Abstract
Transgenic mice have been generated with expression of the herpes virus thymidine kinase gene directed by a 2.7-kb fragment of the IIb murine promoter of the gene encoding the IIb-subunit of the platelet integrin IIbβ3 (Tropel et al, Blood90:2995, 1997). Administration of ganciclovir (GCV) to these mice resulted not only in an acute cessation of platelet production due to the depletion of the megakaryocytic lineage, but also a decrease in erythrocyte and leukocyte numbers. Immunogold staining on ultrathin frozen sections and electron microscopy has now shown that the remaining population of immature hematopoietic cells contain a high proportion of Sca-1+ and CD34+ cells, with CD45R+ cells of the lymphopoietic lineage being maintained. Stromal cells were also preserved. Blood thrombopoietin levels were high. At 4 days of the recovery phase, Sca-1 and CD34 antigen expression decreased with intense proliferation of cells of the three lineages, with megakaryocyte (MK) progenitors being identified by their positivity for glycoprotein IIb-IIIa. These results suggest that transcriptional activity for the IIb gene promoter was present on pluripotent hematopoietic stem cells. At 6 to 8 days after cessation of GCV, numerous mature MK were observed, some of them with deformed shapes crossing the endothelial barrier through thin apertures. Proplatelet production was visualized in the vascular sinus. After 15 days, circulating platelet levels had increased to approximately 65% of normal. Transgenic IIb-tk mice constitute a valuable model to study in vivo megakaryocytopoiesis.
© 1998 by The American Society of Hematology.
MEGAKARYOCYTOPOIESIS starts with the commitment of pluripotent stem cells to the megakaryocytic lineage and continues towards platelet production through cell division, endoreplication, maturation, and fragmentation. Antibodies to platelet glycoprotein (GP) markers such as GPIIb have allowed the identification of small mononuclear cells corresponding to megakaryocytic progenitors not yet expressing the morphological characteristics of mature megakaryocytes (MK).1,2 During MK maturation, platelet glycoproteins such as GPIIb or GPIIIa can be detected in the colony-forming unit-MK (CFU-MK),3 and the expression of GP IIIa correlates with the disappearance of CD34, a progenitor cell marker.4,5 CD34+ GPIIIa− cells give rise to MK colonies after 11 days of maturation, whereas they can be observed as early as day 4 with a maximum at day 7, when CD34+ GPIIIa+ cells are plated. The latter cells have morphological characteristics of blast cells with a high nucleus to cytoplasm ratio.4 More recently, it has been reported that the addition of thrombopoietin (TPO), a cytokine described as essential for MK proliferation and maturation,6,7 reduces the time of maturation of CD34+CD38+ cells in culture to 7 days and allows the formation of proplatelets at the end of this period.8 However, although culture systems have led to an improved understanding of the differentiation process, the cells are maintained under artificial conditions that do not take into account the critical role of the bone marrow environment.
Recently, an inducible transgenic mouse model was developed in which megakaryocytopoiesis could be stopped and then restarted on demand. The promoter region of the human GPIIb gene was used to target the expression of the thymidine kinase (tk) toxigene in the MK.9 In these transgenic mice (HαIIb-tk), administration of the antiherpetic drug, ganciclovir (GCV), results in the elimination of cells expressing tk. MK production can be regulated by controlling the duration of GCV administration. Initial studies of HαIIb-tk transgenic mice indicated that GCV led to the eradication of the megakaryocytic lineage and of the erythroid precursors, suggesting that the GPIIb promoter was transcriptionally active in bipotent progenitor cells.9 More recently, a second type of transgenic mouse (MαIIb-tk) using the 2.7-kb regulatory elements of the murine GPIIb gene containing a longer part of the promoter region was created. This second model confirmed the results obtained with the human promoter and further indicated that GCV induced a complete obliteration of the earliest myeloid progenitors CFU-mix and colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM). These observations were consistent with a transcriptional activity of the αIIb promoter in a totipotent progenitor.10
In the present study, the bone marrow changes induced by GCV in mice transgenic for the tk gene driven by the regulatory elements of the murine αIIb promoter were investigated using electron microscopy and immunogold staining. When the platelet count was decreased by approximately 90%, cells of the MK lineage as well as the mature erythroid and the granulocytic cells were severely reduced in numbers. A pool of cells remaining in the bone marrow of these animals had an immature morphological appearance. Increased amounts of Sca-1+CD45R− mononuclear cells were observed when using a panel of antibodies directed to immature hematopoietic cell markers on ultrathin frozen sections. Such observations suggest an upregulation of immature hematopoietic cells as a result of the intense destruction of more mature cells. The fact that the stromal environment was maintained allowed us to investigate bone marrow recovery after discontinuing GCV and at a time when blood levels of TPO were increased. After 4 days, the number of cells staining positively for Sca-1, an early antigenic marker, was lower, whereas CD34+cells increased. Progressively, cells became more differentiated, and the migration of MK through the endothelial barrier, their fragmentation, and the formation of proplatelets in the vascular sinus were all observed.
MATERIALS AND METHODS
Production and GCV treatment of MαIIb-tk transgenic mice.
Transgenic animals (MαIIb-tk) were created with the transgene composed of the tk gene driven by the 5′ flanking region of the murine αIIb gene.10 The production and screening procedures have been detailed elsewhere.9 10 The antiherpetic drug, GCV, was administered daily to normal and transgenic mice by intraperitoneal injection. GCV doses were 0.1 or 0.05 mg/d/g body weight. Bone marrow samples were analyzed by electron microscopy for each of the following three groups of animals: (1) nontreated nontransgenic mice, (2) GCV-treated nontransgenic mice, and (3) GCV-treated transgenic mice. At least two bone marrow samples were analyzed at each time point during the different phases of treatment with GCV. In particular, bone marrow samples were analyzed when the platelet count was decreased by approximately 90% of normal values and during recovery up to the point when the platelet count had returned to 65% of normal values. This included samplings taken 48 hours, 3 days, and up to 15 days after discontinuing GCV (Table 1).
Effect of GCV on Blood Cell Counts in MIIb-tk Mice
| . | Platelets . | Erythrocytes . | Leukocytes . |
|---|---|---|---|
| Last stage of GCV treatment | 91.4 ± 3.7 | 71.1 ± 13.2 | 82.2 ± 6.9 |
| 48 h to 4 d into recovery | 91.2 ± 4.5 | 52.4 ± 20 | 48.3 ± 7.3 |
| 6 to 8 d into recovery | 62 ± 9.8 | 48 ± 1.2 | 34.6 ± 8.2 |
| 15 to 20 d into recovery | 35.7 ± 2.5 | 21.5 ± 7.7 | 22 ± 0 |
| . | Platelets . | Erythrocytes . | Leukocytes . |
|---|---|---|---|
| Last stage of GCV treatment | 91.4 ± 3.7 | 71.1 ± 13.2 | 82.2 ± 6.9 |
| 48 h to 4 d into recovery | 91.2 ± 4.5 | 52.4 ± 20 | 48.3 ± 7.3 |
| 6 to 8 d into recovery | 62 ± 9.8 | 48 ± 1.2 | 34.6 ± 8.2 |
| 15 to 20 d into recovery | 35.7 ± 2.5 | 21.5 ± 7.7 | 22 ± 0 |
Results represent the percentage decrease in blood cell counts (±SD) after 7 days of GCV administration (0.1 mg/d/g body weight), during the early stage of recovery (48 hours to 4 days after discontinuing GCV), and during the late phase of recovery (6 to 8 days and 15 to 20 days after GCV withdrawal).
Bone marrow fixation and preparation for electron microscopy.
Marrow was removed from the femur bones, with care taken so as not to disturb native structure. Samples were fixed in 1.25% (vol/vol) glutaraldehyde (Fluka AG, Buchs, Switzerland) diluted in 0.1 mol/L phosphate buffer (pH 7.2) for 1 hour at room temperature. After two washings in 0.1 mol/L phosphate buffer (pH 7.2), they were resuspended in the same buffer containing 0.2% (vol/vol) glutaraldehyde at 4°C.
Antibodies.
To characterize progenitor cells, we used rat monoclonal antibodies (MoAbs) directed against glycoproptein CD34 (clone RAM 34) and against the phosphatidylinositol-anchored protein Sca-1 (also termed Ly-6A/E; clone E13-161.7) (both purchased from Pharmingen, San Diego, CA). To identify cells belonging to the B-lymphocyte lineage, we used a rat MoAb directed against CD45R (Ly-5, B220; clone RM2600; Caltag Laboratories, Burlingame, CA). To detect T cells, a rat MoAb directed against CD90 (Thy-1; clone G7; Pharmingen) reacting with Thy-1.1 and Thy-1.2 alloantigens was used. To identify cells belonging to the megakaryocytic lineage, we used a rabbit antibody prepared against GPIIb-IIIa complexes purified from human platelets and cross-reacting with GPIIb-IIIa of mouse platelets (gift from Dr B Steiner, Hoffman-La Roche, Basel, Switzerland).
Standard electron microscopy and immunogold labeling on ultrathin cryosections.
For standard electron microscopy, fragments of washed bone marrow were postfixed in 1% (wt/vol) osmic acid containing 1.5% (wt/vol) potassium ferrocyanide (Sigma Chemical Co, St Louis, MO) for 1 hour at 4°C, dehydrated by graded alcohols and propylene oxide, and finally embedded in Epon (Taab Laboratories, Reading, Berks, UK), as previously described.11 Ultrathin sections were obtained with an Ultracut E ultramicrotome (Reichert, Vienna, Austria) and subsequently stained with uranyl acetate (Merck, Darmstadt, Germany) and lead citrate (Sigma).
For immunogold labeling, bone marrow samples were postfixed in 1.25% (vol/vol) glutaraldehyde for 1 hour at room temperature and, after washing, infused with 2.3 mol/L sucrose (Fluka) before being frozen in propane and then in liquid nitrogen with a Reichert KF 80 freezing system (Leica, Vienna, Austria). Ultrathin sections of approximately 70 nm were cut at -120°C with the Ultracut E ultramicrotome equipped with a FC 4E cryokit attachment and placed on collodion-coated nickel grids. Briefly, the grids were incubated for 10 minutes on drops of washing buffer consisting of phosphate-buffered saline (PBS) supplemented with 0.5% albumin and containing 0.02 mol/L glycine (Sigma). The grids were then incubated for 15 minutes on drops of PBS supplemented with fetal calf serum. Sections were next incubated with one of the previously described antibodies for 1 hour at room temperature. The grids were rinsed twice and incubated with appropriate secondary (goat antirabbit IgG or goat antirat IgG) gold-labeled antibody (1/50 dilution of AuroProbe EM G10; Amersham, Les Ulis, France). To avoid cross-reactions between antibodies of the same species during double-labeling, fixation with 1% (vol/vol) glutaraldehyde in PBS-albumin was performed between each round of staining, as previously described by Youssefian et al.12Finally, after three further washes in buffer and distilled water, the cryosections were stained by uranyl acetate and embedded in a thin film of methylcellulose before being examined in a Jeol JEM-1010 transmission electron microscope at 80 kV (Jeol, Croissy-sur-seine, France).
Spleen fixation and preparation for histological analysis.
Because the spleen is an important hematopoietic organ, spleens of normal and thrombopenic mice were histologically compared. The removed tissues were directly fixed in Bouin’s fixative for 24 hours and embedded in paraffin according to standard procedures. Sections were cut with a Reichert microtome (Leica), stained with hematoxylin and eosin, and observed under a Nikon Microphot-FX microscope (Nikon, Paris, France) at magnifications ranging from ×10 to ×100.
Thrombopoietin assay.
TPO levels were measured in anticoagulated ACD-A plasma from control and thrombocytopenic mice using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, the wells were coated with 8 μg/mL of an MoAb (clone 378120.211) to TPO used as a capture antibody (R&D Systems, Abingdon, UK). After coating overnight at room temperature, wells were incubated with 300 μL of PBS containing 1% (wt/vol) albumin, 5% (wt/vol) sucrose, and 0.05% (wt/vol) NaN3 (blocking solution). Volumes (100 μL) containing TPO standard, serum samples at different dilutions, or a blank solution were then added according to the manufacturer’s instructions. Incubation for 2 hours at room temperature was followed by washing with PBS containing 0.05% Tween-20, pH 7.4. Biotinylated detection antibody (R&D Systems) was added at 200 ng/mL diluted in Tris-buffered saline containing 0.1% albumin, 0.05% Tween-20, and 0.5% (vol/vol) heat-inactivated normal rat serum (Sigma). After 2 hours at room temperature, 100 μL horseradish peroxydase- conjugated streptavidin (dilution 1/1,600; Amersham) was added. Incubation was continued for 20 minutes at room temperature and, after washing, a mixture of H2O2 and tetramethylbenzidine (Sigma) was added. After 30 minutes, the reaction was stopped by adding 0.5 mol/L H2SO4 to each well; a yellow coloration represented a positive reaction. The optical density was determined within 30 minutes by a microtiter plate reader set to 450 nm.
RESULTS
Characterization of the bone marrow changes of MαIIb-tk transgenic mice treated with GCV for 7 days.
In initial experiments, standard electron microscopy was used to examine the effect of GCV on the bone marrow of normal mice. Mature MK were observed in their preferential location, ie, lying on the adventitial surface of endothelial cells delimiting the vascular sinus. Cells showing the different stages of MK maturation were normally represented. Figure 1a illustrates a mature MK in its normal environment, ie, surrounded by cells of the granulocytic lineage. In these bone marrow samples of nontransgenic GCV-treated mice, the different stages of all hematopoietic lineages were seen, showing that this drug per se did not induce any change in bone marrow cellularity. Erythroid and granulocytic lineages were abundant in the hematopoietic spaces. Administration of GCV to transgenic MαIIb-tk mice for 7 days reduced the platelet count to approximately 90% of normal values. A decrease in erythrocytes and leukocytes was also observed (see Table 1). Ultrastructural examination of the bone marrow showed the absence of maturing cells and that megakaryocytic, erythroid, and also granulocytic lineages were depleted (Fig 1b). In contrast, stromal cells and endothelial cells were not affected. Hematopoietic spaces were invaded by many large macrophages, with their processes extended among the few remaining MK and other hematopoietic cells (not shown). Interestingly, we noted an enhanced presence of mononuclear and poorly differentiated cells, suggesting an enrichment of immature cells (Fig 1b).
Electron micrographs illustrating the bone marrow of a normal mouse and a MIIb-tk transgenic mouse both treated with GCV. (a) A mature MK in a nontransgenic mouse surrounded by numerous cells belonging to the granulocytic lineage. An erythroblastic islet can also be seen in the bottom right hand corner. (b) MIIb- tk bone marrow after GCV treatment showing the intense disorganization of the marrow. Note the enhanced presence of small mononuclear cells (arrowheads) having a high nucleus-cytoplasm ratio. MK were absent. Bars = 5 μm.
Electron micrographs illustrating the bone marrow of a normal mouse and a MIIb-tk transgenic mouse both treated with GCV. (a) A mature MK in a nontransgenic mouse surrounded by numerous cells belonging to the granulocytic lineage. An erythroblastic islet can also be seen in the bottom right hand corner. (b) MIIb- tk bone marrow after GCV treatment showing the intense disorganization of the marrow. Note the enhanced presence of small mononuclear cells (arrowheads) having a high nucleus-cytoplasm ratio. MK were absent. Bars = 5 μm.
Identification of the hematopoietic cells remaining in the bone marrow of GCV-induced thrombocytopenic mice.
Immunogold staining of frozen ultrathin sections of marrow from MαIIb-tk mice was performed using MoAbs directed against immature hematopoietic markers such as anti-Sca-1 and anti-CD34. Among the mononuclear cells, we found that a population of small size (from 4 to 7 μm in diameter) with a high nucleus/cytoplasm ratio and a round nucleus with peripheral chromatin condensation were labeled with a MoAb directed against Sca-1 (Fig 2a). The labeling was present on the plasma membrane of the cells. Approximately 20% of the mononuclear cells were Sca-1+, a considerably higher percentage than in normal marrow, in which Sca-1+cells were extremely rare. It should be noted that the proportion of small mononuclear cells in the depleted mice corresponds to approximately 40% of the total bone marrow cell population. Therefore, it can be calculated that the proportion of Sca-1+ cells represents approximately 8% of the total depleted bone marrow cell population.
Immunogold labeling of ultrathin sections of cells remaining in the depleted bone marrow of the transgenic MIIb-tk mice after GCV treatment. Labeling was performed using rat MoAb to murine markers and bound IgG were shown by goat IgG coupled to gold particles. (a) A typical small Sca-1+ cell (4 to 7 μm diameter) with a high nucleus/cytoplasm ratio. Sca-1 labeling (arrowheads) is visible on the plasma membrane of the cell. (b) A CD34+cell labeled on the plasma membrane (arrowheads) also presents the morphological features of an immature cell. (c and d) Double-labeling for Sca-1 and CD45R. Sections were first incubated with MoAb directed against Sca-1 whose binding was shown by goat antibody to rat IgG coupled to 10-nm gold particles. After rapid fixation, sections were then incubated with MoAb directed against CD45R whose binding was shown by goat antibody to rat IgG coupled to 5-nm gold particles. An example of a Sca-1+ CD45R− cell is illustrated in (c). Only 10-nm gold particles (arrowheads) showing the presence of the Sca-1 antigen are present on the plasma membrane of this cell. In (d) is shown an example of a Sca-1− CD45+ cell present in the same preparation. The labeling is intense for CD45R and numerous 5-nm gold particles (arrowheads) are exclusively found associated with the plasma membrane of this cell. Bars = 0.5 μm.
Immunogold labeling of ultrathin sections of cells remaining in the depleted bone marrow of the transgenic MIIb-tk mice after GCV treatment. Labeling was performed using rat MoAb to murine markers and bound IgG were shown by goat IgG coupled to gold particles. (a) A typical small Sca-1+ cell (4 to 7 μm diameter) with a high nucleus/cytoplasm ratio. Sca-1 labeling (arrowheads) is visible on the plasma membrane of the cell. (b) A CD34+cell labeled on the plasma membrane (arrowheads) also presents the morphological features of an immature cell. (c and d) Double-labeling for Sca-1 and CD45R. Sections were first incubated with MoAb directed against Sca-1 whose binding was shown by goat antibody to rat IgG coupled to 10-nm gold particles. After rapid fixation, sections were then incubated with MoAb directed against CD45R whose binding was shown by goat antibody to rat IgG coupled to 5-nm gold particles. An example of a Sca-1+ CD45R− cell is illustrated in (c). Only 10-nm gold particles (arrowheads) showing the presence of the Sca-1 antigen are present on the plasma membrane of this cell. In (d) is shown an example of a Sca-1− CD45+ cell present in the same preparation. The labeling is intense for CD45R and numerous 5-nm gold particles (arrowheads) are exclusively found associated with the plasma membrane of this cell. Bars = 0.5 μm.
The cellularity of these marrow samples was reduced to approximately 35% of the levels present in normal bone marow. Because the Sca-1+ cells expressed the morphological characteristics of immature cells, the presence of CD34 was also examined. CD34+ cells were present but were less abundant than those labeled for Sca-1+. As shown in Fig 2b, these cells exhibited similar morphological characteristics of primitive and immature cells. To assess the proportion of B lymphocytes in the population of Sca-1+ cells, double-staining was performed with an antibody against CD45R. We found that the majority of the Sca-1+ cells were negative for CD45R. A Sca-1+CD45R− cell is shown in Fig 2c. Only a few cells were found to be positive for both markers. The depleted bone marrow also contained a population of CD45R+ but Sca-1−cells, implying that the B-lymphocyte lineage was not affected by GCV treatment (Fig 2d). Overall, these results indicated that a majority of Sca-1+ cells did not belong to the B-lymphocyte lineage and that they presented characteristics of immature hematopoietic cells. Little labeling was seen using the MoAb anti-Thy-1 directed against T cells, thus excluding the possibility that the Sca-1 cells express high levels of this antigen (data not shown). All of these results suggest that a large proportion of the cells remaining in the depleted marrow correspond to immature hematopoietic cells.
Bone marrow characteristics during the early recovery phase.
To investigate the capacity of the mononuclear cells in the depleted marrow to be transformed into more mature hematopoietic cells, we examined bone marrow samples from transgenic mice at different times after discontinuing the GCV treatment. Few modifications of the bone marrow or blood cell counts were observed during the first 48 hours. Sca-1+ cells continued to represent approximately 8% of the bone marrow cells.
Figure 3 shows the major modifications in bone marrow cellularity that were characteristic of days 3 and 4 after discontinuing GCV. In addition to mononuclear cells, we now observed cells of the granulocytic and erythroid lineages. However, mature MK were still rarely found, showing that the recovery of this lineage was slower than that of the others. Although the platelet count remained low, erythrocyte and leukocyte counts were now improved (Table 1). The marrow morphology had become reorganized and now appeared compact (Fig3a). The number of invading macrophages had decreased. The stromal cells appeared well preserved and endothelial cells were seen to be normally delimiting the vascular sinus (Fig 3b). A large number of cells in mitosis were observed, implying an intense proliferation (Fig3c through e). The percentage of Sca-1+ mononuclear cells had returned to the level found in normal bone marrow. In contrast, the concentration of CD45R+ cells remained the same. An increased number of CD34+ cells was observed. Overall, our results suggest that, at this stage, there is an explosion of maturing hematopoietic cells concommitant with the disappearance of the Sca-1 marker. Using antibodies directed against the platelet GPIIb-IIIa complex, we detected the presence of MK precursors that had yet to take on the morphological characteristics of mature MK. Numerous cells of 10 to 15 μm in diameter with a high nucleus/cytoplasm ratio were labeled on the plasma membrane with polyclonal antibody directed against GPIIb-IIIa (Fig 4). In some of these MK precursors, a few α-granules were present and GPIIb-IIIa was already and specifically distributed in their membrane.
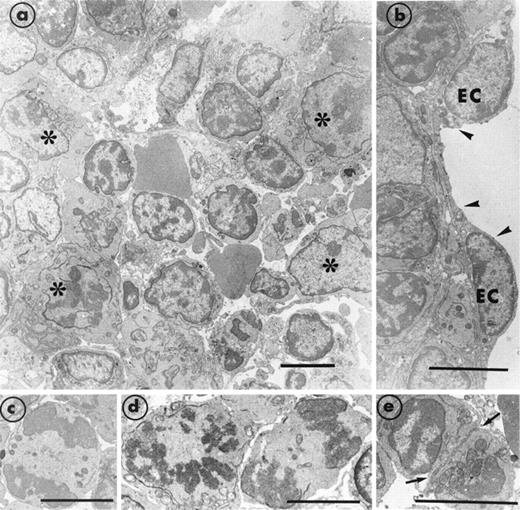
Section of bone marrow obtained from a transgenic MIIb-tk mouse 4 days after discontinuing GCV treatment. (a) The bone marrow has become reorganized and compact. Numerous maturing cells (*) are present. However, mature MK were still rarely observed. (b) A well-preserved endothelial cell (EC) is delimiting a vascular sinus (arrowheads). (c through e) Typical examples of cells in mitosis and in division (arrows) illustrate the intense cell proliferation. Bars = 5 μm.
Section of bone marrow obtained from a transgenic MIIb-tk mouse 4 days after discontinuing GCV treatment. (a) The bone marrow has become reorganized and compact. Numerous maturing cells (*) are present. However, mature MK were still rarely observed. (b) A well-preserved endothelial cell (EC) is delimiting a vascular sinus (arrowheads). (c through e) Typical examples of cells in mitosis and in division (arrows) illustrate the intense cell proliferation. Bars = 5 μm.
Two illustrations of MK progenitors characterized by immunogold staining with polyclonal antibody reacting with murine GPIIb-IIIa on ultrathin frozen sections of MIIb-tk bone marrow 4 days after discontinuing GCV. The size of these MK progenitors is between 10 to 15 μm in diameter. (a) Example of an immature MK having a high nucleus/cytoplasm ratio and a thin cytoplasmic rim. Some staining for GPIIb-IIIa is visible on the plasma membrane (arrowheads) and on the membrane of the few -granules present (arrows). (b) In this immature MK characterized by the presence of GPIIb-IIIa on the plasma membrane (arrowheads), the gold particles are also associated with internal membranes possibly representing a developing demarcation membrane system (arrow). Bars = 0.5 μm.
Two illustrations of MK progenitors characterized by immunogold staining with polyclonal antibody reacting with murine GPIIb-IIIa on ultrathin frozen sections of MIIb-tk bone marrow 4 days after discontinuing GCV. The size of these MK progenitors is between 10 to 15 μm in diameter. (a) Example of an immature MK having a high nucleus/cytoplasm ratio and a thin cytoplasmic rim. Some staining for GPIIb-IIIa is visible on the plasma membrane (arrowheads) and on the membrane of the few -granules present (arrows). (b) In this immature MK characterized by the presence of GPIIb-IIIa on the plasma membrane (arrowheads), the gold particles are also associated with internal membranes possibly representing a developing demarcation membrane system (arrow). Bars = 0.5 μm.
TPO evaluation.
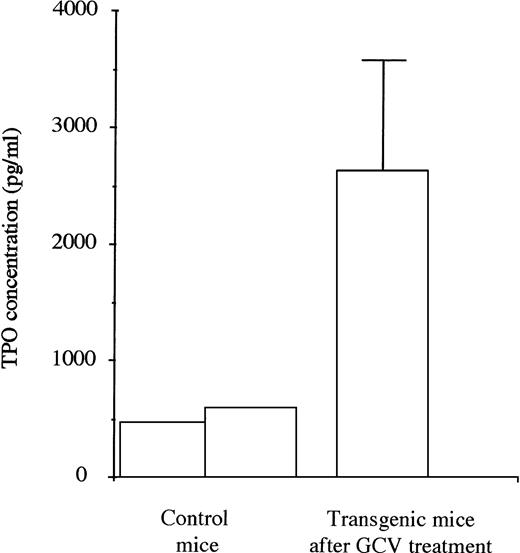
TPO levels were first evaluated in plasma taken from 2 control mice. They were then measured in plasma taken from a series of 5 transgenic mice during the 4 days subsequent to the eradication of the MK lineage after 1 week of treatment with GCV (Fig 5). The mean TPO levels were increased by 5 to 6 times in the treated mice showing stress-recovery conditions.
TPO levels in plasma of untreated control (2) and transgenic mice (5) in the 4 days after the stopping of GCV treatment. For the transgenic mice, high levels of TPO were found when the platelet count and the MK mass were severely decreased.
TPO levels in plasma of untreated control (2) and transgenic mice (5) in the 4 days after the stopping of GCV treatment. For the transgenic mice, high levels of TPO were found when the platelet count and the MK mass were severely decreased.
Characterization of bone marrow cells during the period of intense proliferation.
Eight days after discontinuing GCV, the platelet count had returned to 40% of normal values, whereas after 15 days, the platelet count was approximately 65% of initial levels (see Table 1). Examination of the bone marrow at 8 days showed an intense hematopoiesis. We focused our attention on megakaryocytopoiesis. The MK concentration was significantly increased when compared with the marrow of control mice. MK were often located in clusters close to each other. The mature MK were larger than the MK found in normal marrow. They had an usual distribution of α-granules and demarcation membrane systems. The distribution of GPIIb-IIIa complexes on mature MK was normal, indicating that not only do the platelet counts return to initial values, but also that GPIIb-IIIa synthesis was also normal after discontinuing GCV (results not shown). As megakaryocytopoiesis was enhanced, the frequency of MK crossing the endothelial barrier was higher and fragmentation into proplatelets was often observed. A characteristic of MK crossing the endothelial barrier was the intense deformation of their shape. Figure 6a shows an example of a very mature MK with the cell and also the nucleus, exhibiting a deformed shape with a narrowing at the point of passage between two endothelial cells. In the vascular sinus, the fragmentation of the cytoplasm and the nucleus can be seen. Constrictions were present delimiting the platelet territories. Figure 6b schematizes these findings, showing the passage of MK from the marrow compartment to the vascular sinus.
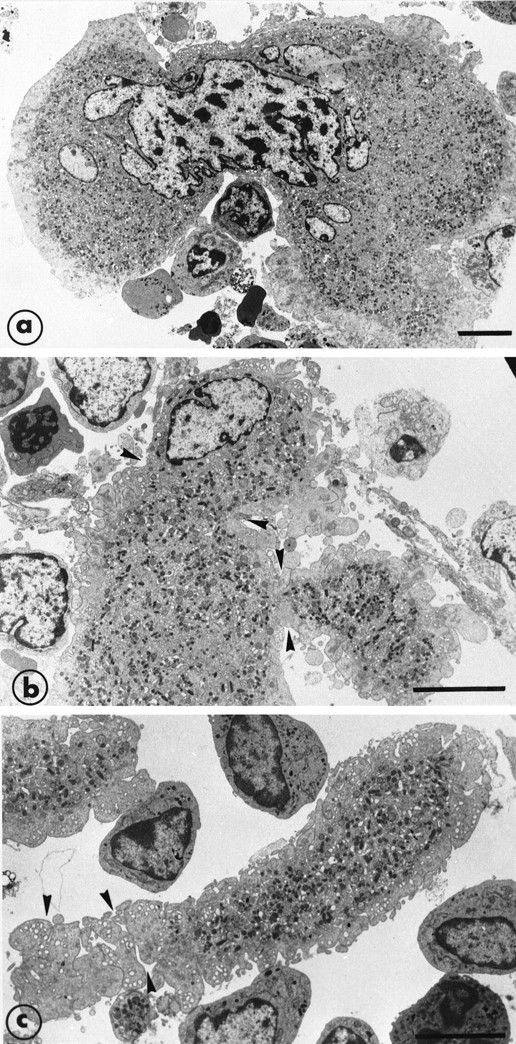
After 8 days of recovery, the bone marrow was enriched in mature MK that were sometimes to be seen crossing through the endothelial barrier. (a) This MK has a highly deformed shape with half of the nucleus and cytoplasm inside the bone marrow and the other part in the vascular sinus delimited by endothelial cells. There is a zone of strangulation due to the small aperture of the endothelial barrier. In the vascular compartment, the cytoplasm is fragmented into proplatelet fragments on which constrictions (arrowheads) seem to delineate the platelet territories. (b) The cartoon schematizes the high capacity for shape deformation of the MK crossing through a very small aperture of the endothelial barrier. Bars = 5 μm.
After 8 days of recovery, the bone marrow was enriched in mature MK that were sometimes to be seen crossing through the endothelial barrier. (a) This MK has a highly deformed shape with half of the nucleus and cytoplasm inside the bone marrow and the other part in the vascular sinus delimited by endothelial cells. There is a zone of strangulation due to the small aperture of the endothelial barrier. In the vascular compartment, the cytoplasm is fragmented into proplatelet fragments on which constrictions (arrowheads) seem to delineate the platelet territories. (b) The cartoon schematizes the high capacity for shape deformation of the MK crossing through a very small aperture of the endothelial barrier. Bars = 5 μm.
Figure 7a, illustrates another example of the capacity of MK to change their shape and to migrate through the very small apertures of the endothelial barrier. In some MK, the cytoplasm showed constrictions that appeared to delineate large proplatelet territories (Fig 7b). Separated large fragments were observed only in the vascular compartment (Fig 7c).
Illustration of the step leading to the final maturation of MK from the deformation of the cytoplasm to the separation of proplatelet fragments. (a) A further example of cytoplasm and nucleus deformation of a large mature MK. (b) An extremity of a mature MK shows deformation and constriction zones (arrowheads) and the presence of projections suggesting the subsequent and early separation of the proplatelet fragments. (c) An elongated proplatelet fragment separated from the cytoplasm. As indicated by arrowheads, this particular zone is fragmenting into platelet-size fragments and is still surrounded by the organelle-free zone. Bars = 5 μm.
Illustration of the step leading to the final maturation of MK from the deformation of the cytoplasm to the separation of proplatelet fragments. (a) A further example of cytoplasm and nucleus deformation of a large mature MK. (b) An extremity of a mature MK shows deformation and constriction zones (arrowheads) and the presence of projections suggesting the subsequent and early separation of the proplatelet fragments. (c) An elongated proplatelet fragment separated from the cytoplasm. As indicated by arrowheads, this particular zone is fragmenting into platelet-size fragments and is still surrounded by the organelle-free zone. Bars = 5 μm.
Megakaryocytopoiesis in the spleen in GCV-treated transgenic mice.
Examination of sections of spleens showed major differences between those of GCV-treated normal and transgenic mice. During treatment, the spleens of transgenic animals were disorganized, with a diminution of the red pulp corresponding to hematopoietic tissue. For the transgenic mice, when platelet counts were at their lowest, the number of MK per section was much decreased compared with control mice (Table 2). During bone marrow recovery, the number of MK in the spleen increased, showing that, as observed in the bone marrow, spleen hematopoiesis was impaired during GCV treatment and then recovered after GCV was discontinued.
Evaluation of Platelet Number and Spleen MK Count in Control Mice and Transgenic Mice During and After GCV Treatment
| . | Mean Decrease in Platelet Count (%) . | Mean No. of MK Per Spleen Section . |
|---|---|---|
| Control mice treated with GCV | Unchanged | 20 ± 6 |
| Transgenic mice last stage of GCV treatment | 93% | 1 ± 1 |
| 48 h to 6 d into recovery | 78% | 2 ± 1 |
| 15 to 20 d into recovery | 40% | 16 ± 1 |
| . | Mean Decrease in Platelet Count (%) . | Mean No. of MK Per Spleen Section . |
|---|---|---|
| Control mice treated with GCV | Unchanged | 20 ± 6 |
| Transgenic mice last stage of GCV treatment | 93% | 1 ± 1 |
| 48 h to 6 d into recovery | 78% | 2 ± 1 |
| 15 to 20 d into recovery | 40% | 16 ± 1 |
Each group was composed of 2 mice; the mean number of MK was evaluated on a minimum of 6 sections per spleen.
DISCUSSION
In the present report, the morphological features of the bone marrow of MαIIb-tk mice were examined during and after GCV administration. After 7 days of GCV treatment, there was a profound disorganization of the marrow associated with an increased concentration of macrophagic cells. The MK lineage and, to a lesser extent, the mature cells of the erythroid and granulocytic lineages were deficient. Using immunogold labeling, a proportion of about 8% of the remaining bone marrow cells were shown to be Sca-1+ cells, most of which were CD45R− and did not express Thy-1. The CD34+cells were less numerous, suggesting that the majority of cells were very immature.
Before characterizing the remaining cells more intensively, an important point was to determine whether the bone marrow changes were not simply the result of the effect of the GCV itself. Bone marrow samples of nontransgenic GCV-treated mice were therefore examined. We found that they were unchanged compared with nontreated control marrow, indicating that GCV had no toxic effect on bone marrow cellularity at the dose used in these experiments. Furthermore, we have previously reported that the phosphorylated and active form of GCV did not diffuse randomly from transgene expressing cells to control cells.10 In mixed cultures of bone marrow cells from control animals and transgenic mice, the GCV toxicity was restricted to cells expressing the transgene. Finally, the fact that the remaining cell populations showed specific antigenic characteristics indicated that there was not a random destruction process. Overall, our results suggest that the toxic effect of GCV was restricted to cells expressing the transgene.
A specific subpopulation of the marrow cells in the GCV-treated transgenic mice expressed Sca-1 and had morphological characteristics of undifferentiated cells. These Sca-1 cells were of small size (∼4 to 7 μm in diameter), with a high nucleus/cytoplasm ratio and a round nucleus with peripheral chromatin condensation. Their cytoplasm contained large mitochondria and these cells resembled the Sca-1+ stem cells isolated from the peripheral blood of mice by Yamamoto et al.13 The Sca-1 antigen, also termed Ly-6A/E, is a phosphatidylinositol-anchored protein that represents a convenient marker for primitive hematopoietic stem cells, particularly in mice expressing the Ly-6b haplotype.14 The mice used in our study resulted from a mating between two mouse strains, C57BL and DBA/2, both expressing the Ly-6bhaplotype. Sca-1 combined with other markers such as Thy-1 and c-kit is always found to be expressed on immature hematopoietic cells.15 16
Because B and T lymphocytes also express the Ly-6A/E molecule, we performed a double-staining using MoAbs directed against Sca-1 and CD45R to recognize the B-lymphocyte lineage or against the T-cell marker, Thy-1, a marker of T cells. The results of the double-staining showed that the majority of the Sca-1+ cell populations did not express either of these antigenic markers, confirming that they corresponded to immature hematopoietic cells, as suggested by their morphological features. The proportion of Sca-1+ cells was increased compared with normal bone marrow, suggesting an upregulation process of these cells in association with the inhibition of hematopoiesis due to the toxic effect of GCV. Such a regulation was previously shown by Nishio et al,17 in which the administration of 5-fluorouracil resulting in the elimination of proliferating progenitors increased the proportion of Sca-1+ cells, reaching a maximum after 3 days.
The present results that show the destruction of the megakaryocytic lineage but also of maturing cells of granulocytic and erythroblastic lineages and the enrichment of immature hematopoietic cells such as Sca-1+ cells, and to a lesser extent CD34+cells, suggest that hematopoiesis was discontinued at an early stage of differentiation. They also imply the transcriptional activity of the GPIIb promoter in very immature hematopoietic cells, probably in cells expressing CD34 but not in Sca-1+ cells. Several studies have shown that CD34 is coexpressed with a megakaryocytic marker such as GPIIIa on MK progenitors.4,5 Furthermore, our observations are in agreement with the results previously obtained by Tropel et al10 showing that the addition of GCV to bone marrow cultures of transgenic mice in vitro inhibits the growth of primitive progenitors cells possessing megakaryocytic, erythroid, and myeloid potential such as CFU-GEMM, cells that are known to express CD34+.18 These results are also consistent with previously published observations of Fraser et al19 showing that pluripotent hematopoietic stem cells reacted with antisera containing anti-GPIIb and anti-GPIIIa activities.
The transgenic mice used in this study expressed high amounts of tk, resulting in the inhibition of three hematopoietic lineages. In transgenic mice expressing tk under the control of the human αIIb promoter, with low levels of tk, only platelet and erythrocyte counts were found to be decreased.9 In these bone marrow samples, the number of immature hematopoietic Sca-1+ cells were also increased but to a lower extent than in MαIIb-tk mice (results not shown).
Bone marrow reorganization showed significant signs of proliferation 4 days after stopping the GCV (Fig 3). The decrease in the number of the Sca-1+ cells and the enrichment of CD34+ cells confirmed that GCV treatment had affected a population of cells expressing CD34. According to Morel et al,20 15% of Sca-1+ cells from normal bone marrow express low levels of CD34, and this population is more immature than those expressing Sca- 1+CD34bright, implying that CD34 antigen is a later marker than Sca-1. The studies of Osawa et al21 are in favor of this hypothesis, because it was demonstrated that the CD34low/−c-Kit+Lin−Sca-1+population is capable of long-term reconstitution, whereas CD34+c-Kit+Lin−Sca-1+cells are capable of short-term reconstitution. This observation also can explain the enhanced presence of CD34+ cells 4 days after the arrest of the inhibition of hematopoiesis. Finally, after 6 to 8 days of recovery, the bone marrow was very active, with the different lineages abundantly present. That GCV induced a depletion of marrow cells followed by a progressive restitution of all the bone marrow lineage confirms that the Sca-1+ cells remaining during GCV administration represented a population of very immature hematopoietic cells with the capacity of multilineage repopulation.
The eradication and the recovery of hematopoietic cells observed in our model may be compared with the bone marrow depletion seen after 5-fluorouracil treatment of mice. In both cases, cessation of treatment was followed by increased cell proliferation observed after 4 days.17 Nevertheless, in our model, the depletion concerned preferentially the MK lineage with a 90% decrease in platelet count. In the 5-fluorouracil model, the decrease in platelet count was less, possibly explaining why the return to normal platelet values occurred earlier (6 to 11 days after a single injection of 5-fluorouracil)22,23 compared with the 15 to 20 days necessary to reach 65% of the initial platelet level in our study. In models in which bone marrow primitive Lin−Sca-1+ cells were transplanted into lethally irradiated mice, the time of bone marrow reconstitution was longer, between 2 and 3 months.21,24,25 To explain these differences in the recovery time, it should be noted that, in the case of toxigene eradication, even if the remaining cells were very immature in their majority, the population was more heterogeneous. Furthermore, these cells were already in their environment, whereas for transfused cells, their homing into bone marrow is an additional necessary step. It should also be noted that the stromal cells in the toxigene model were preserved,26 whereas irradiation can modify bone marrow stroma.27 28
In fact, the large amounts of TPO that we have observed can also explain the relatively rapid recovery under the stress conditions of the toxigene model. TPO alone or in combination with other cytokines acts on the proliferation of hematopoietic progenitors.29-31 This cytokine can also speed up the process of cell maturation.22,32,33 About 8 days after discontinuing GCV, we observed in the bone marrow an enrichment in mature MK of large size. Some of them with deformed shape were crossing the endothelial barrier and were producing platelets. In the presence of an increased concentration of TPO, Cramer et al8 showed that the time of MK differentiation and maturation from human CD34+CD38+ progenitors was shortened to 7 days in culture. During the thrombocytopenic phase, the TPO level was greatly upregulated, consistent with the fact that the TPO level is directly regulated by the platelet and megakaryocyte mass.34-36 Increased amounts of TPO have been shown to increase the size, ploidy, and number of MK in the marrow.22,37,38 Since the discovery of TPO, numerous studies have described platelet formation in vitro.8,39 Our model provided us with the opportunity to observe this last step of MK fragmentation in vivo. Migration of entire cells with a highly deformed shape crossing through a small aperture of the endothelial barrier was observed as previously described.40 We did not see the very long thin pseudopods extending from mature cells as described in vitro, showing that the process is not totally equivalent.8,41Proplatelet formation was shown to be initiated during the passage of cells through the endothelial barrier. Nevertheless, it seems that, as described in vitro,8 some constriction zones observed in the cytoplasmic extension separate the platelets. We also found large fragments not yet individualized into platelets; perhaps disruption of these fragments in the pulmonary capillary circulation explains the alternative theory of platelet production as suggested by Trowbridge,42 because the whereabouts of their fragmentation into platelets is still a matter of debate.
In conclusion, the morphological and immunological study of bone marrow of the transgenic mice showed the destruction of the MK lineage associated with the inhibition of erythroid and granulocytic lineages and the enrichment in immature hematopoietic cells after treatment with GCV. From these observations, we imply that the transcriptional activity of GPIIb was present not only in MK, but also in more immature cells having the potential capacity to differentiate into erythroid and granulocytic lineages. This activity is downregulated in the more mature cells of these lineages. The reconstitution of the bone marrow cells after stopping GCV is associated with the high proliferation of cells of the MK lineage; their increased number makes it possible to study the different phases of MK maturation, including platelet production. This model would also be useful to investigate the distribution of other glycoproteins during megakaryocytopoiesis, including the vitronectin receptor (αvβ3) as already studied by us.43
ACKNOWLEDGMENT
The authors thank Dr B. Steiner for providing the polyclonal antibody to GPIIb-IIIa complexes used in this study. We also acknowledge the technical advice of Eric Labouerye during the examination of the spleens. We thank Amgen for providing financial support for measuring TPO levels.
Supported by funding from the CNRS, Université de Bordeaux II (MRESN), the Conseil Régional d’Aquitaine. C.P. was financed through a doctoral scholarship from the French Groupe d’Etudes d’Hémostase et Thrombose (from Synthelabo) and currently receives a doctoral grant from the Société Française d’Hématologie.
Address reprint requests to Paquita Nurden, MD, UMR 5533 CNRS, Hôpital Cardiologique, 33604 Pessac, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal