Abstract
Angiogenesis, the formation of new capillary blood vessels, is a feature of a variety of pathological processes. To study the effects of a specific group of hormones (all ligands of the steroid/retinoid/thyroid hormone receptor superfamily) on the angiogenic process in humans, we have used a model system in which human microvascular endothelial cells from foreskin (hMVEC) are cultured on top of a human fibrin matrix in the presence of basic fibroblast growth factor and tumor necrosis factor-α. This model mimics the in vivo situation where fibrin appears to be a common component of the matrix present at sites of chronic inflammation and tumor stroma. Our results show that testosterone and dexamethasone are strong inhibitors and all-trans retinoic acid (at-RA) and 9-cis retinoic acid (9-cis RA) are potent stimulators of the formation of capillary-like tubular structures. These effects are mediated by their respective nuclear hormone receptors as demonstrated by the use of specific synthetic receptor agonists and antagonists. 17β-estradiol, progesterone, and 1,25-dihydroxyvitamin D3 did not affect or only weakly affected in vitro angiogenesis, which may be related to the lack of significant nuclear receptor expression. Although hMVEC express both thyroid hormone receptors α and β, no effect of thyroid hormone on tube formation was found. The effects of testosterone, dexamethasone,at-RA, and 9-cis RA on tube formation were accompanied by parallel changes in urokinase-type plasminogen activator (u-PA) expression, at both mRNA and antigen levels. Exogenous suppletion of the medium with single chain u-PA enhances tube formation in our in vitro model, whereas quenching of u-PA activity (but not of tissue-type plasminogen activator activity) or of u-PA binding to u-PA receptor by specific antibodies suppressed basal and retinoid-stimulated tube formation. Moreover, addition of scu-PA to testosterone- or dexamethasone-treated hMVEC restored the suppressed angiogenic activity for a substantial part. Aprotinin, an inhibitor of plasmin activity, completely inhibited tube formation, indicating that the proteolytic properties of the u-PA/u-PA receptor complex are crucial in this process. Our results show that steroid hormones (testosterone and dexamethasone) and retinoids have strong, but opposite effects on tube formation in a human in vitro model reflecting pathological angiogenesis in the presence of fibrin and inflammatory mediators. These effects can be explained by hormone-receptor–mediated changes in u-PA expression, resulting in enhanced local proteolytic capacity of the u-PA/u-PA receptor complex.
© 1998 by The American Society of Hematology.
ANGIOGENESIS is the formation of new capillary blood vessels by a process of sprouting from existing microvascular vessels. It has a role during development and in the normal physiology of reproduction, formation of collaterals, and wound healing, but is also important under pathological conditions, where it contributes to the pathogenesis of a number of diseases such as diabetic retinopathy, tumor growth, and rheumatoid arthritis.1-5 In each system, the formation of new capillaries involves a series of discrete, but overlapping events, including (1) localized degradation of the endothelial cell basal lamina; (2) endothelial cell migration and extracellular matrix invasion; (3) endothelial cell proliferation; and (4) formation of capillary lumina and reconstitution of the basal lamina.6 7Given the (patho)physiological importance of angiogenesis, it is of clinical relevance to identify factors that either stimulate or inhibit those processes and to elucidate their mode of action.
Several reports have pointed to an effect of steroid hormones and retinoids (retinoic acid derivatives) on angiogenic activity. However, results have been conflicting, and both stimulatory and inhibitory activities of these compounds have been found.8-17 It is not clear at present, whether the variation in response to hormones relates to differences in assay systems, species, the parameter measured, or hormone concentration and metabolism. Many studies have been performed in nonhuman angiogenesis models and focused on individual components of the angiogenic process (for instance, proliferation or migration), rather than on the complete response of capillary tube formation. To gain more insight into the effect of hormones on angiogenesis in humans, we have developed an in vitro model, in which human foreskin microvascular endothelial cells (hMVEC) can be induced to invade a three-dimensional fibrin matrix thereby forming capillary-like tubular structures, which can be quantified by computer-assisted video analysis.18 In this in vitro model, both a growth factor (basic fibroblast growth factor [bFGF] or vascular endothelial cell growth factor) and a factor to induce urokinase-type plasminogen activator (u-PA), for example tumor necrosis factor-α (TNF-α), are necessary to induce capillary-like tubular structures. This model mimics the in vivo situation where fibrin appears to be a common component of the matrix present at sites of chronic inflammation and tumor stroma.19 Electron microscopy of cross sections showed that the capillary-like tubular structures have a lumen and are very much like capillary structures in vivo.18Tube-formation in this model was shown to be dependent on u-PA activity and plasmin activity, which are thought to play a role in the regulation of the first steps of angiogenesis.
We have used this in vitro model to examine the angiogenesis-modulating activity of a group of hormones acting via binding to a specific class of nuclear receptors, the steroid/retinoid/thyroid hormone receptor superfamily.20 Our results show both blocking (testosterone and dexamethasone) and stimulating (all-trans and 9-cisretinoic acid) activities of the hormones tested. In all cases, hormone-induced changes in tube formation are paralleled and can be mimicked by corresponding changes in u-PA levels. Our findings provide evidence for an important role for hormones and their respective nuclear receptors in the formation of tube-like structures by influencing the expression of u-PA and thereby the local proteolytic capacity of the endothelial cell.
MATERIALS AND METHODS
Materials.
17β-estradiol, 2-methoxyestradiol, progesterone, testosterone, dexamethasone, 3,3,5-triiodo-l-thyronine (T3), all-transretinoic acid (at-RA), RU486 (mifepristone), dimethyl sulfoxide (DMSO), and charcoal-stripped, delipidated bovine calf serum were purchased from Sigma (St Louis, MO). R1881 (methyltrienolone) was obtained from NEN (Boston, MA). 1,25-Dihydroxyvitamin D3 (Ro 21-5535) was kindly provided by Dr M.R. Uskoković (Hoffmann-LaRoche, Nutley, NJ) and 9-cis retinoic acid (9-cis RA) and the retinoic acid receptor α (RARα) antagonist, Ro 41-5253, were kindly provided by Drs M. Klaus and C. Apfel (Hoffmann-LaRoche, Basel, Switzerland). Hydroxyflutamide was a gift from Dr T. Lavecchia (Schering-Plough, Kenilworth, NJ). 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphtyl)-ethenyl] benzoic acid (3-methyl-TTNEB) and (E)-4-[2-(5,5,8,8,-tetramethyl-5,6,7,8-tetrahydro-2-naphtalenyl-1-propenyl] benzoic acid (TTNPB) were kindly provided by Dr S. Karathanasis, American Cyanamid Company (Pearl River, NY) and by Dr M. Issandou, Glaxo Wellcome (Les Ulis, France), respectively. Stock solutions of hormones (10 mmol/L) were prepared in DMSO and stored at −20°C. Stock solutions were diluted with incubation medium to the final test concentrations immediately before the start of an experiment. All experiments involving retinoids, R1881, and hydroxyflutamide were performed in subdued light, and the tubes containing these test compounds were covered with aluminium foil. bFGF was obtained from Intergen (Purchase, NY), thrombin from Leo Pharmaceutical Products (Weesp, The Netherlands), and human fibrinogen from Chromogenix AB (Mölndal, Sweden). Factor XIII was kindly provided by Dr H. Keuper (Centeon Pharma, Marburg, Germany). Human recombinant TNF-α was a gift from Dr J. Travernier (Biogent, Ghent, Belgium), and contained 2.45 × 107 U/mg protein and <40 ng lipopolysaccharide per μg protein. Single chain u-PA was generously provided by Dr A. Molinari (Farmitalia, Milan, Italy). The amino terminal fragment of u-PA (ATF, amino acids 1-143) was provided by Abbott (Abbott Park, IL). Aprotinin was purchased from Pentapharm Ltd (Basel, Switzerland). Rabbit polyclonal anti–u-PA antibodies and rabbit polyclonal anti–t-PA antibodies were prepared in our laboratory. The u-PA receptor (u-PAR) blocking monoclonal antibody H-2 was a gift from Dr U. Weidle (Boehringer-Mannheim, Penzberg, Germany). Technovit 8100 was obtained from Heraeus Kulzer (Wehrheim, Germany). Enzyme-linked immunosorbent assay (ELISA) kits for determination of t-PA antigen (Thrombonostika t-PA) and PAI-1 antigen (Imulyse) were obtained from Organon Teknika (Boxtel, The Netherlands) and Biopool (Umeå, Sweden), respectively. Deoxycytidine 5[α-32P] triphosphate (dCTP) was from Amersham (Buckinghamshire, UK). Oligonucleotides used for reverse transcriptase-polymerase chain reaction (RT-PCR) were purchased from Isogen Bioscience (Maarssen, The Netherlands). Other materials used have been specified in the methods described or in the related references.
Cell culture.
hMVEC were isolated, cultured, and characterized as previously described.21 The cells were cultured in gelatin-coated dishes in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20 mmol/L HEPES (pH, 7.3), 10% (vol/vol) human serum, 10% (vol/vol) heat-inactivated newborn calf serum (NBCS), 150 μg/mL crude endothelial growth factor,22 5 IU/mL heparin, 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Subcultures were obtained by trypsin/EDTA treatment of confluent monolayers at a split ratio of 1:3.
In vitro angiogenesis model.
Human fibrin matrices were prepared by addition of 0.1 U/mL thrombin to a mixture of 2.5 U factor XIII, 2 mg fibrinogen, 2 mg Na-citrate, 0.8 mg NaCl, and 3 μg plasminogen per mL DMEM medium without indicator. A total of 300 μL of this mixture was added to 1 cm2 wells. After clotting at room temperature, the fibrin matrices were soaked with 0.5 mL DMEM supplemented with 10% (vol/vol) heat-inactivated human serum, 10% (vol/vol) heat-inactivated NBCS, and penicillin/streptomycin. Endothelial cells were seeded at high density to obtain confluent monolayers, and, unless stated otherwise, cultured in DMEM without indicator supplemented with 10% (vol/vol) heat-inactivated human serum, 10% (vol/vol) heat-inactivated NBCS, 2 mmol/L L-glutamine, penicillin/streptomycin, 20 ng/mL bFGF, and 20 ng/mL TNF-α (referred to as incubation medium). Incubations were for 8 to 12 days, and test compounds were added together with incubation medium where appropiate. The conditioned medium was collected and replaced every 2 to 3 days. Invading cells and the formation of capillary-like tubular structures (“tube formation”) of endothelial cells in the three-dimensional fibrin matrix were analyzed by phase contrast microscopy and the total number, the total area, and the total length of capillary-like tubular structures of six randomly chosen microscopic fields/well (11.6 mm2/field) were measured using an Olympus microscope equipped with a monochrome CCD camera (MX5) connected to a computer with Optimas image analysis software (Tokyo, Japan). Because all three measured parameters correlated well with each other (r > .96), only data for total length of capillary-like structures are shown. For determination of t-PA secretion, cells were cultured on gelatin-coated 1-cm2 wells in parallel to cells grown on fibrin matrices. In experiments with nuclear receptor antagonists, the antagonist was added to the medium 1 hour before the hormone.
Histochemistry.
Matrices were fixed in 2% (vol/vol) p-formaldehyde in phosphate-buffered saline (PBS) (0.15 mol/L NaCl; 10 mmol/L Na2HPO4; 1.5 mmol/L KH2PO4, pH 7.4), embedded in Technovit 8100, sectioned at 3 μm, and stained with hematoxylin and floxin.
ELISAs.
u-PA antigen was determined by a previously described u-PA ELISA, which recognizes single-chain u-PA, two-chain u-PA, and u-PA/PAI-1 complex with the same efficiency.18 t-PA and PAI-1 antigen determinations were performed by commercially available immunoassay kits (Thrombonostika t-PA and Imulyse).
Northern blot analysis.
Total RNA from hMVEC (30 cm2) was isolated by the isothiocyanate/phenol/acid extraction method of Chomcynski et al.23 The RNA was dissolved in H2O, and the RNA concentration was determined spectrophotometrically. Equal amounts (7.5 to 10 μg) of RNA were separated on a formaldehyde/agarose gel and were subsequently capillary transferred to a Hybond N membrane according to the instructions of the manufacturer (Amersham). Hybridization was performed in 7% (wt/vol) sodium dodecyl sulfate (SDS), 1 mmol/L EDTA, 0.5 mol/L Na2HPO4/NaH2PO4 buffer, (pH, 7.2) overnight at 63°C with 25 ng of probe labeled with the random primer method (Mega prime kit, Amersham). The membranes were subsequently washed three to four times during 20 minutes with 2× SSC/1% (wt/vol) SDS (1× SSC: 0.15 mol/L NaCl, 0.015 mol/L Na3 citrate) when using the u-PA and u-PAR probes, twice with 2× SSC, and twice with 1× SSC when using the other probes. The filters were exposed to an Amersham Hyper film-MP film with an intensifying screen at −80°C.
cDNA probes.
The following cDNA fragments were used as probes in the hybridization experiments: a 1.0-kb fragment of the human u-PA cDNA (a gift from Dr W-D. Schleuning, Schering AG, Berlin, Germany),24 a 585-bpBamHI fragment of the human u-PAR cDNA (a gift from Dr F. Blasi, Milano, Italy),25 a 2.7-kb Sma I fragment of the human androgen receptor cDNA (a gift from Dr A.O. Brinkmann, Rotterdam, The Netherlands),26 a 2.6-kb KpnI/Dra I fragment of the human glucocorticoid receptor (a gift from Dr S. Wissink, Utrecht, The Netherlands),27 and a 1.2-kb Pst I fragment of hamster actin cDNA.28
Oligonucleotide primers.
The following primer sequences were used in the RT-PCR to detect receptor mRNA: for the estrogen receptor α mRNA: sense 5′-TGATGGGGAGGGCAGGGGTGAAGTG-3′ (aa 272-279) and antisense 5′-TAGGCGGTGGGCGTCCAGCATCTCC-3′ (aa 541-549), as described by Perrot-Applanat et al.29 For the estrogen receptor β mRNA: sense 5′-TTGTGCGGAGACAGAGAAGTGC-3′ (aa 175-182) and antisense 5′-GGAATTGAGCAGGATCATGGCC-3′ (aa 349-355).30 For the progesterone receptor mRNA: sense 5′-GTGGGCGTTCCAAATGAAAGCCAAG-3′ (aa 660-667) and antisense 5′-QAATTCAACACTCAGTGCCCGGGACT-3′ (aa 897-905).29 For the thyroid receptor α mRNA: sense 5′-AGTGGGATCTGATCCACATTGC-3′ (aa 164-171) and antisense 5′-GATCTTGTCCACACACAGCAGG-3′ (aa 332-338).31For the thyroid receptor β: sense 5′-GGGAGCTCATCAAAACTGTCAC-3′ (aa 214-221) and antisense 5′-GGCTACTTCAGTGTCATCCAGG-3′ (aa 359-366).32For the vitamin D3 receptor: sense 5′-AGACACACTCCCAGCTTCTCTG-3′ (aa 171-180) and antisense 5′-ACGTCTGCAGTGTGTTGGACAG-3′ (aa 359-366).33
RT-PCR.
RT-PCR was performed under standard conditions following the specifications recommended by the supplier. In short, cDNAs were synthesized in one reaction mixture containing 1 μg total RNA, 0.45 μg oligo dT primer, and RT-II-superscript (Life Technologies, Paisley, UK); the cDNAs were then heated for 8 minutes at 95°C. Subsequently, the cDNAs were treated with RNAse H (25 U/mL) for 25 minutes at 37°C. Next, the cDNAs (1 μL of a 10× diluted cDNA reaction mixture) were amplified in the presence of 5% (vol/vol) DMSO and 5% W-1 (vol/vol) (Life Technologies) with the corresponding primers. The amplifications were performed for 30 cycles for all receptors. The denaturation was performed for 60 seconds at 94°C. Primer extension was performed for 60 seconds at 60°C for 4 cycles, then at 58°C for 4 cycles, next at 56°C for 4 cycles, and finally at 55°C for 18 cycles for the progesterone receptor and the thyroid receptors, α and β. For the estrogen receptors α and β and for the vitamin D receptor, primer extension was performed for 60 seconds at 60°C for 10 cycles and then at 58°C for 20 cycles. The DNA-synthesizing step was performed at 72°C for 1 minute. For the estrogen receptors α and β, PCR was also performed for 55 cycles, with a cycling profile of 1 minute at 94°C, 1 minute at 62°C, and 1 minute at 72°C. After 20 cycles with a primer concentration of 28 nmol/L, additional primers were added to reach a final concentration of 380 nmol/L. Aliquots of the PCR reaction mixture were separated on an agarose gel, stained with ethidium bromide, and visualized with a UV transilluminator.
RESULTS
Effect of steroid hormones, retinoids, thyroid hormone, and 1,25-dihydroxyvitamin D3 on tube formation.
To determine the effect of the various hormones on tube formation, hMVEC were seeded on three-dimensional fibrin matrices and maintained in standard incubation medium containing 10% (vol/vol) human serum, 10% (vol/vol) NBCS, 20 ng/mL TNF-α and 20 ng/mL bFGF, and varying concentrations of the appropiate hormone. Tube formation required the presence of both bFGF and TNF-α, and none of the hormones tested showed any angiogenic activity by itself or in combination with either bFGF or TNF-α (data not shown). Tube formation usually became detectable after 3 to 5 days of cell culture, and the onset of this process was not or hardly affected by the presence of the hormones. Effects of hormones on tube formation were most apparent after 8 to 12 days of culture and are illustrated in Fig1 (A through E) for the most potent compounds, namelyat-RA, testosterone, and dexamethasone. Very similar results were obtained when the human serum/NBCS was replaced by 20% (vol/vol) delipidated calf serum (data not shown). Histologic analysis of cross-sections perpendicular to the surface of the matrix shows that the tube-like structures are located in the fibrin matrix underneath the endothelial cell monolayer (Fig 1F). At a hormone concentration of 1 μmol/L, tube formation was strongly suppressed by testosterone and dexamethasone to 32% ± 9% and 30% ± 17% of control values, respectively (Fig 2). The synthetic androgen receptor agonist R1881 also effectively inhibited tube formation (see Fig 8A). A total of 1 μmol/L 17β-estradiol and its metabolite, 2-methoxyestradiol, only weakly inhibited tube formation to 82% ± 11% and 74% ± 19%, respectively, whereas progesterone (1 μmol/L) had no significant effect (85% ± 17%) (Fig 2).at-RA (a ligand for the retinoic acid receptor, RAR) and 9-cis RA (a ligand for both RAR and the retinoid X receptor, RXR) both stimulated tube formation, with 9-cis RA being an even more potent stimulator (377% ± 80%) than at-RA (278% ± 40%) (Fig 2B). Comparable results were obtained with the RAR-specific synthetic ligand TTNPB and the RXR-specific ligand TTNEB (data not shown), indicating that both RARs and RXRs are able to mediate the effect of retinoids on tube formation. Neither thyroid hormone (T3) nor 1,25-dihydroxyvitamin D3 significantly affected tube formation at a concentration of 1 μmol/L (Fig 2B), but at a concentration of 10 μmol/L 1,25-dihydroxyvitamin D3 increased tube formation to 161% ± 35% (data not shown). Effective hormones influenced tube formation in a concentration-dependent manner with ED50 values of 20 nmol/L for testosterone, 8 nmol/L for dexamethasone, 60 nmol/L for at-RA, and 70 nmol/L for 9-cis RA, and maximal effects were reached at hormone concentrations of 0.1 to 1.0 μmol/L.
Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).
Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).
Effect of steroid hormones, retinoids, thyroid hormone, and 1,25-dihydroxyvitamin D3 on tube formation. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α. (A) Shows the effect of vehicle, 0.01% (vol/vol) DMSO (con) or 1 μmol/L 17β-estradiol (E2), testosterone (test), progesterone (prog), dexamethasone (dex), or 2-methoxyestradiol (2-ME) and (B) shows the effect of 1 μmol/L of 1,25-dihydroxyvitamin D3 (D3), thyroid hormone (T3), all-transretinoic acid (at-RA), or 9-cis retinoic acid (9-cis RA) on tube formation. The data represent the average ± SD of three to six experiments with the number of experiments indicated in parentheses for each condition, with each experiment performed in duplicate, and are expressed as percentage of control values. Total tube-length/cm2 under control conditions ranged from 32 to 329 mm/cm2 (average = 130 mm/cm2) between the different experiments. * P< .05, *** P < .0001.
Effect of steroid hormones, retinoids, thyroid hormone, and 1,25-dihydroxyvitamin D3 on tube formation. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α. (A) Shows the effect of vehicle, 0.01% (vol/vol) DMSO (con) or 1 μmol/L 17β-estradiol (E2), testosterone (test), progesterone (prog), dexamethasone (dex), or 2-methoxyestradiol (2-ME) and (B) shows the effect of 1 μmol/L of 1,25-dihydroxyvitamin D3 (D3), thyroid hormone (T3), all-transretinoic acid (at-RA), or 9-cis retinoic acid (9-cis RA) on tube formation. The data represent the average ± SD of three to six experiments with the number of experiments indicated in parentheses for each condition, with each experiment performed in duplicate, and are expressed as percentage of control values. Total tube-length/cm2 under control conditions ranged from 32 to 329 mm/cm2 (average = 130 mm/cm2) between the different experiments. * P< .05, *** P < .0001.
Role of u-PA in hormone-modulated tube formation.
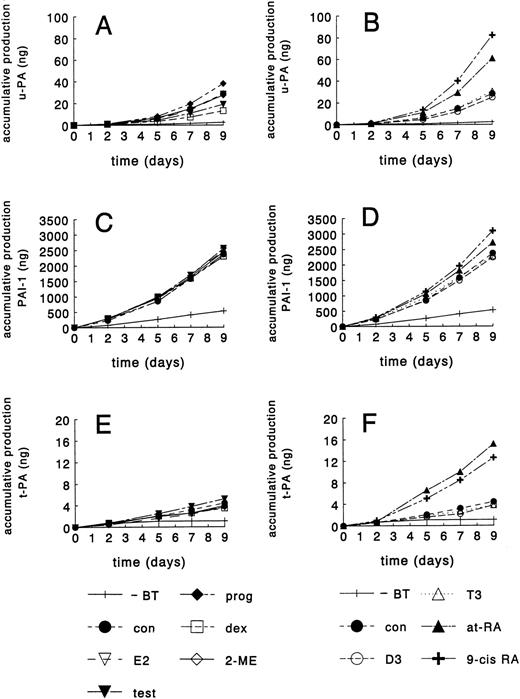
Because we had previously demonstrated that tube formation in our in vitro angiogenesis model depends on u-PA activity,18 we evaluated the effect of the various hormones on the accumulation of u-PA in the medium (Fig 3). Under standard incubation conditions, a continuous increase in u-PA accumulation rate was observed from day 2 onward over a 9-day period (Fig 3A and B). At a concentration of 1 μmol/L, testosterone and dexamethasone lowered u-PA accumulation to 66% ± 4% and 52% ± 6% of control values, respectively, after 9 days, at-RA and 9-cis RA enhanced the u-PA accumulation to 236% ± 56% and 284% ± 18%, respectively (Figs 3A and B). The other hormones tested, 17β-estradiol, 2-methoxyestradiol, progesterone, T3, and 1,25-dihydroxyvitamin D3, did not change u-PA production significantly in our in vitro model. The effect of the various hormones on u-PA accumulation thus highly parallels the effect on tube formation. Similarly as found for u-PA, PAI-1 accumulation rate increased in time. Of all the hormones tested, only 9-cis RA and at-RA slightly increased PAI-1 levels (Fig 3C and D). Because t-PA binds to fibrin, we performed parallel experiments with hMVEC grown on gelatin-coated dishes to evaluate the effect of the various experimental conditions on t-PA accumulation. t-PA production proceeded at a constant rate. Both at-RA and 9-cis RA stimulated t-PA production to 374% ± 91% and 278% ± 25% of control values, respectively, whereas the other compounds did not affect t-PA production (Fig 3E and F). Omitting bFGF and TNF-α from the standard incubation medium resulted in a very low, constant production rate of u-PA, PAI-1, and t-PA. In all, these findings are consistent with a role of u-PA (but not of t-PA) in mediating the hormonal effect on tube formation.
Accumulation of u-PA, PAI-1, and t-PA in hormone-treated hMVEC-conditioned medium. hMVEC were cultured on three-dimensional fibrin matrices (for determination of u-PA and PAI-1 production) or gelatin-coated dishes (for determination of t-PA production) in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α in the presence of vehicle (con) or the indicated hormones (1 μmol/L). (A and B) Show the accumulative production in the medium of u-PA (ng/well), (C and D) show the accumulative production of PAI-1 (ng/well), and (E and F) show the accumulative production of t-PA (ng/well). u-PA, PAI-1, and t-PA antigen were determined in the conditioned medium by ELISA as described in Materials and Methods. The data shown are from one representative experiment (of five performed). Under control conditions, the cumulative levels of u-PA, PAI-1, and t-PA after 9 days of culture varied between 20 to 49 ng/well, 2,300 to 2,440 ng/well, and 3.4 to 5.4 ng/well, respectively in the different experiments. For comparison, u-PA, PAI-1, and t-PA antigen levels were also determined when cells were cultured in incubation medium from which bFGF and TNF-α had been omitted (−BT). E2, 17β-estradiol; test, testosterone; prog, progesterone; dex, dexamethasone; 2-ME, 2-methoxyestradiol; D3, 1,25-dihydroxyvitamin D3; T3, thyroid hormone; at-RA, all-trans retinoic acid; 9-cis RA, 9-cisretinoic acid.
Accumulation of u-PA, PAI-1, and t-PA in hormone-treated hMVEC-conditioned medium. hMVEC were cultured on three-dimensional fibrin matrices (for determination of u-PA and PAI-1 production) or gelatin-coated dishes (for determination of t-PA production) in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α in the presence of vehicle (con) or the indicated hormones (1 μmol/L). (A and B) Show the accumulative production in the medium of u-PA (ng/well), (C and D) show the accumulative production of PAI-1 (ng/well), and (E and F) show the accumulative production of t-PA (ng/well). u-PA, PAI-1, and t-PA antigen were determined in the conditioned medium by ELISA as described in Materials and Methods. The data shown are from one representative experiment (of five performed). Under control conditions, the cumulative levels of u-PA, PAI-1, and t-PA after 9 days of culture varied between 20 to 49 ng/well, 2,300 to 2,440 ng/well, and 3.4 to 5.4 ng/well, respectively in the different experiments. For comparison, u-PA, PAI-1, and t-PA antigen levels were also determined when cells were cultured in incubation medium from which bFGF and TNF-α had been omitted (−BT). E2, 17β-estradiol; test, testosterone; prog, progesterone; dex, dexamethasone; 2-ME, 2-methoxyestradiol; D3, 1,25-dihydroxyvitamin D3; T3, thyroid hormone; at-RA, all-trans retinoic acid; 9-cis RA, 9-cisretinoic acid.
To further substantiate that hormonal modulation of tube formation in our in vitro model is related to changes in u-PA levels, we performed experiments in which the amount of u-PA available for angiogenesis was altered by the addition of specific antibodies or exogenous single chain u-PA (scu-PA). Figure 4A shows that the addition of antibodies, which neutralize u-PA activity, completely inhibited basal and at-RA–stimulated tube formation, whereas antibodies, which neutralize t-PA activity, were without effect. Similarly, antibodies directed against u-PAR strongly inhibited basal (by 75% ± 26%) and at-RA–stimulated tube formation (by 68% ± 19%) (Fig 4B). The structures, which were formed in the presence of u-PAR antibody, were very small and resembled single invading cells rather than capillary-like tubes. The addition of exogenous scu-PA to control cultures (mimicking retinoid-stimulated u-PA production) dose-dependently increased tube formation, and exogenous scu-PA could also restore for a great part the testosterone- and dexamethasone-inhibited tube formation (Fig 4C). Together these findings indicate that differences in u-PA levels explain the observed hormone effects on tube formation.
Effects of antibodies against u-PA, t-PA, and u-PAR and of addition of single chain u-PA on hormone-modulated tube formation. hMVEC were cultured on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and the appropriate hormone. Activity blocking antibodies to u-PA, t-PA, and u-PAR were added at the start of an experiment, whereas single chain u-PA was added from day 2 onward. After 8 to 11 days, the total length of capillary-like tubular structures was determined as described in Materials and Methods. (A) Effect of solvent (PBS), antibodies to u-PA (α uPA, 150 μg/mL) or t-PA (α tPA, 150 μg/mL) and rabbit IgG isolated from pooled normal serum (con-ab, 150 μg/mL) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% (vol/vol) DMSO) or all-trans retinoic acid (RA, 1 μmol/L). (B) Effect of solvent (PBS), antibody to u-PAR (α uPAR, 25 μg/mL) and antibody to FITC (con-ab, 25 μg/mL) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% DMSO) or all-trans retinoic acid (RA, 1 μmol/L). (C) Effect of addition of solvent (PBS) and scu-PA (10 or 30 ng scu-PA) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% DMSO), testosterone (test, 1 μmol/L), or dexamethasone (dex, 1 μmol/L). The data represent the average of two experiments performed in duplicate wells (with ranges given by error bars) and are expressed as percentages of control values (tube-length/cm2 in the presence of bFGF and TNF-α).
Effects of antibodies against u-PA, t-PA, and u-PAR and of addition of single chain u-PA on hormone-modulated tube formation. hMVEC were cultured on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and the appropriate hormone. Activity blocking antibodies to u-PA, t-PA, and u-PAR were added at the start of an experiment, whereas single chain u-PA was added from day 2 onward. After 8 to 11 days, the total length of capillary-like tubular structures was determined as described in Materials and Methods. (A) Effect of solvent (PBS), antibodies to u-PA (α uPA, 150 μg/mL) or t-PA (α tPA, 150 μg/mL) and rabbit IgG isolated from pooled normal serum (con-ab, 150 μg/mL) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% (vol/vol) DMSO) or all-trans retinoic acid (RA, 1 μmol/L). (B) Effect of solvent (PBS), antibody to u-PAR (α uPAR, 25 μg/mL) and antibody to FITC (con-ab, 25 μg/mL) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% DMSO) or all-trans retinoic acid (RA, 1 μmol/L). (C) Effect of addition of solvent (PBS) and scu-PA (10 or 30 ng scu-PA) on tube formation of hMVEC in incubation medium supplemented with vehicle (con, 0.01% DMSO), testosterone (test, 1 μmol/L), or dexamethasone (dex, 1 μmol/L). The data represent the average of two experiments performed in duplicate wells (with ranges given by error bars) and are expressed as percentages of control values (tube-length/cm2 in the presence of bFGF and TNF-α).
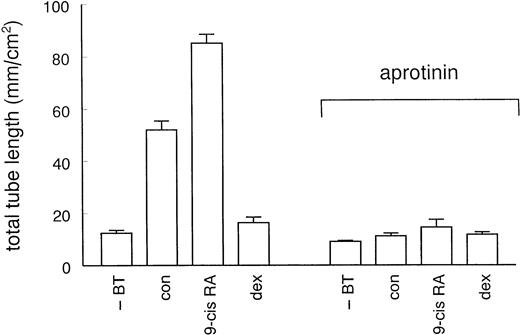
To examine whether the effect of u-PA involves proteolytic activation of plasminogen by receptor-bound u-PA, experiments were performed in the presence of aprotinin (100 KIU/mL), an inhibitor of plasmin activity. Aprotinin completely inhibited tube formation in the presence of bFGF and TNF-α alone, as well as in the additional presence of 9-cis RA or dexamethasone (Fig 5), indicating that plasmin activity is critically important for the formation of tubular structures in the fibrin matrix. To further demonstrate that receptor-bound u-PA activity is required, we performed experiments with the amino terminal fragment (ATF) of u-PA. ATF, which lacks the catalytic domain, binds similar to the u-PA receptor as u-PA, but was found to be inactive in stimulating tube formation (data not shown).
Effect of aprotinin on hormone-modulated tube formation. hMVEC were cultured on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and the appropriate hormone (1 μmol/L 9-cis RA or 1 μmol/L dexamethasone) or vehicle (con). For comparison, cells incubated in the absence of bFGF and TNF-α (−BT) were included. An inhibitor of plasmin activity, aprotinin (100 KIU/mL), was added at the start of the experiment. Total tube-length/cm2 ± standard error of mean (SEM) was determined of triplicate wells as described in Materials and Methods.
Effect of aprotinin on hormone-modulated tube formation. hMVEC were cultured on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and the appropriate hormone (1 μmol/L 9-cis RA or 1 μmol/L dexamethasone) or vehicle (con). For comparison, cells incubated in the absence of bFGF and TNF-α (−BT) were included. An inhibitor of plasmin activity, aprotinin (100 KIU/mL), was added at the start of the experiment. Total tube-length/cm2 ± standard error of mean (SEM) was determined of triplicate wells as described in Materials and Methods.
u-PA and u-PAR mRNA expression.
Northern blotting studies were conducted to examine whether the effect of hormones on u-PA protein levels were reflected at the mRNA level. After a 96-hour exposure of hMVEC to the various hormones, testosterone and dexamethasone were found to decrease u-PA mRNA levels by approximately twofold, whereas at-RA and 9-cis RA increased u-PA mRNA levels twofold and threefold, respectively (Fig 6). The other hormones did not significantly influence u-PA mRNA levels. Hybridization with the cDNA for u-PAR showed a very similar induction pattern, with the exception of dexamethasone, which did not affect u-PAR mRNA levels. Northern blotting analysis of u-PA and u-PAR mRNA expression after 8 hours of incubation very much resembled that of the 96-hour hormone exposure, except for testosterone, which did not cause any early change in u-PA and u-PAR mRNA expression. Omitting bFGF and TNF-α from the incubation medium resulted in a twofold to threefold decrease in u-PA and u-PAR mRNA levels.
Northern blot analysis of u-PA and u-PAR mRNA of hMVEC cultured in the presence of steroid hormones, retinoids, thyroid hormone, or 1,25-dihydroxyvitamin D3. hMVEC were cultured on gelatin-coated dishes in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α and 0.01% (vol/vol) DMSO (con) or 1 μmol/L of 17β-estradiol (E2), progesterone (prog), testosterone (test), dexamethasone (dex), 1,25-dihydroxyvitamin D3 (D3), thyroid hormone (T3), all-trans retinoic acid (at-RA), or 9-cisretinoic acid (9-cis RA). After 2 days, the media were refreshed. After 4 days, RNA was isolated and analyzed (7.5 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for u-PA, u-PAR, and actin mRNA. For comparison, RNA was also isolated and analyzed from cells before the addition of hormones or vehicle (t = 0). Signals for u-PA and u-PAR were quantified by densitometry and adjusted for the corresponding actin mRNA signals. Data are expressed relative to that found in control (con) cells. Results are means of two independent experiments.
Northern blot analysis of u-PA and u-PAR mRNA of hMVEC cultured in the presence of steroid hormones, retinoids, thyroid hormone, or 1,25-dihydroxyvitamin D3. hMVEC were cultured on gelatin-coated dishes in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α and 0.01% (vol/vol) DMSO (con) or 1 μmol/L of 17β-estradiol (E2), progesterone (prog), testosterone (test), dexamethasone (dex), 1,25-dihydroxyvitamin D3 (D3), thyroid hormone (T3), all-trans retinoic acid (at-RA), or 9-cisretinoic acid (9-cis RA). After 2 days, the media were refreshed. After 4 days, RNA was isolated and analyzed (7.5 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for u-PA, u-PAR, and actin mRNA. For comparison, RNA was also isolated and analyzed from cells before the addition of hormones or vehicle (t = 0). Signals for u-PA and u-PAR were quantified by densitometry and adjusted for the corresponding actin mRNA signals. Data are expressed relative to that found in control (con) cells. Results are means of two independent experiments.
Nuclear hormone receptors.
Northern analysis was performed to demonstrate the expression of mRNAs coding for RARα, RXRα, androgen receptor (AR), and glucocorticoid receptor (GR) (Fig 7). Two transcripts for RARα (3.6 kb and 2.8 kb) and one for RXRα (4.8 kb) were evident in cultured hMVEC. The human AR mRNA migrated as two species of approximately 10 kb and 7 kb and one band for GR mRNA (7 kb) was found. To determine whether the effect of testosterone, dexamethasone, andat-RA on u-PA production and tube formation was mediated by their respective nuclear receptors, experiments in the presence of receptor-specific antagonists were performed. As shown in Fig 8A, the specific androgen receptor antagonist, hydroxyflutamide,34,35 counteracted the inhibitory effect of the synthetic androgen, R1881. For blocking glucocorticoid receptor-mediated transcriptional activity, we used RU486 (mifepristone). RU486 is a synthetic progestin and glucocorticoid antagonist that binds with high affinity to progesterone and glucocorticoid receptors.36 RU486 prevented the suppressing effect of dexamethasone on tube formation (Fig 8B). The RAR antagonist Ro41-5253 completely inhibited the at-RA–induced increase in tube formation (Fig 8C). Ro41-5253 is a retinoid that specifically antagonizes the transactivation of RARs by retinoids, having a preference for RARα.37 38 These effects of the receptor-specific antagonists on tube formation were paralleled by similar changes in u-PA production (data not shown).
Nothern blot analysis of RARα, RXRα, androgen receptor (AR), and glucocorticoid receptor (GR) mRNA in hMVEC. hMVEC were cultured on gelatin-coated dishes in incubation medium without bFGF and TNF-α. RNA was isolated and analyzed (10 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for RARα, RXRα, AR, and GR mRNA. The sizes of the various transcripts were calculated by comparison to the migration of an RNA ladder.
Nothern blot analysis of RARα, RXRα, androgen receptor (AR), and glucocorticoid receptor (GR) mRNA in hMVEC. hMVEC were cultured on gelatin-coated dishes in incubation medium without bFGF and TNF-α. RNA was isolated and analyzed (10 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for RARα, RXRα, AR, and GR mRNA. The sizes of the various transcripts were calculated by comparison to the migration of an RNA ladder.
Effects of nuclear receptor antagonists on R1881-, dexamethasone- and at-RA–modulated tube formation. hMVEC were cultured for 8 to 12 days on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and to which was added 1 nmol/L R1881 (R1881) (A), 10 nmol/L dexamethasone (dex) (B), or 10 nmol/L at-RA (RA) (C). Receptor antagonists (hydroxyflutamide, RU486 or Ro41-5253; final concentration 1 μmol/L for hydroxyflutamide and RU486 and 10 μmol/L for RO41-5253) or vehicle (0.1% or 0.01% DMSO) were added 1 hour before the hormones. (A) Effect of solvent (DMSO, 0.01% [vol/vol]) or hydroxyflutamide (OH-flu, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with R1881 (R1881, 1 nmol/L). (B) Effect of solvent (DMSO, 0.01% [vol/vol]) or RU486 (RU486, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with dexamethasone (dex, 10 nmol/L). (C) Effect of solvent (DMSO; 0.1 % [vol/vol]) or Ro41-5253 (Ro41-5253, 10 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with all-trans retinoic aicd (at-RA, 10 nmol/L). The data represent the average ± standard deviation of three experiments performed in duplicate and are expressed as percentages of control values (tube-length/cm2 in the presence of bFGF and TNF-α).
Effects of nuclear receptor antagonists on R1881-, dexamethasone- and at-RA–modulated tube formation. hMVEC were cultured for 8 to 12 days on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and to which was added 1 nmol/L R1881 (R1881) (A), 10 nmol/L dexamethasone (dex) (B), or 10 nmol/L at-RA (RA) (C). Receptor antagonists (hydroxyflutamide, RU486 or Ro41-5253; final concentration 1 μmol/L for hydroxyflutamide and RU486 and 10 μmol/L for RO41-5253) or vehicle (0.1% or 0.01% DMSO) were added 1 hour before the hormones. (A) Effect of solvent (DMSO, 0.01% [vol/vol]) or hydroxyflutamide (OH-flu, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with R1881 (R1881, 1 nmol/L). (B) Effect of solvent (DMSO, 0.01% [vol/vol]) or RU486 (RU486, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with dexamethasone (dex, 10 nmol/L). (C) Effect of solvent (DMSO; 0.1 % [vol/vol]) or Ro41-5253 (Ro41-5253, 10 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with all-trans retinoic aicd (at-RA, 10 nmol/L). The data represent the average ± standard deviation of three experiments performed in duplicate and are expressed as percentages of control values (tube-length/cm2 in the presence of bFGF and TNF-α).
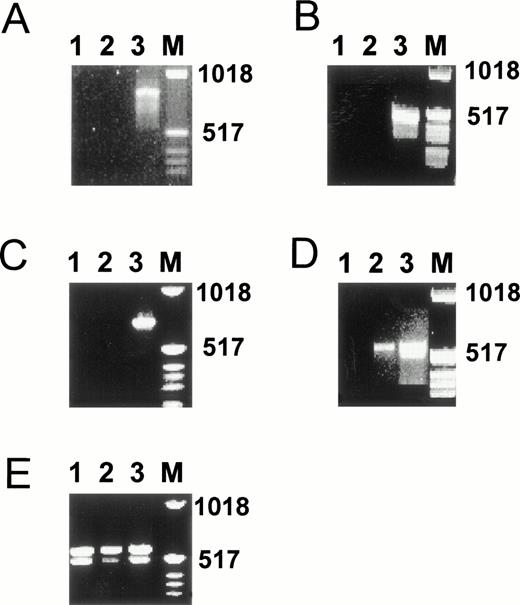
To determine whether the weak effects of some of the hormones tested are related to the low expression levels of the corresponding nuclear receptors, we performed RT-PCR analysis on RNA isolated from hMVEC cultured in incubation medium for 4 days. Indeed, the levels of progesterone receptor mRNA, estrogen receptor α mRNA, and estrogen receptor β mRNA in hMVEC were below the detection limit of our assay (Fig 9A through C). Also, in cells cultured in the absence of bFGF and TNF-α, mRNA for these receptors could not be detected. Only a very weak band for the vitamin D3 receptor could be demonstrated (Fig 9D). Unexpectedly, both thyroid hormone receptor α and β were clearly expressed (Fig 9E).
Presence of estrogen receptors α and β, progesterone receptor, thyroid hormone receptors α and β, and vitamin D receptor mRNA in hMVEC as determined by RT-PCR. hMVEC were cultured for 4 days on gelatin-coated dishes in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α or in incubation medium from which bFGF and TNF-α was omitted. RNA was isolated from these cells and cDNAs were synthesized using 1 μg total RNA and oligo dT primer as described in Materials and Methods. The cDNAs were amplified with primers for estrogen receptor α (A), estrogen receptor β (B), progesterone receptor (C), vitamin D receptor (D), and thyroid hormone receptors α and β (E) as described in Materials and Methods. The expected length of the amplified DNA fragment of the estrogen receptor α is 832 nt, of the estrogen receptor β 541 nt, of the progesterone receptor 737 nt, of the thyroid hormone receptor α 523 nt, of the thyroid hormone receptor β 458 nt, and of the vitamin D receptor 579 nt. As a positive control for the expression of estrogen receptor α, progesterone receptor, thyroid hormone receptors α and β, and vitamin D receptor mRNA, RNA isolated from MCF7 cells was used. As a positive control for the expression of the estrogen receptor β mRNA, RNA isolated from the SV-HFO osteoblast cell line was used. Lane 1, incubation medium from which bFGF and TNF-α had been omitted; lane 2, incubation medium containing bFGF and TNF-α; lane 3, appropiate positive control; M, molecular weight marker.
Presence of estrogen receptors α and β, progesterone receptor, thyroid hormone receptors α and β, and vitamin D receptor mRNA in hMVEC as determined by RT-PCR. hMVEC were cultured for 4 days on gelatin-coated dishes in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α or in incubation medium from which bFGF and TNF-α was omitted. RNA was isolated from these cells and cDNAs were synthesized using 1 μg total RNA and oligo dT primer as described in Materials and Methods. The cDNAs were amplified with primers for estrogen receptor α (A), estrogen receptor β (B), progesterone receptor (C), vitamin D receptor (D), and thyroid hormone receptors α and β (E) as described in Materials and Methods. The expected length of the amplified DNA fragment of the estrogen receptor α is 832 nt, of the estrogen receptor β 541 nt, of the progesterone receptor 737 nt, of the thyroid hormone receptor α 523 nt, of the thyroid hormone receptor β 458 nt, and of the vitamin D receptor 579 nt. As a positive control for the expression of estrogen receptor α, progesterone receptor, thyroid hormone receptors α and β, and vitamin D receptor mRNA, RNA isolated from MCF7 cells was used. As a positive control for the expression of the estrogen receptor β mRNA, RNA isolated from the SV-HFO osteoblast cell line was used. Lane 1, incubation medium from which bFGF and TNF-α had been omitted; lane 2, incubation medium containing bFGF and TNF-α; lane 3, appropiate positive control; M, molecular weight marker.
DISCUSSION
The present study demonstrates that steroid hormones and retinoids can have strong, but different effects on the formation of capillary-like tubular structures by human microvascular endothelial cells cultured on top of a human fibrin matrix in the presence of bFGF and TNF-α. We found that testosterone and dexamethasone almost completely block tube formation in this in vitro model, while at-RA and 9-cisRA strongly potentiate the effect of bFGF and TNF-α. These compounds are likely to exert their action via their respective nuclear receptors, as demonstrated by the use of receptor-specific antagonists. 17β-Estradiol, progesterone, thyroid hormone (T3), and 1,25-dihydroxyvitamin D3 showed no or only minor effects on in vitro angiogenic activity, which, with the exception of T3, could be related to the absence of significant nuclear receptor expression.
We found that testosterone and dexamethasone decreased, andat-RA and 9-cis RA increased u-PA mRNA and antigen levels. These alterations in u-PA expression not only parallel, but are also likely to be responsible for the observed changes in angiogenic activity for the following reasons. First, exogenous suppletion of the medium with scu-PA enhances tube formation in our in vitro model, whereas quenching of u-PA activity (but not t-PA activity) or u-PA binding to u-PAR by specific antibodies suppresses basal and retinoid-stimulated tube formation. Second, addition of scu-PA to testosterone- and dexamethasone-treated hMVEC restores the suppressed angiogenic activity for a substantial part.
It is likely that the effect of u-PA on tube formation involves proteolytic activation of plasminogen by receptor-bound u-PA, because inhibition of plasmin by aprotinin decreases the formation of capillaries. This does not exclude the possibility, however, that the u-PA/u-PA receptor system is also relevant for other aspects of the angiogenic process. After proteolytic disruption of the cell-matrix interaction, the cell has to create simultaneously new attachment sites by which it “pulls” itself into the matrix. Experiments by Yebra et al39 indicate that the u-PA/u-PA receptor complex has a cooperative effect with the integrin αvβ5 in promoting cell migration by providing an additional receptor for attachment to matrix proteins. Consequently, enhancement of the number of occupied u-PA receptors not only provides the endothelial cell with an enhanced local proteolytic capacity, but also provides the cell the increased capacity to form new attachment sites.
Our results exclude a major contribution of u-PA in the angiogenic process through signalling via the u-PA receptor.40,41 This cellular activation by u-PA appears to be mediated through the ATF of u-PA, which lacks the catalytic domain, but binds similar to the u-PA receptor as u-PA. However, ATF was unable to replace u-PA in our in vitro angiogenesis model in stimulating tube formation (see also Koolwijk et al18).
The small effect of 17β-estradiol on tube formation, despite the lack of an effect on u-PA production, may be related to its conversion to 2 methoxyestradiol (2-ME). 2-ME has been shown to inhibit in vitro angiogenesis, probably by interfering with tubulin polymerization.15 42 We found 2-ME to inhibit tube formation in our model without affecting u-PA expression. Inasmuch as the absence of a direct effect of 17β-estradiol on angiogenesis is related to the male origin of the endothelial cells (viz. human foreskin) is not clear at present. For a proper assessment of the role of sex steroids in angiogenesis, it may be relevant to extend our present experiments to endothelial cells of female origin.
The mechanism by which the steroid hormones and retinoids alter the TNF-α–stimulated u-PA expression in hMVEC is not known, but may be related to activation of the NFκB/Rel system by TNF-α.43,44 In unstimulated endothelial cells, NFκB/Rel family complexes are retained in the cytoplasm by the binding of inhibitory proteins, including IκBα. Endothelial activation evokes dissociation of IκBα and allows translocation of the transcription factors to the nucleus. Two functional NFκB elements at −1580 and −1865 bp have been identified in the human u-PA promoter.45 We have found, using a recombinant adenovirus expressing IκBα, that overexpressed IκBα inhibits cytokine-stimulated NFκB activation and u-PA expression in human umbilical vein endothelial cells (Kooistra and De Martin, unpublished data, 1997). The dexamethasone-binding glucocorticoid receptor can directly or indirectly bind and inactivate the NFκB transcription factor, analogous to the mechanism responsible for the negative interaction between the glucocorticoid receptor and the AP-1 transcription complex.46,47 More recent studies have also demonstrated transcriptional activation of the IκBα gene by dexamethasone.48,49 The resulting increase in IκBα protein synthesis then inhibits NFκB translocation to the nucleus. Testosterone, whose androgen receptor belongs to the same subclass of nuclear receptors as the glucocorticoid receptor, might also act by maintaining IκB levels, as suggested by Keller et al50for the repressive effect of androgens on the expression of interleukin-6. Alternatively or additionally, formation of androgen receptor/NFκB complexes may be responsible for the inhibiting effects of androgens on NFκB-mediated transcription activation.
Our finding that at-RA and 9-cis RA by themselves only slightly increase u-PA expression, but strongly upregulate the TNF-α–induced u-PA synthesis, resembles the results described by Harant et al51 for the activation of IL-8 gene transcription in the human melanoma cell line A3. It was shown that stimulation with at-RA and TNF-α resulted in enhanced NFκB binding compared with that induced by TNF-α alone and also resulted in changes in the composition of the NFκB complexes bound to the IL-8 NFκB site.
Although the present study underlines the importance of u-PA in determining tube formation in a fibrin matrix, we cannot exclude the possibility that the steroids and retinoids alter expression of other relevant parameters as well. One indication is that at-RA and 9-cis RA increase and testosterone decreases u-PAR mRNA levels in our model system. As discussed above, the u-PAR provides the cell a mechanism for localized proteolysis,52,53 which may be helpful for a tightly controlled and restricted proteolysis, both in time and in space, which is essential in the angiogenic process. Moreover, u-PAR can act as an adhesion receptor for vitronectin,54 thereby providing the cell an adhesive interaction with components of the extracellular matrix, which is another important requirement for angiogenesis to occur. Other possible regulators of the angiogenic process that are susceptible to regulation by steroids and retinoids include thrombospondin, integrins, and matrix-degrading metalloproteases (MMPs). Thrombospondin-1, shown to suppress the angiogenic response in vivo and in vitro, is regulated by progesterone in the human endometrium.55-57 Because the glucocorticoid and progesterone receptor share the same nucleotide binding sequence, dexamethasone could also enhance secretion of thrombospondin-1 in hMVEC. Similarly, at-RA has been shown to induce the expression of integrins like αvβ3 and β4.58,59These integrins are cell-membrane proteins that connect the cell cytoskeleton with matrix proteins such as fibrin, can transduce signals into the cell, and have been implicated in angiogenic processes.60 The expression of members of the MMP family, which are involved in the breakdown of the extracellular matrix, can be inhibited by glucocorticoids and retinoids.61-63 This repression has been shown to be mediated by the AP-1 binding site, present in the promoters of these genes.64 65
Changes in effecters other than u-PA may be particularly relevant in angiogenesis models based on nonfibrin matrices like collagen or mixed matrices and may also explain some of the seemingly conflicting results between different studies. For example, Pepper et al66showed that at-RA inhibited tube formation in their in vitro model using bovine endothelial cells cultured on a collagen matrix, despite the at-RA–induced increase in u-PA production. Because collagen type-1 matrices are degraded effectively only by MMPs, these results are probably due to a reduced production of MMPs in the presence of at-RA. Retinoids were also shown to be effective inhibitors of angiogenesis in the CAM assay.9 10 However, this model system reflects embryonic angiogenesis, where, to our knowledge the formation of blood vessels is independent of the presence of inflammatory mediators and where the composition of the extracellular matrix is probably very distinct from the matrix present in, for example, wounds and tumors.
Our in vitro model system reflects pathological angiogenesis in humans in the presence of fibrin and inflammatory mediators. Our results are therefore probably most relevant for angiogenic recanalization of a fibrin clot, neovascularization in tumor stroma, and the formation of new blood vessels in the temporary fibrin matrix present at sites of chronic inflammation. The different effects of the diverse hormones on angiogenesis in this model system underline the importance of the microenvironment in the angiogenic process. Our findings provide insight into the mechanism whereby normally circulating hormones influence the angiogenic activity of endothelial cells and are relevant for developing tools to influence pathological angiogenesis.
ACKNOWLEDGMENT
The authors thank Erna Peters for performing pilot in vitro angiogenesis experiments, Karin Toet for performing the t-PA ELISAs, and Marielle Kroon for performing the histochemical studies.
Supported by Grant No. 92.324 from the Netherlands Heart Foundation (The Hague, The Netherlands) and Grant No. 97.1511 from the Dutch Cancer Society (Amsterdam, The Netherlands).
Address reprint requests to Teake Kooistra, PhD, Gaubius Laboratory, TNO-PG, PO Box 2215, 2301 CE Leiden, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001ay.jpeg?Expires=1765884502&Signature=C5u5jz7T6wZNsNllaCIPxaKmgwkBfocukx9v2Qtj3qq6fHp3K~rfSfbc~EDIituVp2bytxCKNJgm3rz9Y8e35BJWCzArIWxESAYNyudqGhzJ5NZ337UE8E7yb68fGypz23i0Fyz-g6dfWytGkeEUqSEweYgzsiO0PwPW~KhAwiabFSm6eznjfegRJqOKsKA9C6aNkp-u7PUJ-zy6MPlNAKcaLgNkoNpZFWSzrrSB2udwZ7HPPdI6lxi0JltaZm0iedz3es83MG-hOIeLXDwl2xDAib7JXHvlg2DXTpR4FT~9oQrfXzMDa1zAz1GXx1UBlGDbqNkj4SPYpOHtYuAJuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001by.jpeg?Expires=1765884502&Signature=ziNMAXgIV8o2wogIxUyjIeeI9OOWjkhaQ2YuStBBscOzrySp5KWUr~qs~SnfpoLdfDdBiLjLa0OkdVChrYMrnTPVTcPbrtcYbIzbFlz2PrKDe0~kj86M8mWiwbWsmVdzQS4~jTENFU2cYYJaPDxnGBBOYNw292W1Rx7ixo-DkLcZHqbaLDZWCe8tZaR57i0REHCwyC0h7oOTrH4Wg4dYcwRuBm32WjRbxJNuWJ0cdoLqQB9XZ4IC6Y7xGHhZJ0J3VVzg8OpKwmYfJ0xVqYV-NxVJSFkYmRBlVSabKQHV5pfClrOgit2USoAXojBpUcyFN7~F5zhCjEi7VmcOZ4Yakg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001cy.jpeg?Expires=1765884502&Signature=MkD289ZPKJjFxcDJhqJvDKGzel46uq9kZxW5LSTtaiWoHgdI~iuV9mk6QOl8C2tSSuJ22Ca5UOtQoRQ3ewayu6LwLigsUBVFz-jGPZKlCB6b~Zr2KOA~FXa9HpSJnKKTiY-a2EYxRYLEteY-JZYWLnRUZ0mPIJ6OzgVhHuVO7zZviJK1TjyWzhSNc9W9MPIiTx46yYHyGrY5gq1CcS9C4Rm5oga1R3v1S77bBIobN2OsGUgCYX-fy4GP1HDj6IVr2eYoYFk2abzw0tDpGzDfD9MFgfDhTcxDAa37ovYP8BsHv9r1FV8b9cWGKBRu8JpaFfBKx3hN7JYAIGQBNpbJZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001dy.jpeg?Expires=1765884502&Signature=hFyL8LpjPUDd3I3slOPw8DqlluhRBYUgkKdGrA-Ii4ICAycj97BDCKTZP6hk6zyEyiQ8FQu6W1xmufZiTIlDA0nrTs~VvEfEFo32Lk0JqF6pRDdp8jjmeWGslRH23GuLG04c0Ae013JmEeYqiqFkulLSFqGqQUbIiQ89wYRAqq-MChovxQrRgl6VpxHanSV73A7ZZZFoT9xuRwqGyR-F-7JuHow4miT~mAd6l-p7HQuiqwjOScwRq5dQvGZCyDUUO7etBizr8saHQ8pFLm5-zFYoGzoVlIH06BglTQhIw6r~fVduV-4a6cBXk7m6aLaEI50nJgAR0-782tJsz7zncg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001ey.jpeg?Expires=1765884502&Signature=b-ShHNzbShXZ4blrDUFqatjnfPICmSYtML~QnJV4ZS22yN8MRA8hz2bcfxiNWebjpY-0zXdXYJ3yxn~ISCykzzWmvq610gNz7mJZGOy7HffqITd76470P0djSWST62Z4GHG71V8e74hV147jkg8UIo~S-i-q79ljH1iWWzmHjWfyzWV9hcdpQyyUcOIzPGqcffE2jha1JxhBFrBRqNZaxwOfu7X4~pDr9eABVazf7YhBAsnx~Tmbv~kgTkRpQilxvR3ZXHRYGI4RN2ApOa~ZL-ekkm4smgiWWA067FFO1ucN4kKmET3NhIymyJy2xbEwJM6WxG0dZIjWtNkDB79AUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Effect of all-trans retinoic acid, testosterone, and dexamethasone on in vitro angiogenesis. hMVEC were cultured on the surface of a three-dimensional fibrin matrix in incubation medium containing 20 ng/mL TNF-α and 20 ng/mL bFGF, supplemented with hormone or vehicle. After 9 days of culture, nonphase contrast photographs were taken with the plane of focus beneath the endothelial surface monolayer. (A) Incubation medium from which bFGF and TNF-α had been omitted; (B) incubation medium + vehicle (0.01% [vol/vol] DMSO); (C) incubation medium + all-trans retinoic acid (1 μmol/L); (D) incubation medium + testosterone (1 μmol/L); (E) incubation medium + dexamethasone (1 μmol/L); (F) incubation medium + vehicle; cross-section through a fibrin matrix perpendicular to the surface of the matrix, stained with hematoxylin and phloxin. Lumens surrounded by endothelial cells are indicated by arrows (original magnification ×40). Bar represents 500 μm (A through E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515001fy.jpeg?Expires=1765884502&Signature=s0b8ZHGbL536MCOTTnRg786Hgy-9F0yoMWwcxaY~bDAxhkJGvpYoLsc30BSUPx-E6k8xDaygA5hSbz5QXZIlY0hifnQ~d9IUyC8WNZqDD9AI7yZU3cMaYnhB-utxm4ilwfuSt59BLFguCO5RY3RL2P0AUHsdsOxgH8YqBRr4as9qTsRGB3Uf~nfSq3wjv64Xvu8dEA08yT5BwAsWzgeJwoZaL250dF5ZiFndipcwdy0xapq~MYdz0g8T0kOYAd2dm1tq~kKIm8I4-I8aCSawCz8TTQ~KZE3h8Kq7TcTRc1-7KxXD~~5xcCg-Pj8Mxq4Hirkzn95qwOhQ~KjJLacQ2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 6. Northern blot analysis of u-PA and u-PAR mRNA of hMVEC cultured in the presence of steroid hormones, retinoids, thyroid hormone, or 1,25-dihydroxyvitamin D3. hMVEC were cultured on gelatin-coated dishes in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α and 0.01% (vol/vol) DMSO (con) or 1 μmol/L of 17β-estradiol (E2), progesterone (prog), testosterone (test), dexamethasone (dex), 1,25-dihydroxyvitamin D3 (D3), thyroid hormone (T3), all-trans retinoic acid (at-RA), or 9-cisretinoic acid (9-cis RA). After 2 days, the media were refreshed. After 4 days, RNA was isolated and analyzed (7.5 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for u-PA, u-PAR, and actin mRNA. For comparison, RNA was also isolated and analyzed from cells before the addition of hormones or vehicle (t = 0). Signals for u-PA and u-PAR were quantified by densitometry and adjusted for the corresponding actin mRNA signals. Data are expressed relative to that found in control (con) cells. Results are means of two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515006w.jpeg?Expires=1765884502&Signature=lk3MAtaghm54eEB-OqTt6roiwYIIkNibmZVdlWyWGBZ4zi2I61vJCGi3kfXYQ-Fd8JoKUDJGNn-AfDlkHNX5pfZaZQFL0F4m6kaUSXlWWjdn0NIVxsBfsX801ncVHgV5eZh715Msf2pcrxTH3xYuOAQiiHyOqJ34iyS980F52yTWaLhfVeLv1-oCDWq~OqLOKk-TqcTdLtNpS8yLBEWSc4oZnQ7AbFjm7ovc79RvkLQJUMMDvwbPkJHX~y~PJPiG2fngnOUIbx0Eq8dnnGW61FBeAOt6BAJtiW9YiWOuV4INXT0lcR5n6agNISP~l2r65Gyxd8kqZ9zQY-8FZ0IFxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Nothern blot analysis of RARα, RXRα, androgen receptor (AR), and glucocorticoid receptor (GR) mRNA in hMVEC. hMVEC were cultured on gelatin-coated dishes in incubation medium without bFGF and TNF-α. RNA was isolated and analyzed (10 μg/lane) by Northern blot analysis using 32-[dCTP]–labeled probes for RARα, RXRα, AR, and GR mRNA. The sizes of the various transcripts were calculated by comparison to the migration of an RNA ladder.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515007w.jpeg?Expires=1765884502&Signature=slWBlJk8seVdXAbRHRBBE-MLRxGGEUYQN4-xrJ7lOJyCZwd9wZf5dLeH6CM27YQonRzbNP1Obc1CugJ8sJ0hilhII7h6CILqagSRNtt75PdrpOhQjDqeo-lMDlTnhthr-G2WHvyptjojhptvzcQ1efROhsTeYTHfjwjMYZFL2bch15Xg-qKe3sf1doeMGx447pjJHyYtdK72IFW-K52WB9XBwnNT58nI8meVySD11muctalPNSY90F0HKHSatzwGpkrx7x4Y4VkxewwL7zU0T-d0j9rTwSVzTEN1vzNl1vMz4AM9imz8I7RjDOSpWjqpr4mGWWGdTG7rQSxhRR1vig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Effects of nuclear receptor antagonists on R1881-, dexamethasone- and at-RA–modulated tube formation. hMVEC were cultured for 8 to 12 days on three-dimensional fibrin matrices in incubation medium containing 20 ng/mL bFGF and 20 ng/mL TNF-α, and to which was added 1 nmol/L R1881 (R1881) (A), 10 nmol/L dexamethasone (dex) (B), or 10 nmol/L at-RA (RA) (C). Receptor antagonists (hydroxyflutamide, RU486 or Ro41-5253; final concentration 1 μmol/L for hydroxyflutamide and RU486 and 10 μmol/L for RO41-5253) or vehicle (0.1% or 0.01% DMSO) were added 1 hour before the hormones. (A) Effect of solvent (DMSO, 0.01% [vol/vol]) or hydroxyflutamide (OH-flu, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with R1881 (R1881, 1 nmol/L). (B) Effect of solvent (DMSO, 0.01% [vol/vol]) or RU486 (RU486, 1 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with dexamethasone (dex, 10 nmol/L). (C) Effect of solvent (DMSO; 0.1 % [vol/vol]) or Ro41-5253 (Ro41-5253, 10 μmol/L) on tube formation of hMVEC in incubation medium alone (con) or supplemented with all-trans retinoic aicd (at-RA, 10 nmol/L). The data represent the average ± standard deviation of three experiments performed in duplicate and are expressed as percentages of control values (tube-length/cm2 in the presence of bFGF and TNF-α).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/3/10.1182_blood.v92.3.927/5/m_blod41515008x.jpeg?Expires=1765884502&Signature=1t95ZXhUj-pk-iuKylZdqooDTBm4~u2I7OasfzhtkkSyp04ej0dIJafeF5bGlJOgZoGFhk9rOtC9ax7uJNGEEOMtAJQqx5umR8vAADVexGnNOeb7bUPCFSIJzK8ktn7~hBc0aLTN6o~vd0WG3TV7Lawh3dye6RA5fdyep1uCe0jYgDKhB2sOMDNU8JKTBq3Afyp9npEGK6rsj~-RH-zk~Ou7WDQTVcGpOefS5mKRKt-fNlIjLlQsAhOrQ3I9q2G14MfZxqkkf9PYafhjqaWgAxpDx7yrEPlWp0xNsi7e7JqMM0EoqUhH3KTiVRsCkDIUL4mkQhMCOzVBPtBLM8DmMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal