GENETIC IRON OVERLOAD disorders are prevalent yet poorly understood. Over the past 3 years, the discoveries of the hereditary hemochromatosis gene and an intestinal iron transporter have significantly advanced our understanding of iron metabolism. Furthermore, they have suggested novel links between molecules involved in immune defense and iron homeostasis. These findings lay the foundation for developing novel strategies for understanding, diagnosing, and treating patients afflicted with iron overload disorders.
NORMAL IRON METABOLISM
All mammalian cells have an absolute requirement for iron, most likely because iron is abundant, comprising 5% of the earth’s crust, and versatile, existing in two interconvertible redox states. Our Paleolithic ancestors enjoyed a rich meat diet, high in readily absorbable heme iron. Iron deficiency would have been a rare disorder for early humans.1 However, dietary iron deficiency likely became prevalent with the development of agriculture and the consequent decrease in intake of animal products over the past 10,000 years. Hereditary hemochromatosis (HHC), a disorder characterized by inappropriately high intestinal iron absorption, may have conferred a selective advantage in an era when dietary iron was relatively scarce.2 Twentieth century industrialization has been accompanied by a return to increased meat consumption1 and an increase in life expectancy. Under these circumstances, iron loading is no longer an advantage, and HHC has become a prominent disease.
Most iron in mammals exists either as heme, present in heme proteins, or as ferritin, a mobilizable storage form. Only a small fraction enters and leaves the body on a daily basis. Most iron is recycled from the breakdown of effete red blood cells by macrophages of the reticuloendothelial system (RES). At any given time, approximately 0.1% (3 mg) of total body iron circulates in an exchangeable plasma pool. In normal individuals, essentially all circulating plasma iron is bound to transferrin (Tf). The mechanism(s) by which Tf acquires iron from intestinal absorptive cells and RES cells is unknown. Tf is a powerful chelator, binding iron with a dissociation constant of 1022 mol/L−1.3 This chelation serves three purposes: it renders iron soluble under physiologic conditions, it prevents iron-mediated free radical toxicity, and it facilitates transport into cells. Radioactive tracer studies indicate that at least 80% of the iron bound to circulating Tf is delivered to the bone marrow.4 However, when the iron binding capacity of plasma Tf is saturated, as in patients with HHC, the excess non-Tf bound iron is rapidly taken up by hepatocytes and other cells.
Iron is taken into erythroid cells by receptor-mediated endocytosis of Tf (reviewed in Harford et al5). Specific receptors (TfRs) on the outer face of the plasma membrane bind diferric-Tf with high affinity. Once internalized, endosomes are acidified to pH 5.5 to 6.0 through the action of an ATP-dependent proton pump.6-9Endosomal acidification weakens binding of iron to Tf and produces conformational changes in both Tf and TfR, strengthening their association.10,11 Iron release may also be facilitated by a plasma membrane oxidoreductase.12 13 The apo-Tf-TfR complex is recycled back to the plasma membrane, where apo-Tf is discharged, thereby completing an elegant and efficient cycle. Previously, it was not clear how iron exited from the transferrin cycle endosome. However, recent results have given new insights into this process and demonstrated a surprising link between the Tf cycle and intestinal iron absorption (see below).
Iron homeostasis is regulated strictly at the level of intestinal absorption. In normal individuals, intestinal iron absorption is influenced by body iron stores, hypoxia, and erythropoietic activity. The signals regulating absorption remain obscure. Oxidation state, intraluminal pH, and ancillary nutrients such as ascorbic acid can affect the efficiency of uptake. Ferrous iron is most efficiently absorbed. A ferric reductase present on duodenal microvillus membranes may promote absorption by converting dietary ferric iron to the ferrous form.14,15 The intestinal iron transport system also transports cobalt and manganese, the transition metals that flank iron in the periodic table.16 17
HHC
Since the late 1800s, the clinical triad of skin hyperpigmentation, hepatic cirrhosis, and adult onset diabetes has been recognized as evidence of iron overload. It is now clear that HHC is the most prevalent genetic disease in individuals of northern European descent. Patients with this disorder chronically absorb a small excess of iron, and middle-aged homozygotes frequently have 10 times normal body iron stores. As iron stores exceed the body’s capacity for effective chelation, free iron accumulates. Unbound iron is highly toxic, owing to its participation in the generation of free radicals and reactive oxygen intermediates. These molecules provoke peroxidation of membrane lipids leading to cellular injury, ultimately resulting in severe damage to the liver, heart, joints, and endocrine organs. Liver damage may be exacerbated by alcohol consumption or viral hepatitis. The onset of the disease is insidious, and the initial manifestations are often nonspecific symptoms, including fatigue and arthropathy. If untreated, hemochromatosis invariably progresses and is ultimately fatal. However, if diagnosed before end stage organ damage, phlebotomy is an effective and life-saving treatment. Red blood cells are removed at regular intervals to deplete iron stores, and bone marrow erythropoiesis compensates effectively.
The cloning of the causative gene in HHC represents a true tour de force of modern molecular biology. In the mid-1980s the fortuitous discovery of an increased frequency of the HLA-A3 allele in hemochromatosis patients quickly allowed investigators to demonstrate linkage between the phenotype and the HLA complex on human chromosome 6p.18 However, despite this lucky start, nearly 12 years elapsed before the gene was found. Premier human genetics labs around the world devoted years to the effort, but were foiled by severe linkage disequilibrium in the candidate genomic region. Finally, using a novel approach, a small biotech company, Mercator Genetics (now called Progenitor, Menlo Park, CA), found a compelling candidate gene.19
On the basis of the HLA-A3 association, the Mercator group assumed a strong “founder effect” in hemochromatosis. They postulated that a unique mutation had occurred in the remote past in one ancestor common to most (but not all) affected patients. On the basis of this assumption, they analyzed polymorphic genetic markers throughout a large candidate region to determine which alleles were most frequently present in hemochromatosis patients. Assuming that the original mutation arose on a single chromosome from a single individual, it should be associated with a particular set of alleles of nearby polymorphic markers. They took advantage of the fact that alleles of markers close to the gene were more likely to have been maintained in modern hemochromatosis patients, whereas alleles of more remote markers would have changed through historical recombination events. They performed this analysis looking first at the frequency of occurrence of polymorphic alleles of each marker. Only later did they examine the pattern of alleles in each individual (haplotype), using the haplotype analysis as a means of deducing historic recombination events. This strategy allowed a more precise localization of the causative gene than had previously been possible using linkage studies alone, because information could be assimilated from a large number of individuals who were not known to be related to each other. After narrowing the candidate interval to 250 kb, the Mercator group performed an exhaustive screen for genes within the region, using cDNA selection, exon trapping, and direct genomic sequencing. A single gene emerged as the only plausible candidate for the disease gene. It encodes a protein, HFE (originally called HLA-H), that resembles atypical HLA class I molecules, consistent with the localization of the gene near the HLA cluster.19 This explained an earlier finding that mice lacking β2-microglobulin, a protein associated with HLA class I molecules, developed iron overload resembling that of patients with hemochromatosis.20,21 However, how an atypical class I-like molecule could affect iron homeostasis remained a mystery. Recently, targeted disruption of the HFE gene has provided confirmation of the identity of this gene as the HHC locus and proved that HHC results from a loss of protein function. Zhou et al22 found that HFE knockout mice develop iron overload with features similar to human HHC patients.
Two point mutations have been found within HFE in hemochromatosis patients, but only one of these (Cys282Tyr) has been definitively associated with hemochromatosis and corresponds to the founder mutation.19,23-25 It is thought to have arisen in a single individual, approximately 60 to 70 generations in the past.26 It is most prevalent in individuals of western European descent and rare in individuals of African descent. The discovery of the Cys282Tyr mutation in HFE has had enormous impact on the diagnosis of hemochromatosis, offering a simple polymerase chain reaction (PCR)-based genotyping assay that will identify most (but not all) patients unambiguously. This will complement the use of iron saturation measurements and supplant HLA typing in the majority of HHC patients and allow identification of young homozygotes and heterozygous carriers without biochemical evidence of iron overload. However, it is still important to consider the entire clinical context as a significant proportion of hemochromatosis patients lack the Cys282Tyr mutation, and individuals have been reported who are homozygous for Cys282Tyr but do not show clinical evidence of hemochromatosis.27
A second HFE polymorphism, His63Asp, has been found in more diverse ethnic backgrounds and may represent an older mutation.28Its clinical significance is less clear, and there are conflicting data as to whether it contributes to iron overload in Cys282Tyr/His63Asp compound heterozygotes (reviewed in Cuthbert29).
The functional role of HFE in iron metabolism remains obscure, but several recent clues have emerged. The crystal structure of normal HFE protein strongly resembles that of class I MHC molecules. Similar to class I molecules, HFE interacts with β2-microglobulin through a domain termed α3 that resembles an Ig constant-like domain.30 However, in contrast to true class I proteins, HFE is not capable of binding short peptides, because the portion of the protein similar to the peptide binding groove in class I molecules has been narrowed. Studies in cells transfected with HFE expression constructs have shown that the Cys282Tyr mutation interferes with binding of β2-microglobulin and consequently prevents normal membrane localization of HFE.31 32 This suggests that normal HFE attenuates iron absorption through some activity on the cell surface.
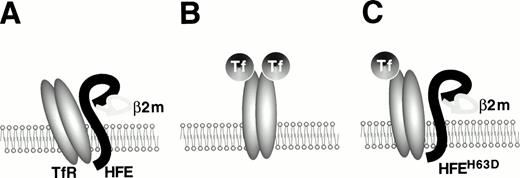
Three recent studies have reported evidence of a physical interaction between HFE and TfR. TfR was initially found to colocalize with HFE protein by immunohistochemical analysis of human placental syncytiotrophoblasts and to associate with the HFE/β2-microglobulin complex.33 This was confirmed by a more complete biochemical analysis that indicated that normal HFE protein bound to TfR and decreased its affinity for diferric transferrin, whereas Cys282Tyr HFE did not bind to TfR.34 Interestingly, the His63Asp mutant form of HFE also bound to TfR, but did not decrease its affinity for transferrin. This is the first functional evidence that the His63Asp mutation is physiologically significant. Finally, HFE protein covalently linked to a biosensor chip has a high measured affinity for binding TfR at pH 7.5, similar to the pH of the cell surface, but not at pH 6.0, which is similar to the pH within transferrin cycle endosomes.30 A complex forms with a stoichiometry of one TfR homodimer per HFE molecule. The stoichiometry of transferrin in the quarternary complex has not yet been determined. Figure 1 summarizes our current understanding of HFE/TfR interactions.
The TfR-HFE complex. The structure of the ternary complex formed by transferrin, TfR, and HFE has not yet been elucidated fully. This cartoon represents our current understanding. (A) Wild-type HFE protein, associated with β2-microglobulin, binds to TfR with high affinity and decreases binding of diferric transferrin.34(B) C282Y HFE protein does not associate with β2-microglobulin and therefore is not expressed in mature form on the cell surface, leaving TfR free to bind transferrin. (C) H63D HFE protein is expressed on the cell surface, but it does not decrease TfR affinity for transferrin to the same extent as wild-type HFE does.
The TfR-HFE complex. The structure of the ternary complex formed by transferrin, TfR, and HFE has not yet been elucidated fully. This cartoon represents our current understanding. (A) Wild-type HFE protein, associated with β2-microglobulin, binds to TfR with high affinity and decreases binding of diferric transferrin.34(B) C282Y HFE protein does not associate with β2-microglobulin and therefore is not expressed in mature form on the cell surface, leaving TfR free to bind transferrin. (C) H63D HFE protein is expressed on the cell surface, but it does not decrease TfR affinity for transferrin to the same extent as wild-type HFE does.
Despite this progress, it is still not known how HFE regulates iron transport. It is unlikely that HFE acts as an iron transporter directly. It was formerly believed that dietary iron entered the intestinal cell through the transferrin cycle. This hypothesis has been disproved experimentally. The transferrin gene is not expressed in intestinal cells, and transferrin found in the lumen is derived from plasma.35 It is unlikely that plasma transferrin entering bile performs a chelating function in the intestinal lumen, because it is fully saturated with iron before it leaves the biliary system.36 Furthermore, hypoxia, which greatly increases iron absorption, has no effect on intestinal transferrin levels.37 Experiments indicate that endogenous transferrin cannot donate iron to intestinal mucosal cells, and TfRs have been found on basolateral surface, rather than at the brush border membrane.38 39
However, it is possible that TfR is involved in the transfer of iron across the basolateral (serosal) surface of the absorptive, epithelial enterocyte. Intestinal cells show regulated expression of basolateral TfR that increases in iron deficiency and decreases in iron overload.40 However, surface TfR activity is most prominent in crypt cells and decreases with differentiation of the absorptive epithelium. More differentiated cells demonstrate a redistribution of TfRs from the surface to intracellular sites.41 This makes it unlikely that the TfR is directly responsible for basolateral iron transfer.41 At least two interpretations remain that are not mutually exclusive. It is possible that TfRs are important for supplying iron to rapidly developing enterocyte precursors in the crypts. Alternatively, they may serve to transduce some sort of signal from the body to the future absorptive cells to communicate the need for iron absorption. This latter interpretation is particularly interesting in light of the recently discovered interaction of HFE and TfRs.
Several lines of evidence indicate that patients with HHC have increased iron transfer at the basolateral surface of the absorptive cell.42-44 A larger portion of a oral radiolabeled iron dose is retained in HHC patients than in normal individuals. This indicates that iron taken up by the enterocytes is efficiently transferred to the plasma and not held within the enterocytes as ferritin, to be lost through mucosal sloughing. These findings suggest that mutations in HFE somehow promote basolateral iron export, perhaps because of a failure to interact with the TfR.
However, it remains to be shown that normal HFE interacts with TfR in intestinal cells. The HFE/TfR interaction has been demonstrated in the placenta and in transfected cells; in both cases, TfR was expressed at very high levels. Furthermore, normal HFE protein was detected in an intracellular, paranuclear compartment in small intestine cells, and it was exclusively found in the crypts.45 Although it is intriguing to postulate that HFE interacts with TfR in intestinal cells in a regulatory fashion, presumably to transduce information from circulating mediators as to the state of body iron stores, it is not clear that HFE and TfR are present in the same subcellular location.
IDENTIFICATION OF THE INTESTINAL IRON TRANSPORTER, Nramp2 (DCT1)
Another big piece in the iron overload puzzle came 1 year after HFE was discovered, when two groups, using two quite different approaches, simultaneously reported the identification of an intestinal iron transporter. Both used iron-deficient rodents as experimental models.
Microcytic anemia mice (gene symbol mk) have an autosomal recessive defect in iron metabolism that includes marked impairment of intestinal iron transport.46-48 They have no abnormalities in Tf, TfR, ferritin, or the RNA binding iron regulatory proteins. The phenotype suggested that the causative mutation was likely to be in an intestinal transporter functioning at the brush border.48-50 Using a positional cloning strategy, Fleming et al51 localized the mk mutation to theNramp2 gene on mouse chromosome 15 and found a glycine to arginine (Gly185Arg) missense mutation unique to animals with themk phenotype. Nramp2 had been an orphan protein with no known function cloned based on its homology to Nramp1, a molecule involved in macrophage host defense.52 53
In parallel experiments, Gunshin et al54 searched for an intestinal iron transporter using a Xenopus oocyte expression cloning system and found a single cDNA that stimulated iron transport. This cDNA encoded rat Nramp2 (referred to as DCT-1 in their report). They went on to show that Nramp2(DCT1) transported a variety of other heavy metals as well, including cobalt, manganese, lead, zinc, and copper, and that protons were cotransported. These data concurred with earlier physiology studies that indicated that the human intestinal iron transporter also transports other metals.16,17 55 Iron-deficient rats showed a dramatic increase in the amount of Nramp2(DCT1) mRNA in small intestine enterocytes, consistent with a role for Nramp2(DCT1) as a major iron transport protein. Taken together, the genetic and biochemical characterizations of Nramp2(DCT1) make a compelling argument that it is the primary intestinal transporter involved in apical iron uptake.
Mammalian cells transfected with Nramp2 cDNA expression constructs have markedly increased iron uptake as compared with control transfectants. Transfection of Nramp2 cDNA carrying themk mutation confers minimal iron uptake capability, indicating that the Gly185Arg mutation is highly deleterious to Nramp2(DCT1) function.56 Surprisingly, a second mutant animal with autosomal recessive iron deficiency, the Belgrade rat (gene symbolb), also carries a mutation in Nramp2(DCT1).57 Homozygous mutant b rats have a well-characterized defect in the transferrin cycle in erythroid cells, as well as defective intestinal iron transport.58-64 Their phenotype resembles that of mk mice in having defects at both levels, but the intestinal abnormality was better studied in mkanimals, and the erythroid abnormality was better understood inb animals. The identification of a mutation within the b Nramp2 gene confirms that Nramp2(DCT1) is not only important in intestinal iron absorption, but also for erythroid use (Fig 2). Nramp2(DCT1) functions in export of iron from Tf cycle endosomes. Remarkably, the b mutation results in exactly the same amino acid change (Gly185Arg) as themk mutation. This alteration in Nramp2(DCT1) protein seems to be the unique cause of the mk-like phenotype in rodents.
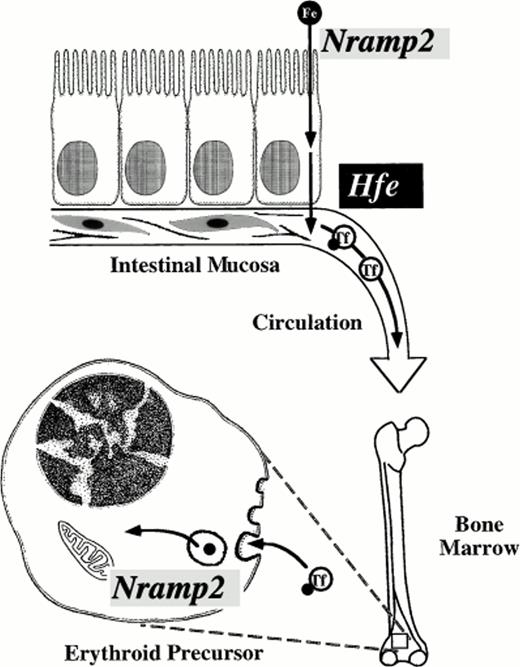
Critical steps in mammalian iron transport. Key iron transport steps are diagrammed, showing the route dietary iron follows from the intestinal lumen to the cytoplasm of an erythroid precursor cell. Nramp2(DCT1) acts as an iron transporter at two steps. It is required for transfer of iron across the intestinal brush border and for export of iron from transferrin cycle endosomes in the bone marrow. The function of Hfe is not well understood, but it appears to regulate basolateral iron transfer from enterocyte to plasma. Transferrin (Tf) chelates circulating iron in plasma.
Critical steps in mammalian iron transport. Key iron transport steps are diagrammed, showing the route dietary iron follows from the intestinal lumen to the cytoplasm of an erythroid precursor cell. Nramp2(DCT1) acts as an iron transporter at two steps. It is required for transfer of iron across the intestinal brush border and for export of iron from transferrin cycle endosomes in the bone marrow. The function of Hfe is not well understood, but it appears to regulate basolateral iron transfer from enterocyte to plasma. Transferrin (Tf) chelates circulating iron in plasma.
The mk and b phenotypes are severe, suggesting that Nramp2(DCT1) function is essential for normal intestinal iron transport, at least in mice and rats. It has not yet been determined whether Nramp2(DCT1) expression or activity is altered in patients with HHC. There is compelling evidence that Nramp2(DCT1) acts as the apical transporter that brings iron into enterocytes, and HHC seems to affect basolateral iron transport (Fig 2). However, it is possible that HHC patients have increased Nramp2(DCT1) function consequent to more rapid export of iron from enterocytes or as a result of an altered regulatory mechanism. This will need to be investigated. However, whether or not HHC alters Nramp2(DCT1) function, the mk and b animal models suggests that Nramp2(DCT1) would be an ideal target for pharmacological blockade of iron absorption. It may be possible to develop drugs that could be administered orally to specifically block Nramp2(DCT1) function in patients with iron overload disorders. At present, hemochromatosis patients must undergo phlebotomy every few weeks to maintain a slightly iron-deficient state. Perhaps an oral, nonabsorbable agent might be developed that could inhibit Nramp2(DCT1) without systemic side effects. Such a drug could offer a significant improvement in the quality of life for patients with hemochromatosis. It might also find uses in treating siderosis associated with thalassemia and other metal intoxication disorders such as lead poisoning.
FUTURE DIRECTIONS
Although great strides have been made towards understanding iron transport and associated disorders, many questions remain. Despite intensive investigation and its tantalizing association with the TfR, it is still unclear how HFE functions in normal individuals. To date, only two functionally significant mutations in HFE have been discovered; is it possible that additional mutations will be found that contribute to iron overload or iron deficiency disorders? What is the etiology of iron overload in patients without Cys282Tyr mutations?
Nramp2(DCT1) is not only expressed in intestinal cells; it is expressed in most if not all tissues and seems to be particularly actively expressed in certain brain structures.54 What role does it play in iron uptake in the central nervous system? Does its function contribute to the pathology associated with increased metal deposition in patients with Parkinson’s disease, Alzheimer’s disease, and other conditions?65 Are there human patients with mutations in Nramp2(DCT1)?
It now seems likely that the role of Nramp1 in host defense also involves movement of metal ions. Nramp1 is expressed exclusively in macrophages and is localized to phagolysosomes.53,66 Normal Nramp1 causes an attenuation of replication of a variety of ferrophilic intracellular pathogens, including several Mycobacteriaspecies, Leishmania, and Salmonella typhimurium. A point mutation in Nramp1 leads to rampant pathogen proliferation, but no alteration of normal host killing mechanisms.53 Nramp1 carrying this mutation is undetectable in macrophages.66 These observations strongly suggest that Nramp1 may act by removing a necessary metal, presumably iron, from the intracellular compartment containing the invaders, thus starving them of an essential nutrient.
What function does Nramp1 serve in normal, uninfected cells? Intriguingly, it is expressed exclusively in reticuloendothelial macrophages. These cells play a fundamental role in iron metabolism, by phagocytosing effete red blood cells, breaking down their hemoglobin, and recycling iron to transferrin for delivery back to the erythron.67 The bulk of iron used for erythropoiesis has passed through this recycling pathway. Perhaps Nramp1 plays a special role in this process. Accordingly, it has been established that various inflammatory cytokines that are associated with anemia of chronic disease68 also affect the regulation of Nramp1.69 One might speculate that the failure of normal iron recycling in anemia of chronic disease might be a consequence of alterations in the expression or activity of Nramp1. This needs to be investigated.
Nramp2(DCT1) is abundantly expressed in the proximal tubules and collecting ducts of the kidney, suggesting that it might be involved in metal reabsorption.54 This raises the intriguing possibility that there may be a potential excretory system for iron after all and that it is overcome by constitutive reabsorption. If so, it is conceivable that reabsorption could be blocked pharmacologically, resulting in a urinary iron leak. This type of therapeutic maneuver could be invaluable in dealing with patients with siderosis due to a chronic transfusion requirement, for example, in patients with myelodysplastic syndromes or thalassemia major.
The severe mk and b phenotypes indicate that Nramp2(DCT1) is a key transmembrane iron transporter, at least in intestinal cells and erythroid cells. Nramp2(DCT1) may be involved in iron transport in other cell types as well. Nonetheless, there is indisputable biochemical evidence for multiple iron transport activities in mammalian cells, and there are clearly several iron transporters operating in yeast. Database searches to date have not shown additional mammalian Nramp-like proteins; it appears likely that other mammalian iron transport molecules will be unrelated. One candidate molecule, SFT (stimulator of Fe transport), was recently isolated in an expression cloning screen using K562 erythroleukemia cell mRNA.70 This molecule appears to promote iron uptake, but there is no direct evidence that it is, itself, an iron transporter. A high-affinity transmembrane iron transporter found in yeast, FTR1, is clearly important for iron homeostasis in that organism, but it has no known mammalian homolog at this time.71
Finally, there are two other iron overload disorders, not yet mentioned in this review, that are still poorly understood. African iron overload, also known as Bantu siderosis, is a prevalent condition in sub-Saharan Africa that leads to massive iron accumulation and ensuing complications.72 The phenotype of African iron overload differs subtly from that of HHC on a pathologic level, as iron first accumulates in reticuloendothelial cells. Furthermore, though there is a genetic predisposition, African iron overload is not linked to the HLA complex.73 It is not yet known whether African-Americans have an increased susceptibility to iron overload as a result of this same gene. A different disorder, neonatal hemochromatosis, is a devastating iron loading disease of the perinatal period that results in liver failure and almost certain death in affected patients. Liver transplant is apparently futile, because iron has been shown to accumulate in the donor organ.74 Future efforts must be directed towards working out the pathogenesis of these diseases and using our new knowledge in their treatment.
ACKNOWLEDGMENT
The authors thank Mark Fleming for stimulating discussions and for help in preparing Fig 2.
N.C.A. is an Assistant Investigator of the Howard Hughes Medical Institute. J.E.L. is supported by a National Institutes of Health (NIH) K08 award. Some studies described in this review are funded by NIH R01 DK53813 to N.C.A.
Address reprint requests to Nancy C. Andrews, MD, PhD, Howard Hughes Medical Institute, Enders 720, Children’s Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail: andrews_n@a1.tch.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal