To the Editor:
It was recently pointed out that platelet transfusion thresholds of 10,000 platelets/μL, as opposed to 20,000/μL, reduce platelet transfusions by approximately 20% in leukemic patients without increasing any major risk of bleeding.1Others2,3 have suggested that an even lower transfusion threshold of 5,000 platelets/μL may be sufficient. We believe that the main difficulties in determining the suitable threshold for platelet transfusion are presently caused by (1) the technical insufficiencies of conventional automated blood analyzers and (2) the existence of platelet-derived microparticles (or membranes) that can improve hemostasis.4 Without any consideration of these factors, determining platelet transfusion thresholds may remain costly guesswork.
(1) Most instruments used in laboratories to establish the platelet count do not recognize platelets but are only capable of counting particles within a defined size limit. Therefore, microcytic erythrocytes, schizocytes, cell debris, air bubbles, chemical precipitates, etc, mingle with electronic noise and true platelets, which, especially at low platelets levels and in the case of drug-induced leukemic cell destruction, can cause major errors in platelet counting.5,6 Rebulla et al,1 authors of a recent study, who chose a prophylactic transfusion level of 10,000 platelets/μL and not the lower 5,000 platelet/μL threshold, admitted doing so mainly because they could not trust the accuracy of platelet counts at low levels in automated blood analyzers.
We have shown in the past that the use of fluorescent platelet-specific antibodies (ie, anti-CD61, anti-CD41, or anti-CD42b) and multiparameter flow cytometry in establishing platelet counts can bypass the problems most blood analyzers have at low platelet levels.5Immunodetection of cells/plateletes with flow cytometry is no longer an elusive method but is part of everyday routine in many laboratories. Manufacturers of automated blood analyzers have recognized this new trend and are starting to integrate the immunodetection of cells/platelets into their routine repertoire. This will hopefully improve the accuracy of automated platelet counts in the future.
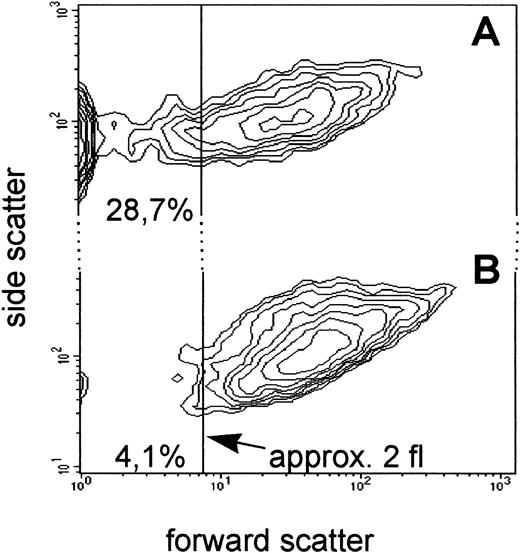
(2) Perhaps as important as the accurate enumeration of platelets is the identification of platelet-derived microparticles that fall below the normal platelet size-threshold of approximately 2 fL.6In a survey of 14 patients with acute myelogenous leukemia receiving myelotoxic chemotherapy, we observed that the levels of platelet-derived microparticles (defined as the percentage of CD61+/CD42b+ particles smaller than 2 fL; Fig1) in the same patient may vary from near 0% to 76% of the total platelet events. Levels of microparticles in normal controls (n = 10) ranged from 0.4% to 7.9% (mean, 4.8%). At the time of diagnosis, the percentage of microparticles often distinctly increased (up to 13-fold) above the normal mean. Bleeding episodes were not observed during chemotherapy when counts of normal-size (>2 fL) platelets were between 5,000 and 10,000/μl and microparticle levels were similar or higher than those of normal-size platelets. Bleeding episodes (gastrointestinal bleeding and hematuria) seen in 4 patients during chemotherapy were associated with reduced platelet microparticle levels (<2%) and total platelet events of 2,300, 2,700, 3,400, and 4,900/μL, respectively.
Flow cytometric analysis (contour plot) of platelets and platelet-derived microparticles in a patient with acute myelogenous leukemia (A) and a normal individual (B) using anti-CD61 and anti-CD42b antibodies. Whole blood was treated with platelet-specific fluorescent antibodies as described previously.5CD61+/CD42b+ events (n = 50,000), representing platelets and platelet-derived microparticles, were gated and are shown in forward and side scatter mode. Normal-size platelets (>2 fL) and platelet-derived microparticles (<2 fL) were defined as shown in the graph by a size-threshold of 2 fL, established with the help of fluorescent calibration beads.
Flow cytometric analysis (contour plot) of platelets and platelet-derived microparticles in a patient with acute myelogenous leukemia (A) and a normal individual (B) using anti-CD61 and anti-CD42b antibodies. Whole blood was treated with platelet-specific fluorescent antibodies as described previously.5CD61+/CD42b+ events (n = 50,000), representing platelets and platelet-derived microparticles, were gated and are shown in forward and side scatter mode. Normal-size platelets (>2 fL) and platelet-derived microparticles (<2 fL) were defined as shown in the graph by a size-threshold of 2 fL, established with the help of fluorescent calibration beads.
These observations indicate that a platelet transfusion threshold of 5,000/μL formerly suggested by Gmür et al2 (who used a time-consuming microscopic platelet counting method) is more appropriate than the 10,000/μL platelet threshold suggested recently by Rebulla et al1 provided that automated technology based on immunodetection is used for platelet counting. This may lead to a further considerable reduction in platelet transfusions and costs (despite the possible investment needed to update laboratory equipment). Furthermore, our results suggest that the favorable role of platelet-derived microparticles in hemostasis needs to be given more consideration in platelet transfusions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal