To the Editor:
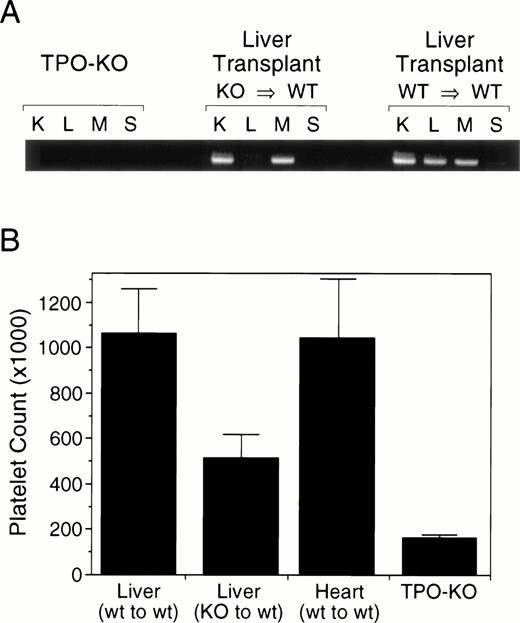
Maintenance of an appropriate number of circulating platelets is required for hemostasis and blood coagulation. The control of platelet production is mediated by thrombopoietin (Tpo) through its effect on the proliferation of megakaryocyte progenitors and on megakaryocyte ploidy.1,2 The levels of Tpo available to stimulate platelet production are thought to be directly regulated by the platelet mass itself.3 In this model, Tpois constitutively produced and released in the circulation, where it is being cleared by platelets through c-mpl–mediated binding.4 But, to date, the site(s) of Tpo production remains to be defined. Using Northern blot analysis, the liver and kidneys appear to be the major site of Tpo mRNA expression.5 However, using more sensitive methods, such as reverse transcription-polymerase chain reaction (RT-PCR), Tpo transcripts can be detected in most tissues, including the bone marrow stroma, which could produce Tpo directly in the megakaryocyte microenvironment. To evaluate the contribution of the liver to the Tpo production required to maintain a normal platelet count, we have generated tissue-specific knockout mice by transplanting the liver of Tpo-deficient mice6 into wild-type recipients.7 As shown in Fig1A, RT-PCR analysis of wild-type mice shows the presence of Tpo transcripts in all tissue analyzed, whereas there is no detectable transcript in any tissue from Tpo-KO mice. In wild-type mice transplanted with a Tpo-KO liver, the Tpo transcripts were absent from the liver only. Circulating Tpo levels were assessed by a c-Mpl–dependent proliferation assay5 and were below the detection limit in both wild-type and transplanted animals (data not shown). Blood cell counts analysis up to 12 weeks after the operation indicated that the circulating platelet levels of the transplanted mice were intermediate between those of normal and Tpo-KO mice, suggesting that the liver contributes approximately 60% of the Tpo required for maintenance of a normal platelet count (Fig 1B). All other blood parameters analyzed, including red blood cells and total white blood cells, were normal in animals transplanted with a wild-type or Tpo-KO liver.
RT-PCR analysis and platelet counts in liver specific Tpo-KO mice. Liver from Tpo-KO mice or wild-type littermates were transplanted in normal recipients, as previously described. (A) Tpo mRNA expression was analyzed in the kidney (K), liver (L), muscle (M), and spleen (S) by RT-PCR. (B) Platelet counts were measured from retroorbital bleeds 12 weeks after transplant; n = 6 mice per group.
RT-PCR analysis and platelet counts in liver specific Tpo-KO mice. Liver from Tpo-KO mice or wild-type littermates were transplanted in normal recipients, as previously described. (A) Tpo mRNA expression was analyzed in the kidney (K), liver (L), muscle (M), and spleen (S) by RT-PCR. (B) Platelet counts were measured from retroorbital bleeds 12 weeks after transplant; n = 6 mice per group.
Thrombocytopenia associated with liver cirrhosis has historically been attributed to hypersplenism; however, lower than normal platelet counts seem to persist in many patients, even after splenectomy or portal decompression.8,9 The present results suggest that thrombocytopenia associated with liver disease such as cirrhosis may be attributable to reduced Tpo production. This is consistent with the finding that Tpo levels in these patients are not elevated even with their reduced platelet count, suggesting a potential defect in Tpo production.10 We have previously shown using quantitative PCR that Tpo mRNA levels were reduced in cirrhotic liver.10The reduction in Tpo production may not be restricted to decrease of mRNA but may be combined with a posttranscriptional decrease in active protein, as has been documented for other liver-derived proteins.11 Our data demonstrate that the liver is the major site of Tpo production and that altered hepatic Tpo production will lead to a significant reduction in platelet levels. Secondary sites of Tpo production are likely to include the kidneys, which express significant amount of Tpo mRNA and are also responsible for erythropoietin production. The bone marrow stroma could provide part of the remaining Tpo, because it would be directly delivered in the megakaryocyte microenvironment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal