Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a growth factor for acute myeloblastic leukemia (AML) cells. Murine double minute 2 (MDM2) oncoprotein, a potent inhibitor of wild-type p53 (wtp53), can function both to induce cell proliferation and enhance cell survival, and is frequently overexpressed in leukemias. Therefore, we focused on the importance of MDM2 protein in GM-CSF–dependent versus GM-CSF– independent growth of AML cells. The TF-1 AML cell line, which has both wtp53 and mutant p53 genes, showed GM-CSF–dependent growth; deprivation of GM-CSF resulted in G1 growth arrest and apoptosis. MDM2 mRNA and protein were highly expressed in proliferating TF-1 cells in the presence of GM-CSF and decreased significantly with deprivation of GM-CSF. In contrast, p53 protein increased with GM-CSF deprivation. Ectopic overexpression of MDM2 in TF-1 AML cells conferred resistance to GM-CSF deprivation, and is associated with decreased p53 protein expression. Moreover, a variant of TF-1 cells that grows in a GM-CSF–independent fashion also expressed high levels of MDM2 and low levels of p53. These results suggest that GM-CSF–independent growth of AML cells is associated with overexpression of MDM2 protein and related modulation of p53 expression.
© 1998 by The American Society of Hematology.
AMPLIFICATION OF THE murine double minute 2 (MDM2) gene, originally cloned from spontaneously transformed BALB/c 3T3 cells,1,2 was reported in human sarcomas.3The MDM2 oncogene encodes a 90 kilodalton (kd) nuclear phosphoprotein that is induced by wild-type p53 (wtp53) after DNA damage3,4 and inactivates p53 function,5functioning as a p53 negative feedback regulator.6-9 Mice deficient in MDM2 die early in development, whereas mice deficient in both MDM2 and p53 develop normally and are viable, suggesting that a critical role of MDM2 in development is the modulation of p53.10,11 In addition to p53, MDM2 also interacts with retinoblastoma protein (pRB) and E2F-1/DP1.12,13 Therefore, MDM2 can function both to enhance cell survival and to induce cell proliferation. Although p53 alterations are common in human solid tumors, they are infrequent in hematologic malignancies.14Conversely, overexpression of MDM2 protein is frequently observed in hematologic malignancies, particularly in patients with poor prognosis and advanced disease,15-22 but is rare in most common epithelial cancers.23,24 Importantly, MDM2 overexpression is not always related to alterations of p53,25,26suggesting that MDM2 can impact on the growth and survival of tumor cells independent of p53. Moreover, MDM2 gene transfection has been shown to transform cultured rat astrocytes and NIH3T3 cells,27 28 which express only wtp53, showing a tumorigenic role for MDM2.
Cytokines can both stimulate growth and inhibit apoptosis in normal as well as malignant hematologic cells.29-31 For example, granulocyte-macrophage colony-stimulating factor (GM-CSF) is a major growth factor for acute myeloblastic leukemia (AML) cells29,30; interleukin-3 (IL-3)/GM-CSF suppresses apoptosis of leukemia cells, and IL-6 inhibits p53-induced apoptosis in murine AML cells.32-34 The mechanisms by which cytokines produce their growth stimulatory and antiapoptotic effects include upregulation of Bcl-2 by IL-3 in AML cells,35 deregulated overexpression of Bcl-2 following IL-3 withdrawal,36inhibition of Fas via SAP kinase by IL-6 in multiple myeloma (MM) cells,37 and rescue from dexamethasone-induced G1 growth arrest via p21 WAF1 by IL-6.38 On the other hand, growth factor independence of human myeloid leukemia cell lines is associated with increased Raf-1 protooncogene phosphorylation.39 However, the mechanisms underlying GM-CSF–dependent versus GM-CSF–independent growth of AML cells remain largely unknown.
In the present study, we examined the relationship between MDM2 expression and GM-CSF–dependent growth of AML cells. GM-CSF–dependent TF-1 AML cells constitutively express MDM2 with weak p53 expression. Deprivation of GM-CSF induces G1 growth arrest and apoptosis, as well as increased p53 and decreased MDM2 expression. Ectopic overexpression of MDM2 in TF-1 cells confers resistance to GM-CSF deprivation and decreased p53 expression. Moreover, a GM-CSF–independent subclone of TF-1 cells expresses high levels of MDM2 and low levels of p53 proteins. These results suggest that overexpression of MDM2 protein is associated with modulation of p53 expression and function, as well as growth of AML cells in a GM-CSF–independent mechanism.
MATERIALS AND METHODS
Culture and γ-Irradiation of Cells
The TF-1 AML cell line established by Kitamura40 was obtained from American Type Culture Collection (Rockville, MD). Cells cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 25 IU/mL penicillin, 25 mg/mL streptomycin, and 1 ng/mL GM-CSF. Cells were grown at 37°C in a humidified 5% CO2atmosphere. A spontaneous cytokine-independent variant was selected by culturing TF-1 cells (1×106 cells/100 mL) in GM-CSF–free medium for 3 days, separation of viable cells by Ficoll density gradient sedimentation, and cloning by limiting dilution in 96-well tissue culture plates in complete media without GM-CSF. The fastest growing clone was selected and amplified in GM-CSF–free media. Parent TF-1 AML cells were also γ-irradiated (2.0 Gy) and cultured in complete media with GM-CSF (1 ng/mL) to induce expression of wtp53 and p21, respectively.
MDM2 Transfection
The full-length MDM2 gene cloned into BamHI sites of pCMV-Neo-BamHI expression vector was a generous gift of Dr Bert Vogelstein (Johns Hopkins Oncology Center, Baltimore, MD). Transfection of the MDM2 gene or control vector into TF-1 AML cells was performed by lipofectamine-mediated gene transfer using 5 μg of DNA and 5 mL of media containing 15 μg of lipofectamine. An equal volume of culture medium containing 20% FBS was added without removing the transfection mixture and incubated for 48 hours, after which the media was changed. After incubation at 37°C for 7 days, transfected cells were selected by culture with 400 μg/mL G418 in 96-well tissue culture plates.
RNA Isolation and Northern Blotting
Total cellular RNA was purified from TF-1 AML cells using the single-step acid guanidine-isothiocyanate technique. Equal amounts of total RNA (20 μg/lane) were separated by electrophoresis in a 1% agarose/2.2 mol/L formaldehyde gel, transferred onto nitrocellulose membranes, and hybridized to 32P-labeled probe of an 800 bp Hind III fragment excised from MDM2 cDNA. The hybridizations were performed for 16 to 24 hours at 42°C in 50% (vol/vol) formamide, 2 × SSC (0.15 mol/L sodium chloride, 0.015 mol/L sodium citrate), 1 × Denhardt's solution, 0.1% (wt/vol) sodium dodecyl sulfate (SDS), and 200 μg/mL salmon sperm DNA. Filters were washed and autoradiographed.
Immunoprecipitation (IP) and Western Blotting (WB)
IP and WB were performed as previously described.41-45 For IP, TF-1 AML cells (5 × 106 viable cells/sample) were washed three times with phosphate buffered saline (PBS)[KCl 0.2 g/L, KH2PO4 0.2 g/L, NaCl 8.0 g/L, Na2PO4 • 7H2O 2.16 g/L] and lysed over 30 minutes at 4°C in lysis buffer: 1 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 0.5% Nonidet P-40 (NP-40), 5 mmol/L EDTA, 1 mmol/L phenlymethylsulfonyl fluoride (PMSF), 200 mmol/L Na3VO4, aprotinin, and 1 mmol/L NaF. Mouse monoclonal antibodies (MoAbs) were added to cell lysates and incubated for 16 hours at 4°C to IP protein complexes. Proteins were collected using protein G sepharose and aliquots of each lysate were analyzed by SDS-polyacrylamide gel electrophoresis. After transfer to polyvinylidine difluoride membranes, membranes were blocked in 5% skimmed milk and probed with MoAbs followed by horseradish peroxidase conjugated antimouse MoAbs or horseradish peroxidase conjugated anti-p53 MoAbs. Detection was done using enhanced chemiluminescence system.
Polymerase Chain Reaction (PCR) and Direct DNA Sequencing of the p53 Gene
A 2.8 kb fragment of the p53 gene (exon IV to exon IX) was amplified from the genomic DNA obtained from TF-1 AML cells (LA-PCR kit). The PCR reaction was performed at 94°C for 30 seconds (denaturing), 58°C for 60 seconds (annealing), and 72°C for 60 seconds (extension) for 35 cycles using the following primers: 5′-AGGACCTGGTCCTCTGACTG-3′ and 5′-TAGACTGGAAACTTTCCACTTG-3′. The PCR product was purified and directly sequenced (PRISM DyeDeoxy Terminator Cycle Sequencing Kit FS, Applied Biosystems) using AmpliTaq DNA polymerase, the automated ABIPRISM 310 Genetic Analyzer (Applied Biosystems), and appropriate sequencing primers: 5′-TTCCTCTTCCTACAGTACTC-3 for exon V and exon VI; 5′-CCAAGGCGCACTGGCCTCAT-3′ for exon VII; and 5′-CCTATCCTGAGTAGTGGTAA-3′ for exon VIII and exon IX.
Cell Cycle Analysis
The effect of GM-CSF deprivation on cell cycle distribution of TF-1 AML cells was examined at different time points (0, 2, 4, 8, and 16 hours) using propidium iodide (PI, Sigma, St. Louis, MO) staining followed by flow cytometric analysis, as previously described.46 47 Briefly, cells (0.5 × 106) were suspended in 0.5 mL of 3.4 mmol/L sodium citrate, 10 mmol/L NaCl, 0.1% NP-40, and 50 ng/mL PI before analysis by flow cytometry (>10,000 cells/sample, 620 nm) (Ortho-Clinical Diagnostics K. K., Koto-ku, Tokyo, Japan).
Apoptosis Assays
TF-1 control vector transfectants, GM-CSF–independent TF-1 AML cells, and TF-1 MDM2 transfectants (1 × 106 cells/mL) were incubated with GM-CSF (1 ng/mL) for 2 days, washed, and cultured in media with GM-CSF (0.0, 0.1, 0.2, and 1.0 ng/mL) or without GM-CSF. The percentage of apoptotic cells was determined by acridine orange (100 μg/mL) and ethidium bromide (100 μg/mL) staining,47 and fluorescence microscopy at 490 nm excitation wavelength. Assays for DNA fragmentation were done as previously described.47 Briefly, genomic DNA was isolated from TF-1 AML cells (2 × 106), separated on a 1.5% agarose gel, stained with ethidium bromide, and photographed under ultraviolet illumination.
Reagents
The following primary MoAbs or polyclonal antibody (PoAb) were used: anti-MDM2 MoAb recognizing amino acid residues 154-167 of human MDM2 protein, horseradish peroxidase conjugated anti-p53 MoAb recognizing pantropic p53 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-p53 MoAbs recognizing both wild-type and mutant p53, anti-p21 PoAb (Santa Cruz), anti-p21 MoAb (Oncogene Science, Cambridge, MA), and antiactin MoAb (Oncogene). Secondary antibodies conjugated horseradish peroxidase and chemiluminescence system were from Amersham (Arlington Heights, IL). Human recombinant GM-CSF was kindly provided by Kirin-Brewery Co, Ltd (Shibuya-ku, Tokyo, Japan). RPMI1640 and culture-related materials were purchased by Gibco BRL (Grand Island, NY). Acridine orange and ethidium bromide were purchased from Sigma (St Louis, MO).
RESULTS
Effect of GM-CSF Deprivation on Cell Cycle Distribution of TF-1 AML Cells
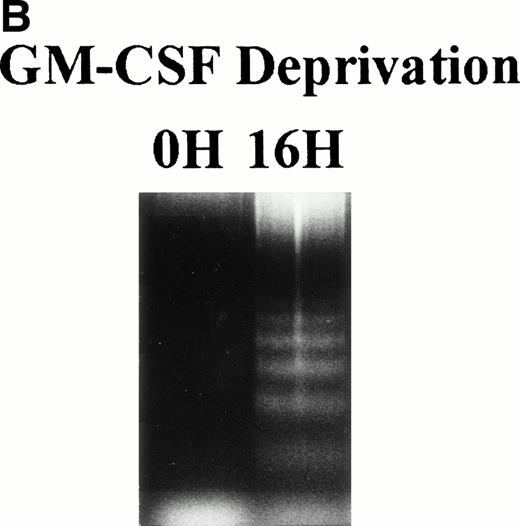
TF-1 AML cells were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh culture media without GM-CSF. Cell cycle distribution was determined at 0, 2, 4, 8, and 16 hours after GM-CSF deprivation by PI staining and flow cytometric analysis (Fig 1A ). The percentage of G1 cells increased from 41% (0 hours) to 68% (16 hours) was coupled with decrease in percentage of S and G2M. Alternatively, percentage of sub-G0 phase increased from 8% (0 hours) to 26% (16 hours). Apoptotic cells, measured by DNA fragmentation, also increased after deprivation of GM-CSF (Fig 1B).
Effect of GM-CSF deprivation on cell cycle distribution and apoptosis of TF-1 AML cells. TF-1 AML cells (3 × 105cells/mL) were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media without GM-CSF. (A) Cell cycle profile was determined by PI staining and flow cytometric analysis at 0, 2, 4, 8, and 16 hours after GM-CSF deprivation. (B) Apoptosis of TF-1 cells before and 16 hours after deprivation of GM-CSF was analyzed by DNA fragmentation.
Effect of GM-CSF deprivation on cell cycle distribution and apoptosis of TF-1 AML cells. TF-1 AML cells (3 × 105cells/mL) were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media without GM-CSF. (A) Cell cycle profile was determined by PI staining and flow cytometric analysis at 0, 2, 4, 8, and 16 hours after GM-CSF deprivation. (B) Apoptosis of TF-1 cells before and 16 hours after deprivation of GM-CSF was analyzed by DNA fragmentation.
Effect of GM-CSF Deprivation on Expression of MDM2 mRNA in TF-1 AML Cells
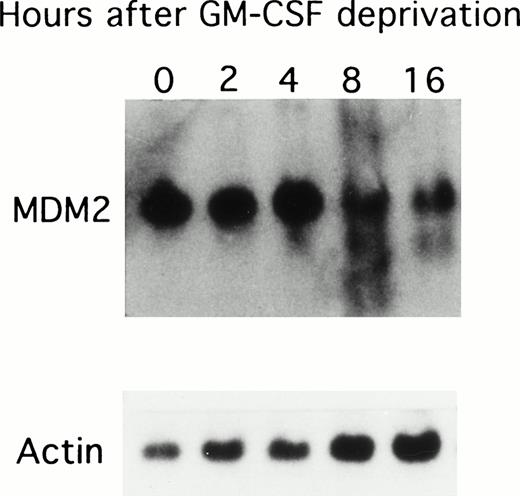
Because GM-CSF deprivation led to apoptosis of TF-1 AML cells, we next correlated MDM2 mRNA expression with cell cycle changes in these cells deprived of GM-CSF (Fig 2). MDM2 mRNA was constitutively expressed in TF-1 cells, and its expression decreased after 8 hours of GM-CSF deprivation. Rehybridization against β-actin mRNA presented equal mRNA loading.
Effect of GM-CSF deprivation on expression of MDM2 mRNA in TF-1 AML cells. TF-1 AML cells (3 × 105 cells/mL) were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media without GM-CSF. Viable cells obtained by Ficoll density gradient sedimentation were prepared at each time point (0, 2, 4, 8, and 16 hours), and 10 μg of RNA from each sample were separated by Northern blotting and hybridized with an MDM2 specific cDNA probe. Rehybridization with β-actin confirmed equal mRNA loading.
Effect of GM-CSF deprivation on expression of MDM2 mRNA in TF-1 AML cells. TF-1 AML cells (3 × 105 cells/mL) were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media without GM-CSF. Viable cells obtained by Ficoll density gradient sedimentation were prepared at each time point (0, 2, 4, 8, and 16 hours), and 10 μg of RNA from each sample were separated by Northern blotting and hybridized with an MDM2 specific cDNA probe. Rehybridization with β-actin confirmed equal mRNA loading.
Effect of GM-CSF Deprivation on Expression of MDM2 and p53 Proteins, as Well as p53 Protein Binding to MDM2
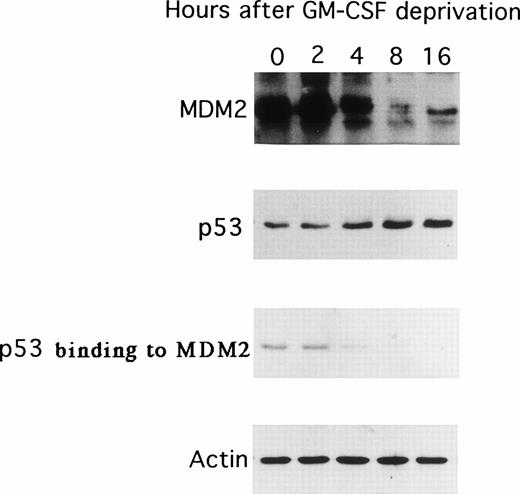
Because GM-CSF deprivation of TF-1 cells was associated with decreased MDM2 mRNA expression, we next examined the expression of p53 and MDM2 proteins as well as the binding of p53 to MDM2 resulting from GM-CSF deprivation. As can be observed in Fig 3, the expression of MDM2 protein decreased at 8 and 16 hours; in contrast, p53 protein expression increased over the same interval after GM-CSF deprivation. In addition, the binding of p53 protein to MDM2 decreased to undetectable levels at 8 hours. The expression of actin was not altered during the 16-hour cultures and confirmed equal protein loading.
Effect of GM-CSF deprivation on expression of MDM2 and p53 proteins, as well as p53 protein binding to MDM2. Total cell lysates were obtained from TF-1 AML cells (5 × 106 viable cells/sample) at 0, 2, 4, 8, and 16 hours after GM-CSF deprivation and immunoprecipitated with anti-MDM2 or anti-p53 MoAbs, and immunoblotted with the same MoAb. In addition, the same cell lysates were immunoprecipitated with anti-MDM2 MoAb and immunoblotted with anti-p53 MoAb. IP and WB with antiactin MoAb confirmed equal protein loading.
Effect of GM-CSF deprivation on expression of MDM2 and p53 proteins, as well as p53 protein binding to MDM2. Total cell lysates were obtained from TF-1 AML cells (5 × 106 viable cells/sample) at 0, 2, 4, 8, and 16 hours after GM-CSF deprivation and immunoprecipitated with anti-MDM2 or anti-p53 MoAbs, and immunoblotted with the same MoAb. In addition, the same cell lysates were immunoprecipitated with anti-MDM2 MoAb and immunoblotted with anti-p53 MoAb. IP and WB with antiactin MoAb confirmed equal protein loading.
Status of p53 in TF-1 AML Cells
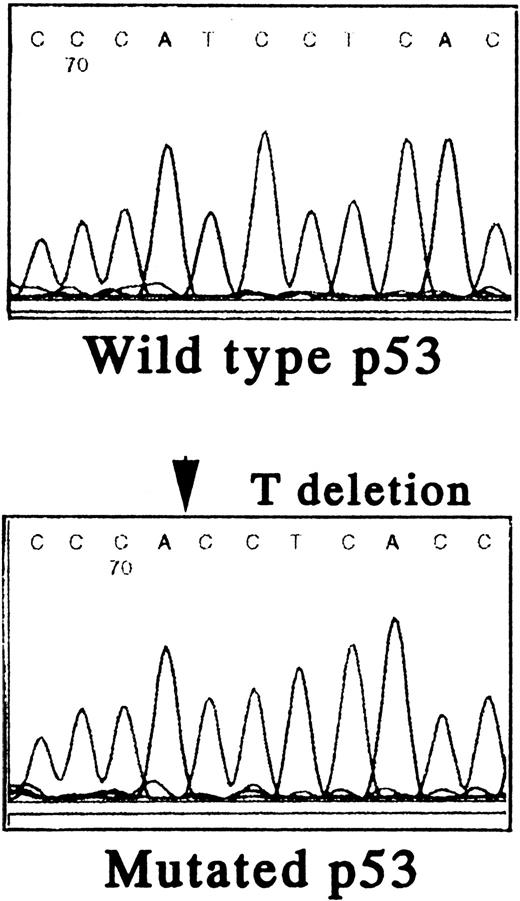
To further define the relationship between MDM2, p53, and the biologic sequelae of GM-CSF deprivation, we first characterized the p53 gene in TF-1 AML cells. Using PCR and direct DNA sequencing, we confirmed that TF-1 cells are hemizygous for both wild-type and mutant p53 genes. There was a 1 bp deletion at codon 251 in exon VII (ATCAC), but no abnormalities were detected in exons IV, V, VI, VIII, and IX (Fig 4A).
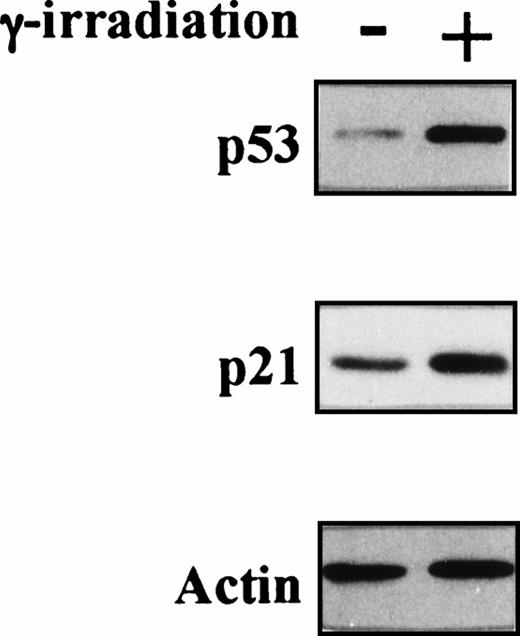
Status of p53 in TF-1 AML cells. Amplicons from exon IV to exon IX of the p53 gene were obtained by PCR from genomic DNA of TF-1 AML cells, and the DNA was directly sequenced using appropriate sequencing primers. (A) The upper panel shows the wtp53 sequence of TF-1 cells, and the lower panel shows a 1 bp deletion at codon 251 in exon VI (ATC∅︀AC). (B) TF-1 cells were cultured for 2 days in fresh media with GM-CSF (1 ng/mL). After exposure to γ-irradiation (mock or 2.0 Gy), cells were cultured for an additional 2 hours or 16 hours . Total cell lysates from 5 × 106 viable cells/sample were immunoprecipitated with anti-p53 MoAb after 2 hours of culture and immunoblotted with horseradish peroxidase conjugated p53 MoAb. Similarly, cell lysates were immunoprecipitated with anti-p21 MoAb after 16 hours of culture and immunoblotted with anti-p21 MoAb. Equal protein loading was confirmed by IP and WB for actin. {/ANNT;4224n;;2112n;2112n}A {/ANNT;4224n;;2112n;124256n}B
Status of p53 in TF-1 AML cells. Amplicons from exon IV to exon IX of the p53 gene were obtained by PCR from genomic DNA of TF-1 AML cells, and the DNA was directly sequenced using appropriate sequencing primers. (A) The upper panel shows the wtp53 sequence of TF-1 cells, and the lower panel shows a 1 bp deletion at codon 251 in exon VI (ATC∅︀AC). (B) TF-1 cells were cultured for 2 days in fresh media with GM-CSF (1 ng/mL). After exposure to γ-irradiation (mock or 2.0 Gy), cells were cultured for an additional 2 hours or 16 hours . Total cell lysates from 5 × 106 viable cells/sample were immunoprecipitated with anti-p53 MoAb after 2 hours of culture and immunoblotted with horseradish peroxidase conjugated p53 MoAb. Similarly, cell lysates were immunoprecipitated with anti-p21 MoAb after 16 hours of culture and immunoblotted with anti-p21 MoAb. Equal protein loading was confirmed by IP and WB for actin. {/ANNT;4224n;;2112n;2112n}A {/ANNT;4224n;;2112n;124256n}B
To confirm the presence of wtp53 in TF-1 AML cells, we next examined p53 and its target, p21WAF-1, protein expressions before and after γ-irradiation of TF-1 cells. As can be observed in Fig 4B, p53 and p21 protein expressions increased at 2 hours and 16 hours after exposure to γ-irradiation, respectively. In contrast, actin protein expression was not altered by γ-irradiation and confirmed equal protein loading.
Effect of Long-Term GM-CSF Deprivation on Expression of MDM2 and p53 Proteins in TF-1 Control Vector Transfectants, in TF-1 MDM2 Transfectants, and in GM-CSF–Independent TF-1 Cells
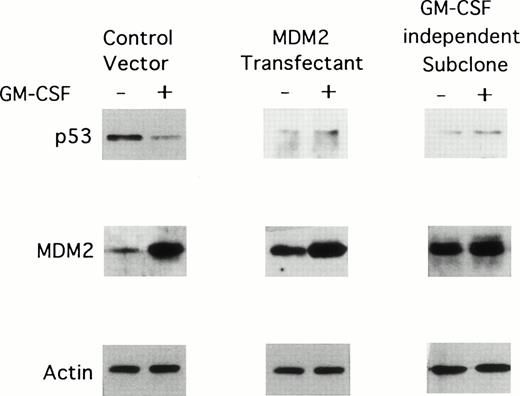
We next determined the effect of long-term (2 weeks) GM-CSF deprivation on MDM2 and p53 protein expression in TF-1 control vector transfectants, TF-1 MDM2 transfectants, and GM-CSF–independent TF-1 cells. As can be observed in Fig 5, all of these cells cultured in GM-CSF weakly express p53 and strongly express MDM2. In the absence of GM-CSF, p53 protein expression decreases in TF-1 MDM2 transfectants and in the GM-CSF–independent TF-1 cells, but increases in TF-1 control vector transfectants. In contrast, MDM2 protein was highly expressed in all cells cultured with GM-CSF and decreases markedly in TF-1 control vector and MDM2 transfectants, but not in GM-CSF–independent TF-1 cells, in the absence of GM-CSF. Actin protein expression is not altered under these culture conditions.
Effect of long-term GM-CSF deprivation on expression of MDM2 and p53 proteins in TF-1 control vector transfectants, in TF-1 MDM2 transfectants, and in GM-CSF–independent TF-1 cells. GM-CSF–independent TF-1 AML cells were cultured in fresh media with GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh media with or without GM-CSF (1 ng/mL) for 16 hours. TF-1 cells transfected with either control vector or the MDM2 gene were cultured in media with G418 (400 μg/mL) and GM-CSF (1 ng/mL) for an additional 16 hours. Expression of MDM2, p53, and actin proteins were examined by immunoblotting.
Effect of long-term GM-CSF deprivation on expression of MDM2 and p53 proteins in TF-1 control vector transfectants, in TF-1 MDM2 transfectants, and in GM-CSF–independent TF-1 cells. GM-CSF–independent TF-1 AML cells were cultured in fresh media with GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh media with or without GM-CSF (1 ng/mL) for 16 hours. TF-1 cells transfected with either control vector or the MDM2 gene were cultured in media with G418 (400 μg/mL) and GM-CSF (1 ng/mL) for an additional 16 hours. Expression of MDM2, p53, and actin proteins were examined by immunoblotting.
Effect of GM-CSF Deprivation on Proliferation and Apoptosis of TF-1 Cells, GM-CSF–Independent TF-1 Cells, as Well as TF-1 Cells Transfected With Either Control Vector or MDM2 Gene
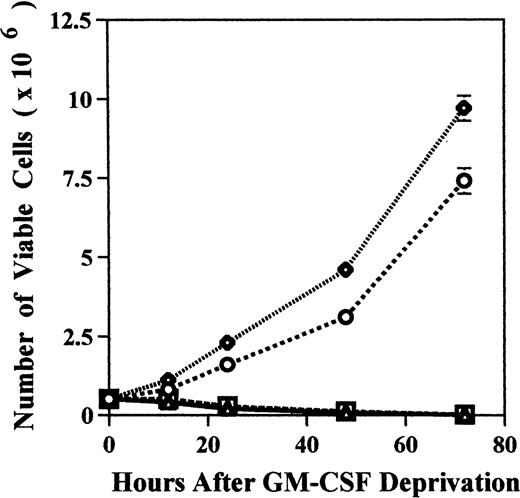
We examined the effect of GM-CSF deprivation on proliferation of MDM2 transfectants by counting the number of viable cells. MDM2 transfected TF-1 cells and GM-CSF–independent TF-1 cells showed continuous proliferation for 72 hours (Fig 6A). In contrast, almost TF-1 cells and control vector transfected TF-1 cells were dead by 72 hours of culture.
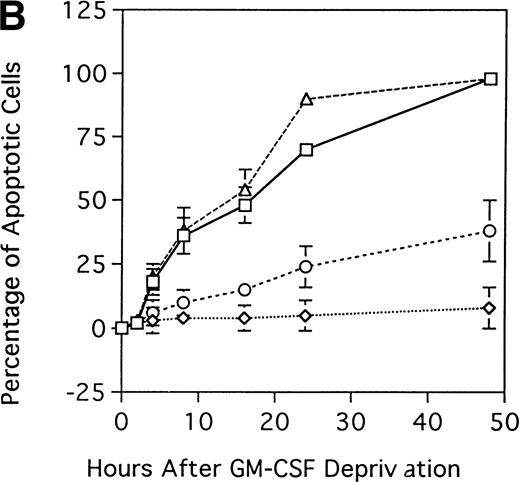
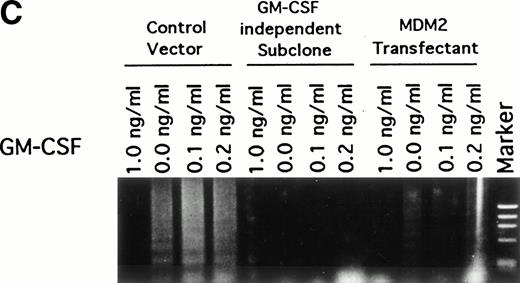
Effect of GM-CSF deprivation on proliferation and apoptosis of TF-1 cells, GM-CSF–independent TF-1 cells, as well as TF-1 cells transfected with either control vector or MDM2 gene. Parent TF-1 cells (▵) and the GM-CSF–independent TF-1 subclone (◊) were cultured in media with GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh medium without GM-CSF. TF-1 control vector transfectants (□) and TF-1 MDM2 transfectants (○) were cultured in media with G418 (400 μg/mL) and GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh media with G418 (400 μg/mL) without GM-CSF. (A) Proliferation of cells cultured in the absence of GM-CSF were determined by counting number of viable cells with trypan blue. Mean standard deviation was determined for three independent experiments. (B) Apoptosis was evaluated with acridine orange/ethidium bromide staining, and the percentage of apoptotic cells was calculated: apoptotic cells / (apoptotic cells + viable cells). Mean standard deviation was determined for three independent experiments. (C) TF-1 control vector transfectants, GM-CSF–independent TF-1 cells, and TF-1 MDM2 transfectants were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media with varying concentrations of GM-CSF (0.0, 0.1, 0.2, and 1 ng/mL). Cells were harvested at 16 hours and apoptosis was evalutated by DNA fragmentation assay. A
Effect of GM-CSF deprivation on proliferation and apoptosis of TF-1 cells, GM-CSF–independent TF-1 cells, as well as TF-1 cells transfected with either control vector or MDM2 gene. Parent TF-1 cells (▵) and the GM-CSF–independent TF-1 subclone (◊) were cultured in media with GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh medium without GM-CSF. TF-1 control vector transfectants (□) and TF-1 MDM2 transfectants (○) were cultured in media with G418 (400 μg/mL) and GM-CSF (1 ng/mL) for 2 days, washed three times with PBS, and resuspended in fresh media with G418 (400 μg/mL) without GM-CSF. (A) Proliferation of cells cultured in the absence of GM-CSF were determined by counting number of viable cells with trypan blue. Mean standard deviation was determined for three independent experiments. (B) Apoptosis was evaluated with acridine orange/ethidium bromide staining, and the percentage of apoptotic cells was calculated: apoptotic cells / (apoptotic cells + viable cells). Mean standard deviation was determined for three independent experiments. (C) TF-1 control vector transfectants, GM-CSF–independent TF-1 cells, and TF-1 MDM2 transfectants were cultured for 2 days in the presence of GM-CSF (1 ng/mL), washed three times with PBS, and resuspended in fresh media with varying concentrations of GM-CSF (0.0, 0.1, 0.2, and 1 ng/mL). Cells were harvested at 16 hours and apoptosis was evalutated by DNA fragmentation assay. A
We next determined whether overexpression of MDM2 gene rescues parent TF-1 cells from apoptosis related to GM-CSF deprivation. Specifically, we examined the percentage of apoptotic cells in GM-CSF–dependent TF-1 cells, in GM-CSF–independent TF-1 cells, as well as in TF-1 control vector and MDM2 transfectants, when cultured in the absence of GM-CSF (Fig 6B). In TF-1 control vector transfectants and in GM-CSF–dependent TF-1 cells, the percentages of apoptotic cells at 48-hours of GM-CSF deprivation were 98% ± 3% and 98% ± 2%, respectively. In contrast, in GM-CSF–independent TF-1 cells and in TF-1 MDM2 transfectants, the percentages of apoptotic cells at 48 hours were significantly decreased (8% ± 8% and 38% ± 12% apoptotic cells, respectively) compared with controls (n = 3, P < .001).
To confirm the antiapoptotic effect of MDM2 in TF-1 cells deprived of GM-CSF, we next assayed DNA fragmentation of these cells under similar culture conditions. DNA fragmentation was observed at low concentrations (0.0, 0.1, and 0.2 ng/mL) of GM-CSF in control vector transfectants that lack MDM2 expression (Fig 6C). In contrast, no DNA fragmentation was observed in the GM-CSF–independent TF-1 cells and only weak DNA fragmentation was observed in TF-1 MDM2 transfectants cultured in the absence of GM-CSF.
DISCUSSION
The results of the present study suggest that GM-CSF–independent growth of AML cells is associated with overexpression of MDM2 protein and related modulation of p53 expression. We first showed that short-term GM-CSF deprivation of the GM-CSF–dependent parental TF-1 AML cells triggered both decreased expression of MDM2 mRNA and protein, and increased p53 expression. Although the mechanisms regulating MDM2 expression are not fully delineated, p53 is known to upregulate MDM2 expression.4-9 However, previous reports have also shown that overexpression of MDM2 in malignant cells may not correlate with status or expression of p53.25,26 This suggests that MDM2 transcription may be regulated, at least in part, by other factors including cytokines such as GM-CSF. Consistent with this view are reports that the withdrawal of GM-CSF in hematopoietic cells results in apoptosis and G1 growth arrest, which is at least partially dependent on p53.48-50 Therefore, GM-CSF signaling might upregulate MDM2 protein, resulting in suppression of apoptosis related to decreased p53 protein.
We next showed that ectopic overexpression of MDM2 in TF-1 GM-CSF–dependent AML cells both increased MDM2 and decreased p53 protein expression, as well as rescued cells from apoptosis related to GM-CSF deprivation. To further confirm the association between this pattern of p53 and MDM2 expression and GM-CSF growth independence, we derived a subclone of TF-1 cells that grew independently of this cytokine. High levels of MDM2 and low levels of p53 protein were expressed in this GM-CSF–independent subclone, further supporting the view that overexpression of MDM2 may downregulate p53 protein expression. Recently, two groups have reported that increased MDM2 protein promotes the rapid degradation of p53 under conditions in which p53 is otherwise stabilized,51 52 also consistent with our results. Our study further showed that more long-term (2 weeks) growth of GM-CSF–independent TF-1 cells was associated with sustained high expression of MDM2 and low levels of p53.
By direct DNA sequencing of p53 (exon IV to exon IX), the TF-1 cell line used in these experiments was found to be hemizygous for p53, possessing a 1 bp deletion (ATCAC) in codon 251 of exon VII consistent with previous reports,53,54 as well as wtp53. We confirmed this novel wtp53 gene by γ-irradiating TF-1 cells and normal PBMNCs and showing increased p53 protein expression in both of these cells. Borellini et al54 reported that p53 protein in TF-1 cells, detected by immunoblotting with two different p53 antibodies, exhibited a mobility identical to p53 from human primary fibroblasts. Moreover, Zhu et al52 showed that p53 function in TF-1 cells was blocked by p53 antisense oligonucleotides. These reports, as well as the current study, suggest that p53 in TF-1 cells may have wild-type function.
What are the implications of the current findings? Our results suggest that MDM2 overexpression may play a role in cytokine independent growth. Our evidence is twofold. First, a GM-CSF–independent subclone from parent TF-1 cells was isolated, which grew in media without GM-CSF and showed high levels of MDM2 and low levels of p53 protein expression that were not altered by addition of GM-CSF. Chao et al55recently derived a GM-CSF TF-1–independent subclone and characterized the role of the Raf/MAP kinase pathway in the antiapoptotic effect of GM-CSF. Second, we also showed that ectopic overexpression of MDM2 overcame apoptosis of TF-1 cells triggered by GM-CSF deprivation. Therefore future studies will delineate the role of the Ras/Raf/MAP kinase and MDM2 overexpression in conferring cytokine-independent growth of AML cells.
ACKNOWLEDGMENT
We thank Dr Bert Vogelstein (Johns Hopkins Oncology Center, Baltimore, MD) for the full-length MDM2 gene.
Address correspondence to Mitsuyoshi Urashima, MD, PhD, Department of Pediatrics, Jikei University School of Medicine, 3-25-8, Nishi-shinbashi, Minato-ku, Tokyo 105, Japan; e-mail:urashima@jikei.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal