To the Editor:
Kaposi’s sarcoma-associated herpesvirus (KSHV) is involved in the pathogenesis of all forms of Kaposi’s sarcoma (KS).1 In acquired immunodeficiency syndrome (AIDS)-associated KS, KSHV detection in peripheral blood mononuclear cells increases with immunosuppression.2 Posttransplant KS are generally due to KSHV reactivation,3 and complete KS remission is often achieved after reduction or cessation of immunosuppressive therapy.4 Serologic studies have shown that 80% to 90% of KS patients have detectable antibodies against KSHV.5 These data clearly demonstrate that KSHV is under immunological control in KS patients.
Recently, KSHV was detected in long-term cultures of bone marrow stromal cells (BMSC) with a phenotype of dendritic cells (DC)6 and in bone marrow (BM) core biopsies from patients with multiple myeloma (MM).7,8 The physiopathological relevance of KSHV in this interleukin-6 (IL-6)–related disease could be that it encodes for a viral IL-6 (vIL-6) able to stimulate the growth of human MM cell lines.9 However, these results contradict what is known about KSHV infection and MM. Epidemiological studies show that KSHV and non-AIDS KS are found at higher incidence in Italy5 and that this is clearly not the case for MM.10 In addition, five groups reported a lack of antibodies against KSHV antigens in MM patients despite a normal humoral response to other herpesvirus.11-15 Finally, we and others were recently unable to found KSHV in DC samples obtained from apheresis cells of MM patients,16,17 and Masood et al14 failed to detect KSHV DNA in long-term BMSC cultures from MM patients. This discrepancy led us to explore the possibility that an extremely low level of KSHV infection in MM patients, leading to variable detection, may be reactivated during severe immunosuppression.
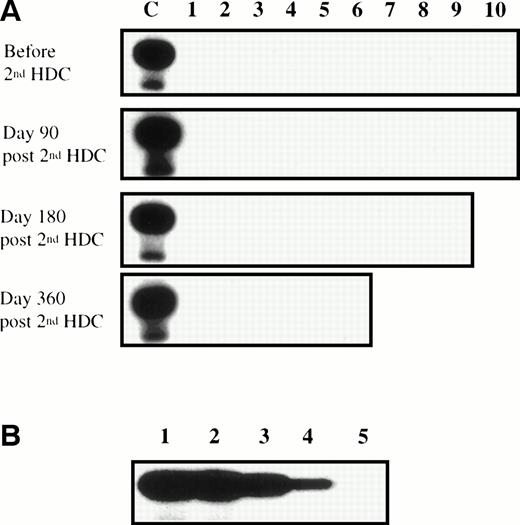
Ten patients with MM were treated with a double high-dose chemotherapy (HDC; 140 mg/m2 melphalan plus 8 Gy total body irradiation) supported by autograft with purified CD34+ cells (reinjection of 4.02 ± 1.03 × 106CD34+/kg; range, 2.88 to 5.73 × 106/kg). CD34+ progenitors were purified by the clinical-grade method from Cellpro (Bothell, WA), leading to a 35.6-fold enrichment in hematopoietic progenitors from a mean value of 2.4% ± 1.08% CD34+ cells (range, 0.99% to 3.47%) before purification to 85.4% ± 7.1% CD34+ cells (range, 72.4% to 92.8%) after purification. The resulting graft was 1,407-fold depleted of T cells (reinjection of 0.11 ± 0.08 × 106CD3+ cells/kg; range, 0.05 to 0.25 × 106/kg). Four of 10 patients relapsed within 1 year. The peripheral blood CD4+ T-cell count was monitored at 3, 6, and 12 months after the second purified autograft. Eight of 10 patients had less than 200 CD4+ cells/μL for at least 3 months, with a mean duration of 7 months for the 6 evaluable over 1 year (Table 1). Many infectious events arose during this first year after second HDC (median of 3 episodes per patient). In particular, 7 of 10 patients suffered from herpesvirus reactivation (Table 1). Because KS has rarely been associated with hematopoietic stem cell transplantation, no kinetic study was available; thus, repeated polymerase chain reaction (PCR) amplification was performed to detect KSHV in BM samples harvested before and 90, 180, and 360 days after the second HDC. KSHV DNA was monitored in 1 μg of genomic DNA (ie, 150,000 cells), in a blinded fashion and in two different laboratories, using a PCR assay against the KS330233 KSHV sequence.1 This sensitive method allowed the detection of KSHV DNA in less than 1 pg of genomic DNA from the KSHV-infected BCBL-1 cell line that corresponds to approximately 5 KSHV genome copies.16 18 KSHV DNA was not detected in any of the 35 BM samples tested (Fig 1). The lack of KSHV detection was not due to the presence of Taq polymerase inhibitors, in particular heparin, because the sensitivity of KSHV PCR was the same when assayed with either DNA harvested from heparinized BM mononuclear cells or DNA from cells collected without heparin (Fig 1).
CD4+ T-Cell Counts and Infectious Events in Autografted MM Patients
| Patient No. . | No. of CD4+ T Cells/μL . | Cumulative No. of Months With CD4+ T Cells <200/μL . | Infectious Events After Second HDC-150 . | |||
|---|---|---|---|---|---|---|
| Before First HDC . | Day 90 After Second HDC . | Day 180 After Second HDC . | Day 360 After Second HDC . | |||
| 1 | 892 | 170 | 291 | 266 | 4 | VZV, CMV |
| 2 | 361 | 91 | 11 | 351 | 6 | VZV, CMV |
| 3 | 350 | 95 | 150 | 146 | 8 | HZV, CMV |
| 4 | 315 | 327 | 307 | 234 | 0 | HSV |
| 5 | 259 | 77 | 75 | 88 | 12 | Other |
| 6 | 369 | 168 | 148 | 160 | 12 | CMV, Other |
| 7 | 430 | 140 | 149 | -151 | 11/11-152 | Other |
| 8 | 593 | 310 | ND | -151 | 0 | CMV |
| 9 | 124 | 64 | 199 | -153 | 6/6-152 | |
| 10 | 412 | 128 | -151 | -151 | 4/4-152 | CMV |
| Mean ± SD | 410.5 ± 207.5 | 157 ± 92.5 | 166.2 ± 99.8 | 207.5 ± 94.9 | ||
| Patient No. . | No. of CD4+ T Cells/μL . | Cumulative No. of Months With CD4+ T Cells <200/μL . | Infectious Events After Second HDC-150 . | |||
|---|---|---|---|---|---|---|
| Before First HDC . | Day 90 After Second HDC . | Day 180 After Second HDC . | Day 360 After Second HDC . | |||
| 1 | 892 | 170 | 291 | 266 | 4 | VZV, CMV |
| 2 | 361 | 91 | 11 | 351 | 6 | VZV, CMV |
| 3 | 350 | 95 | 150 | 146 | 8 | HZV, CMV |
| 4 | 315 | 327 | 307 | 234 | 0 | HSV |
| 5 | 259 | 77 | 75 | 88 | 12 | Other |
| 6 | 369 | 168 | 148 | 160 | 12 | CMV, Other |
| 7 | 430 | 140 | 149 | -151 | 11/11-152 | Other |
| 8 | 593 | 310 | ND | -151 | 0 | CMV |
| 9 | 124 | 64 | 199 | -153 | 6/6-152 | |
| 10 | 412 | 128 | -151 | -151 | 4/4-152 | CMV |
| Mean ± SD | 410.5 ± 207.5 | 157 ± 92.5 | 166.2 ± 99.8 | 207.5 ± 94.9 | ||
CD4+ T-cell count was monitored by flow cytometry.
Abbreviation: ND, not done.
Viral manifestation of varicella (VZV), zooster (HZV), herpes (HSV), other virus (Other), or cytomegalovirus antigen detection (CMV).
Patient died before evaluation.
CD4 count could be evaluated only during the indicated time.
Patient has not reached day 360 after HDC.
Lack of KSHV reactivation during treatment-induced immunosuppression in MM patients. (A) DNA samples extracted from BM before and 90, 180, and 360 days after second HDC were amplified by PCR using the KSHV 330233 primers. Each PCR was performed on 1 μg of genomic DNA and the amplification products were transferred to a nylon membrane and hybridized with a 32P end-labeled internal probe. The positive control (lane C) was the PCR product from the KSHV-infected BCBL-1 cell line. (B) Lanes 1 through 5 contain 10-fold dilutions of BCBL-1 DNA from 1 ng (lane 1) to 0.1 pg (lane 5). BCBL-1 DNA was diluted in the DNA extracted from heparinized BM mononuclear cells from patient no. 1 90 days after the second HDC, so that all PCR were run on 1 μg of total DNA. These data are representative of 10 experiments performed with the heparinized DNA samples extracted from the 10 patients 90 days after second HDC.
Lack of KSHV reactivation during treatment-induced immunosuppression in MM patients. (A) DNA samples extracted from BM before and 90, 180, and 360 days after second HDC were amplified by PCR using the KSHV 330233 primers. Each PCR was performed on 1 μg of genomic DNA and the amplification products were transferred to a nylon membrane and hybridized with a 32P end-labeled internal probe. The positive control (lane C) was the PCR product from the KSHV-infected BCBL-1 cell line. (B) Lanes 1 through 5 contain 10-fold dilutions of BCBL-1 DNA from 1 ng (lane 1) to 0.1 pg (lane 5). BCBL-1 DNA was diluted in the DNA extracted from heparinized BM mononuclear cells from patient no. 1 90 days after the second HDC, so that all PCR were run on 1 μg of total DNA. These data are representative of 10 experiments performed with the heparinized DNA samples extracted from the 10 patients 90 days after second HDC.
Three explanations may account for the discrepancy between the negative PCR with BM aspirates, the negative serological results, the lack of KSHV reactivation in immunosuppressed MM patients, and the positive PCR with stromal cultures and BM biopsies. (1) MM patients could be infected with a variant of KSHV that can escape the immune system or that encodes for antigens not recognized by the available immunological assays. This could explain the failure to detect anti-KSHV antibodies. (2) KSHV could be under a strict T-cell–mediated immune control in MM patients, leading to a very difficult detection by sensitive PCR. In this case, because infected cells remain undetectable in whole BM samples after double HDC and graft of purified CD34+ cells, one could hypothesize that this treatment has not destroyed anti-KSHV–specific CD4+ and CD8+ T cells, contrary to other anti-herpesvirus T cells. (3) KSHV could be not involved in MM patients, and its detection could be linked to false-positive PCR, as pointed out recently by Moore.19
These contradictions need to be elucidated, but our present results emphasize that, if KSHV or a variant of KSHV is really involved in MM, it is not a major factor in relapse occuring in immunosuppressed patients after autologous graft and raises the question of its causal role in MM.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal