|---|---|---|---|---|
| ≥1 in 32 . | <1 in 32 . | ||||
| 30-38 | 25 | ≥50 | 2 | 15 | |
| <50 | 63-150 | 23-151 | .004 | ||
| 20-29 | 17 | ≥50 | 3 | 8 | |
| <50 | 5 | 1 | .050 | ||
| 12-19 | 13 | ≥50 | 4 | 5 | |
| <50 | 4 | 0 | .11 | ||
| Gestation (weeks) . | n . | Cord Platelet Count (109/L) . | Antibody Titer . | P . | |
|---|---|---|---|---|---|
| ≥1 in 32 . | <1 in 32 . | ||||
| 30-38 | 25 | ≥50 | 2 | 15 | |
| <50 | 63-150 | 23-151 | .004 | ||
| 20-29 | 17 | ≥50 | 3 | 8 | |
| <50 | 5 | 1 | .050 | ||
| 12-19 | 13 | ≥50 | 4 | 5 | |
| <50 | 4 | 0 | .11 | ||
P values determined by Fisher's exact test (two sided).
Includes 1 infant with ICH.
No other platelet alloantibodies.
Clinical outcome of alloimmunized pregnancies.
Forty-four of the 47 alloimmunized pregnancies resulted in live births at ≥36 weeks, with 19 spontaneous deliveries, 5 inductions of labor, and 14 Caesarean sections (overall Caesarean rate, 36%) among the 38 live births of babies to women with antenatal antibodies.
Three pregnancies ended in loss of the baby, one at 15 weeks (USN 302) and one as a neonatal death from immaturity after Caesarean section at 25 weeks for severe pre-eclampsia (USN 152, first pregnancy). The third, in a woman not originally typed as HPA-1a negative (SL), resulted in intrauterine death at 29 weeks due to hemorrhage from the cord after blood sampling for investigation of unexplained fetal hydrops. The baby's hemoglobin was 6.1 g/dL, with no detectable red-cell alloantibodies, and, unexpectedly, the platelet count was 6 × 109/L. The father was HPA-1a homozygous, and the titer of maternal anti–HPA-1a was 1:128.
Only one of the remaining nine severely thrombocytopenic infants of alloimmunized women showed evidence of major hemorrhage (infant of USN 347). This infant was delivered by emergency Caesarean section at 37 weeks because of an abnormal heart trace during labor. Cerebral ultrasound scanning on postnatal day one showed a large left occipitoparietal porencephalic cyst with encephalomalacia, consistent with previous hemorrhage. The development of hydrocephalus in this infant required a Rickman reservoir and, later, a ventriculoperitoneal shunt. He subsequently developed infantile spasms controlled on Vigabatrin (Hoechst Marion Rousel Limited, Uxbridge, UK), with peripheral hypertonia, delayed motor and social development, and mild optic atrophy. The remaining infants had petechiae and/or bruising but no other hemorrhage. Cerebral scans of all other babies of mothers with persistent antibodies to HPA-1a, including those with severe thrombocytopenia, as well as of 49 babies of nonalloimmunized DRB3*0101 positive women, were normal.
The stratified risk of HPA-1a alloimmunization and its complications for different patient populations are shown in Table 5.
Stratified Risk (95% Confidence Interval) of HPA-1a Alloimmunization and its Complications in At-Risk Populations
| Population (n) . | ||||||
|---|---|---|---|---|---|---|
| HPA-1a | NK | Negative | Negative | Negative | Negative | Negative |
| HLA-DRB34-1500101 | NK | NK | Positive | NK | Positive | Positive |
| Anti–HPA-1a | NK | NK | NK | Negative | Positive at any time | Persistently positive antenatally |
| Event | 25,379 | 387 | 123 | 341 | 46 | 32 |
| HPA-1a negativity | 1:40 (36.7-42.8) | |||||
| HPA-1a alloimmunization | 1:3474-150(282-451) | 1:8.4 (6.9-12.1) | 1:2.8 (2.3-3.7) | |||
| Thrombocytopenic infant4-151 | 1:3364-150 (250-509) | 1:8.6‡(6.5-12.7) | 1:4.9 (3.6-7.6) | 1:21‡ (13.4-46.3) | 1:2.1 (1.6-3.0) | 1:1.8 (1.4-2.7) |
| Severe thrombocytopenia4-153 | 1:11004-150 (684-2910) | 1:28‡ (18-73) | 1:15 (9.2-46.6) | 1:125‡ (52.3-∞) | 1:5.5 (3.4-14.7) | 1:3.9 (2.4-9.6) |
| Fetal hemorrhage | 1:15,4004-150 (5240-∞) | 1:385 (130-∞) | 1:123 (41.7-∞) | 0 | 1:44 (15.0-∞) | 1:31 (10.6-∞) |
| Population (n) . | ||||||
|---|---|---|---|---|---|---|
| HPA-1a | NK | Negative | Negative | Negative | Negative | Negative |
| HLA-DRB34-1500101 | NK | NK | Positive | NK | Positive | Positive |
| Anti–HPA-1a | NK | NK | NK | Negative | Positive at any time | Persistently positive antenatally |
| Event | 25,379 | 387 | 123 | 341 | 46 | 32 |
| HPA-1a negativity | 1:40 (36.7-42.8) | |||||
| HPA-1a alloimmunization | 1:3474-150(282-451) | 1:8.4 (6.9-12.1) | 1:2.8 (2.3-3.7) | |||
| Thrombocytopenic infant4-151 | 1:3364-150 (250-509) | 1:8.6‡(6.5-12.7) | 1:4.9 (3.6-7.6) | 1:21‡ (13.4-46.3) | 1:2.1 (1.6-3.0) | 1:1.8 (1.4-2.7) |
| Severe thrombocytopenia4-153 | 1:11004-150 (684-2910) | 1:28‡ (18-73) | 1:15 (9.2-46.6) | 1:125‡ (52.3-∞) | 1:5.5 (3.4-14.7) | 1:3.9 (2.4-9.6) |
| Fetal hemorrhage | 1:15,4004-150 (5240-∞) | 1:385 (130-∞) | 1:123 (41.7-∞) | 0 | 1:44 (15.0-∞) | 1:31 (10.6-∞) |
Abbreviation: NK, not known.
Extrapolated from actual observations.
Cord platelet count ≤150 × 109/L.
Based on 284 cord blood platelet counts.
Cord platelet count, <50 × 109/L.
Postnatal recovery.
The recovery of platelet counts in severely thrombocytopenic infants born to alloimmunized mothers was variable. Two infants rapidly recovered without platelet transfusion, with normal platelet counts by postnatal days 6 and 8, whereas a further three babies received a single infusion of HPA-1a–negative donor platelets and recovered normal platelet counts after 2, 4, and 10 days. Multiple platelet transfusions and intravenous immunoglobulin (Sandoglobulin 1 g/kg to 2 g/kg) were provided to the remaining three infants, whose platelet counts required 9, 47 (USN 421), and 82 (USN 347) days to recover. The delay in recovery of platelet counts may relate to the presence of intracerebral hemorrhage in USN 347, but is unexplained in USN 421.
DISCUSSION
Our study has provided baseline data on the natural history of HPA-1a alloimmunization in a cohort of 387 HPA-1a–negative pregnant women, equivalent to approximately 15,000 nonselected pregnancies. In contrast to previous studies with a smaller sample size, this study is of sufficient size to provide statistically reliable data on the incidence of alloimmunization and the effect of anti–HPA-1a antibody on the neonatal platelet count, unbiased by preselection of high-risk pregnancies. It must be considered whether the 75% of HPA-1a–negative women who enrolled for the study were in any way unrepresentative of the whole population. The predominantly white nature of the East Anglian population masks any small effect of ethnic bias, with frequencies of HPA-1a negativity and HLA-DRB3*0101 positivity in the study population as expected for whites. The proportion of multiparous women in the study population (55%) is as expected for this region (data from Rosie Maternity Hospital, Cambridge, UK). Finally, only 1 of the study population reported a previously affected child.
Fetomaternal alloimmunization to HPA-1a was apparent in approximately 1 in 350 pregnancies, corresponding to 11.4% of HPA-1a–negative women. This rate is higher than that observed in most previous studies (0%,16 1.4%,15 and 6%13) but similar to that in another study of 45 HPA-1a–negative women (10%).14
Although the association between HLA-DRB3*0101 positivity and HPA-1a alloimmunization has previously been observed in clinically apparent NAITP,25 the negative predictive value of greater than 99% has helped to clarify its potential usefulness in an unselected white population. We observed only one case of alloimmunization in a woman negative for this allele, as previously observed.5 Although the presence of the HLA-DRB3*0101 allele increased the risk of alloimmunization in HPA-1a–negative women by a factor of 140, its positive-predictive value as a single marker was only 35%.
Maternal parity was not highly predictive of alloimmunization, with 25% of antibody-positive cases in primiparous women, in whom antibodies were detectable as early as 17 weeks, consistent with development of antigens of fetal platelets26 and expression of the β3 integrin on syncytiotrophoblast.27 Previous pregnancy loss before 20 weeks of gestation also appeared to be an potentially immunizing event, with detection of antibodies early in the next pregnancy in two cases. Serial antibody titers showed considerable variation during the evolution of the antibody response during the course of the pregnancy, as previously observed.28 In some women, antibodies that were clearly present early in the pregnancy later became undetectable (transient antibodies), only to emerge again clearly in the postnatal period. The pattern of antibody response was not a reliable predictor of fetal HPA-1a type.
This is the first prospective study of sufficient size to be able to estimate the risk to the fetus due to the presence of maternal HPA-1a alloantibodies, in which there has not been a previously diagnosed sibling. Although the risk of severe disease in siblings of affected cases is high,29 the pathogenicity of HPA-1a alloantibodies in an unselected population is highly variable. First, 15% of babies born to HPA-1a–negative women are themselves HPA-1a negative, and thus unaffected. Second, even with persistent antenatal antibodies and an HPA-1a–positive fetus, a normal cord platelet count was observed in 7 of 26 cases (35%), and a ‘safe’ platelet count (between 50-150 × 109/L30) in a further 10 (38%). Neither cord platelet count nor final antibody titer could be reliably predicted by either parity or timing of immunization, but we have observed for the first time a significant association between severe thrombocytopenia and a third trimester antibody titer of 1:32 or greater using the MAIPA assay, with a positive-predictive value of 75%.
Although two previous studies have not found antibody titer to be a predictor of disease severity,31,32 they lacked the power of this larger series, whereas the second of these measured titers only in the postnatal period, and used an ELISA.32 Our findings are, however, consistent with previous data using the MAIPA technique, in which absorbance values (rather than titer) correlated with infant platelet count.28 Conversely, we observed two infants with mild thrombocytopenia born to mothers with high-titer antibodies; this phenomenon occurs in hemolytic disease of the newborn, and is possibly attributable to maternal IgG antibodies to paternal HLA class-II antigens on fetal monocytes.33 We found neither transient nor postnatal antibodies to be associated with severe thrombocytopenia, although one case classified as mild (USN 412, platelets 52 × 109/L) had postnatal antibodies only.
Thrombocytopenia in infants of HPA-1a–negative women without detectable HPA-1a antibodies is well recognized, and is generally assumed to be due to failure of antibody detection. We found mild thrombocytopenia in 13 infants (including 1 set of twins) of 249 HPA-1a–negative mothers (4.8%). Four of these 12 infants had other potential causes, including alloantibodies to HPA-5b34 and antibodies to GPIIb/IIIa. Extra efforts were made to detect HPA-1a antibodies in these women, using two additional antibody detection assays, but both assays gave negative results in all 12 cases. A point of note is that all 12 women were HLA-DRB3*0101 negative. This HLA distribution must cast some doubt on whether these cases were due to HPA-1a antibodies, because the importance of binding between β3-derived peptides and HLA-DRB3*0101 molecules in the immune response to HPA-1a has now been shown.35 It is of interest that we saw no antibody-negative thrombocytopenic cases in infants of HLA-DRB3*0101–positive women, as might have been expected. However, our observations do not exclude the possibility that in the eight unexplained cases, alloimmunization to other as yet unidentified platelet alloantigens play a role. A further possibility is spurious thrombocytopenia from cord sampling, as the 200 normal controls came, for unavoidable logistical reasons, from venous samples.
There was one fatality most likely due to HPA-1a alloimmunization in our study (patient SL), after diagnostic fetal blood sampling for hydrops fetalis. In this case, the severe fetal thrombocytopenia was unexpected, so platelets were not made available during the procedure. Another infant (of 38-weeks gestation) sustained an antenatal intracerebral hemorrhage (ICH). No additional cases of intracerebral hemorrhage were observed postnatally, but the preventive role of altered obstetric management (lower threshold for Caesarean section) combined with early postnatal platelet transfusion therapy cannot be excluded. There were no obvious predictors of intracerebral hemorrhage in the affected child, the third trimester antibody titer being 1 in 64, as in four other infants without ICH.
Antenatal screening for platelet-specific alloantibodies is not a standard of care for women with no previous history of NAITP. Several observations from this study will help in evaluating different strategies for cost-effectiveness and patient acceptability. For example, HLA DRB3*0101 genotyping could be used to exclude low-risk women from further antibody screening, thus reducing both unnecessary testing and parental anxiety. Antibodies detected before 20 weeks require confirmation with a later specimen, because early transient antibodies are of no clinical significance. Screening will be of value only if a treatment plan can be implemented that prevents ICH and fetal loss, with minimum harm to fetuses who may in any case be unaffected by maternal antibodies. Had infants of all antenatally immunized mothers in this study been subjected to fetal blood sampling, 23 of 33 infants would have been exposed unnecessarily to the risks of the procedure, some perhaps more than once. Even if women with transient antibodies and those with an HPA-1a–negative fetus had been excluded, 17 of 27 samplings would have been unnecessary. Apart from the risks to the fetus, the resource implications of such a strategy are considerable. Antenatal cerebral scanning to look for early ICH has not been evaluated as a means of preventing major bleeds. Postnatal screening has been advocated, measuring cord blood platelet counts in all neonates,36 but this has been shown not to prevent all cases of ICH.14 Although the laboratory costs of postnatal screening are less than antenatal testing,14 this strategy may cease to be cost-effective if the lifetime care costs of missed ICH cases are taken into account. Other problems with neonatal screening include potential nonavailability out of hours, and the observation that as many as 7% of cord samples are unsuitable for counting.14 The additional clinical benefit of detecting thrombocytopenia due to maternal autoantibodies36 remains to be quantified.
As yet, the best strategy for antenatal management of alloimmunized cases is unclear. Of the options for antenatal therapy, intravenous immunoglobulin is of unpredictable efficacy, whereas intrauterine platelet transfusion is associated with a small but finite risk of fetal loss, mainly due to cord hemorrhage (0.6% in one study37). Noninvasive management exposes fetuses to the risk of intruterine ICH or death. The knowledge that HPA-1a antibodies are present may also increase the incidence of Caesarean section (36% in this study from a baseline value of 15%). This has unproven benefit to the fetus and carries its own risks, albeit low, to the mother. Thus preselection of high-risk cases for fetal blood sampling remains in our view the ideal option. Our data on the predictive value of antibody titer offer the possibility of avoiding fetal sampling in the two-thirds of alloimmunized pregnancies with a safe platelet count. The exact role of the inhibitory effects of HPA-1a antibodies on platelet progenitors in culture38 and on the binding of ligands to GPIIb/IIIa in the clinical manifestations of NAITP remain to be elucidated.
The technical aspects of NAITP screening require further refinement, as shown by the initial mistyping of two cases. This phenomenon did not impact on the overall frequency of HPA-1a negativity, which was identical to that of a smaller UK study using a different typing assay.15 It is acceptable for screening assays to have a false-positivity rate, provided that a confirmatory test is available. This could be applied to HPA-1a typing, with the cut off set sufficiently high to prevent any HPA-1a–negative cases from being missed, and rapid confirmation of apparent negatives by an alternative assay. However, this is less than ideal, as is the need to prepare platelet rich plasma before typing, which reduces scope for automation and positive sample identification. The availability of a whole-blood typing assay for HPA-1a39 and a human monoclonal variable domain antibody fragment specific for HPA-1a40 may facilitate large-scale screening programs. As was the case with Rhesus D typing in antenatal screening programs, it is envisaged that the reliability of typing will improve once monoclonal reagents are introduced.
The optimal screening strategy for FAIT screening remains unclear. Future studies of screening will have to be sufficiently large to ensure that any true impact on long-term outcome can be statistically detected, and appropriately costed.
ACKNOWLEDGMENT
We thank the many obstetric, midwifery, paediatric, and blood bank staff who contributed to this study; C. Holmes, C. Milnes, S. Chisholm, A. Pollock, J. Walton, and R. Fagence for help with clinical liaison and provision of HPA-1a–negative platelets; R. Lambert, M. Regtuijt, C. Hurd, and the tissue-typing staff of West Midlands Blood Centre for laboratory support; and J-P. Allain for constructive criticism.
Supported by a grant from the East Anglian Regional Health Authority, Headington, Oxford, UK.
Address correspondence to Lorna M. Williamson, MD, Division of Transfusion Medicine, University of Cambridge, Long Road, Cambridge, CB2 2PT, UK; e-mail: lorna.williamson@nbs.nhs.uk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal