Abstract
Hematopoietic stem cells are capable of extensive self-renewal and expansion, particularly during embryonic growth. Although the molecular mechanisms involved with stem cell maintenance remain mysterious, it is now clear that an intraembryonic location, the aorta-gonad-mesonephros (AGM) region, is a site of residence and, potentially, amplification of the definitive hematopoietic stem cells that eventually seed the fetal liver and adult bone marrow. Because several studies suggested that morphologically defined hematopoietic stem/progenitor cells in the AGM region appeared to be attached in clusters to the ventrally located endothelium of the dorsal aorta, we derived cell lines from this intraembryonic site using an anti-CD34 antibody to select endothelial cells. Analysis of two different AGM-derived CD34+ cell lines revealed that one, DAS 104-8, efficiently induced fetal-liver hematopoietic stem cells to differentiate down erythroid, myeloid, and B-lymphoid pathways, but it did not mediate self-renewal of these pluripotent cells. In contrast, a second cell line, DAS 104-4, was relatively inefficient at the induction of hematopoietic differentiation. Instead, this line provoked the expansion of early hematopoietic progenitor cells of the lin−CD34+Sca-1+c-Kit+phenotype and was proficient at maintaining fetal liver–derived hematopoietic stem cells able to competitively repopulate the bone marrow of lethally irradiated mice. These data bolster the hypothesis that the endothelium of the AGM region acts to mediate the support and differentiation of hematopoietic stem cells in vivo.
© 1998 by The American Society of Hematology.
THE HEMATOPOIETIC stem cell dwells at a diversity of anatomic sites during vertebrate development before it ultimately migrates to its final residence, the bone marrow.1-3 Early evidence suggested that the fetal yolk sac was the primordial locale of hematopoietic stem cells that seeded the fetal liver,4 and recent studies have shown the ability of yolk-sac–derived stem cells to reconstitute the bone marrow of newborn recipients but not adult animals.5 Other data, however, have suggested that an intraembryonic site, the para-aortic splanchnopleur, may be the true initial location of hematopoietic stem cell genesis and amplification. For example, based on studies of chimeric avian embryos, it was shown that the hematopoietic stem cells that seed the fetal liver and, ultimately, the bone marrow are derived from an intraembryonic site and not the extraembryonic yolk sac.6,7 The intraembryonic para-aortic splanchnopleur zone, which goes on to differentiate into the aorta-gonad-mesonephros (AGM) region, is therefore likely to be the site where definitive stem cells are first generated.8,9 Other more recent data have revealed that the para-aortic splanchnopleur/AGM region is of significant interest to hematopoietic stem cell biologists for several additional reasons. Godin and colleagues showed that the para-aortic splanchnopleuric region of mice, in addition to birds, was the earliest site of pluripotent hematopoietic stem cell development.10-13 Other groups also showed that an intraembryonic site in the mouse embryo gave rise to pluripotent stem cells capable of multilineage reconsitution of irradiated animals.14-16 Importantly, Medvinsky and Dzierzak17 established that dissected AGM regions, when placed in air/liquid organ cultures, initiated stem cell production in the absence of the yolk sac and mediated an apparent amplification of functional hematopoietic stem cells, consistent with the hypothesis that this region produces a factor(s) involved with the genesis, maintenance, and/or amplification of these pluripotent cells. In addition to these important findings, morphological studies from a number of investigators have implicated the endothelium of the AGM-localized dorsal aorta as an in vivo site of hematopoietic progenitor cell residence and, potentially, amplification. Thus, using the sialomucin CD34 as a marker for both the endothelium and the hematopoietic stem/progenitor cell, these studies revealed that a specific region of the dorsal aorta, contained within the AGM region, harbored small clusters of CD34+ hematopoietic cells attached to the endothelium in both human and murine embryos (for example, see Fig 1B,C),18,19 similar to hematopoietic clusters observed in the yolk-sac blood islands.19-21Together with the in vitro genesis and expansion data, these morphological studies implied that the endothelium of the AGM-localized dorsal aorta might provide for the support of hematopoietic stem cells in vivo.
A number of laboratories have investigated the role of the embryonic endothelium in hematopoiesis. Yoder and colleagues22 showed that endothelial-like cell lines derived from the yolk sac induced the amplification of mature myeloid cells and progenitors. Fennie and associates 23 showed that CD34+ endothelial cell lines derived from the yolk sac expanded both myeloid and erythroid progenitors as well as mature cell lineages. Lu and collaborators24 established that yolk-sac–derived endothelial cells expanded mature and progenitor myeloid cells and maintained cells capable of differentiating into both B- and T-cell lineages. In addition, these investigators showed qualitative maintenance of cells capable of repopulating all leukocyte lineages of immunocompromised (scid) hosts. Finally, Wineman et al25 and Moore et al26 showed maintenance of competitively repopulating stem cells on a fetal liver–derived stromal cell line for several weeks, although they did not examine the endothelial nature of this stromal cell type. However, although many of these studies attempted to address the ability of embryonic endothelial-like stromal cells to maintain stem and progenitor cells in vitro, they did not examine the proficiency of the endothelial cells derived from the AGM region, a known site of stem cell residence and amplification, to induce the differentiation of progenitors and maintenance of repopulating stem cells. Here we show that endothelial cell lines derived from the AGM region are capable of mediating both the differentiation of hematopoietic progenitor cells along myeloid, erythroid, and lymphoid pathways as well as maintaining hematopoietic stem cells capable of competitively repopulating lethally irradiated adult bone marrow. These data support the hypothesis that the endothelium of the AGM region is involved with embryonic hematopoietic stem cell self-renewal and differentiation.
MATERIALS AND METHODS
Mice.
Timed pregnant mice (C57Bl/6) were purchased from Simonsen laboratory (Gilroy, CA) or Harlan Sprague Dawley laboratory (Dallas, TX). Congenic C57Bl/6, Ly5.2 male mice were purchased from the National Cancer Institute (Frederick, MD). All mice were housed at the Genentech, Inc. animal facility in autoclaved microisolator cages on ventilated cage racks.
Generation of AGM-derived endothelial cell lines.
Eleven-day C57BL/6 embryos (embryo age was determined starting with day 0 on the morning of vaginal plug discovery) were removed from uteri, and 20 AGM regions were dissected with fine tungsten needles in L-15 medium (L-15) with heat-inactivated 5% fetal bovine serum (FBS; GIBCO, Gaithersburg, MD) under the dissecting microscope. Tissues were treated with 0.04% collagenase (Sigma, St Louis, MO) at 37°C for 1 hour and pipetted gently to dissociate the cell clumps. Cells were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM; GIBCO) supplemented with 10% FBS, 0.1 mmol/L nonessential amino acids, 2 mmol/L L-glutamine, penicillin-streptomycin, and 10−4mol/L β-mercaptoethanol (HAVA medium) in 75-cm2 tissue culture flasks (Corning 430720, Corning, NY) to allow for cell adherence. To stimulate the growth of endothelial cells, 150-μg/mL endothelial cell-growth supplement (ECGS; Becton Dickinson, Bedford, MA) and 10 U/mL heparin (Sigma) were added to cultures. Cultures were incubated at 37°C, 5% CO2 for 7 days. Cells were trypsinized and labeled with rabbit anti-murine CD34 polyclonal antibody (anti-CD34), and a CD34+ fraction was isolated using MiniMACS magnetic bead system (Miltenyi Biotec, Auburn, CA) as described previously.23 Subsequently, a CD34-enriched fraction was sorted for CD34+ cells by fluorescein-activating cell sorter (FACS; Coulter, Hialeah, FL) and cultured in gelatin-coated (1.5%; Sigma) 20 × 100 mm dishes (Corning 25020) in HAVA medium23 with 150 μg/mL ECGS, 10 U/mL heparin, and 20% HAVA medium previously conditioned by incubation with adherent AGM-derived cells for 7 days (HAVA-S). After cell growth reached 40% to 50% confluency, cells were infected with polyoma middle T antigen/NEOr retrovirus as described previously23 27 and cultured in HAVA-S with 800 μg/mL G418. G418-resistant colonies appeared after 1-month cultivation, and 80 clones, termed DAS (dorsal aorta-derived stroma) cells, were isolated. The DAS104 cell line was subcloned for highly CD34+ cells by FACS. Thirty DAS104-derived CD34+ cells were cultured in 96-well plates (Falcon 3072) in HAVA medium, and 18 subclones were obtained. Expression of CD34 antigen was examined in each subclone by FACS, and two subclones, DAS104-4 and DAS104-8, were selected for further analysis. Analysis of capillary formation in Matrigel (Biocoat 40234C; Becton Dickinson) was performed as per the manufacturer's instructions.
Antibodies.
The antibodies used in FACS were all obtained from Pharmigen (San Diego, CA) except anti-CD34 polyclonal antibody28 and secondary reagents. The antibodies used in the lineage cocktail are anti-CD3 (145-2C11), anti-CD4 (GK1.5), anti-CD5 (53-7.3), anti-CD8 (53-6.7), anti-B220 (6B2), anti–Gr-1 (8C5), and antierythroid (TER-119). Other antibodies include anti–Sca-1 (E13) labeled with phycoerythrin (PE), anti–c-Kit (2B8) labeled with biotin, fluorescein isothiocyanate- (FITC) or PE-conjugated anti-CD3, anti-CD4, anti-CD8, anti-B220, anti-IgM, anti–Gr-1 and anti–Mac-1 antibody. Secondary reagents include goat antirat IgG labeled with cascade blue (Molecular Probes, Eugene, OR), 670-labeled streptavidin (GIBCO), and donkey antirabbit IgG–labeled FITC (Jackson Immunoresearch, West Grove, PA).
Isolation of fetal liver stem cells.
Fetal liver cells were prepared from day-13 embryos. Livers were dissected and made into a single-cell suspension with a 22-G needle and passed through a nylon mesh screen. Fetal liver mononuclear cells were isolated by density centrifugation over Ficoll solution (1.077 g/mL; Nycomed, Oslo, Norway ). Cells were cultured in 175-cm2flasks (Falcon 3028) overnight, and nonadherent cells were obtained. Cells were suspended in L-15/5% FBS and incubated with CD16/CD32 (2.246) antibody (1:200) (Pharmigen) on ice for 15 minutes. Subsequently, cells were stained with the lineage cocktail of unlabeled antibodies for 30 minutes on ice. After washing twice, cells were stained with goat antirat magnetic beads (Miltenyi Biotec, Auburn CA) and then stained with goat antirat cascade blue. Cells were applied to BS type MACS column (Miltenyi Biotec), and nonadherent fraction was collected. After incubation with normal rat serum (GIBCO) for 15 minutes on ice, cells were stained with anti-CD34 antibody, anti–Sca-1 antibody, and anti–c-Kit antibody. After final washes, cells were suspended in L-15/5% FBS with 1 μg/mL propidium iodide (PI; Sigma) and filtered through a nylon mesh screen. Viable cells were selected by PI exclusion, and four-color FACS for lineage-negative, CD34+, Sca-1+, c-Kit+ cells was performed on a dual-laser Epics Elite ESP cell sorter (Coulter Immunology, Hialeah, FL). After cell sorting, over 95% purity was observed in sorted cells.
In vitro expansion of fetal liver cells on DAS cell lines.
All stromal cell lines were grown in HAVA medium at 37°C in 5% CO2. Stem cells were also cultured on b-End3,27YS Cl-71, or YS Cl-7223 and cocultured under the same conditions as DAS cell lines. Stromal cells were irradiated (20 Gy,137Cs, Gammacell 100; Nordin International Inc, Ontario, Canada) 2 days before coculture and plated to confluence in gelatin-coated 20 × 100–mm dishes (Corning). A total of 3 × 103 sorted fetal liver stem cells (lin−CD34+Sca-1+c-Kit+) was added onto each stromal cell line; cultures were maintained in HAVA medium at 37°C, 5% CO2 in high humidity; and half volume of the medium in each culture was changed every other day. After 7 or 10 days, cells were obtained with 0.04% trypsin-EDTA (GIBCO) or 2% collagenase H (Boehringer Mannheim, Indianapolis, IN), and single cells were obtained using disruption by a 22-G needle and passage through nylon mesh screen. Nonadherent cells were collected after 1-hour incubation on gelatin-coated dishes at 37°C. Numbers of viable cells were scored under the microscope by Trypan blue exclusion, and morphology was examined by Wright-Giemsa (Diff-Quik; Dade Diagnostic, Aguada, Puerto Rico) staining of cytospin preparations.
Irradiated DAS104-4 or DAS104-8 were cultured in gelatin-coated 6-well plates (Falcon 3046), and 1 × 103 fetal liver stem cells were added onto the stromal cells. Cultures were maintained in HAVA medium in the presence of 3 U/mL erythropoietin (EPO; R&D Systems, Minneapolis, MN) or 10 ng/mL interleukin-7 (IL-7; R&D Systems) for 11 days or 12 days. Cells were obtained by vigorous pipetting, and cell differentiation was identified by FACS. Myeloid cell differentiation was based on expression of Gr-1 or Mac-1; B-cell differentiation on B220 or IgM; erythroid differentiation on TER 119; and T-cell differentiation on CD3, CD4, or CD8.
Irradiated DAS104-4 cells were plated to confluence on gelatin-coated 6-well plates (Biocoat 4057; Becton Dickinson) and 1 × 103 fetal liver stem cells were then either seeded directly onto the stromal cells or into transwell apparatus (0.45 μmol/L pore size membrane; Biocoat 4057). Cultures were maintained for 7 days, and cells were obtained with trypsin-EDTA or by vigorous pipetting. Numbers of expanded cells were scored under the microscope in each well.
Long-term reconstitution assays.
All mice (C57Bl/6) used as donors in this experiment were Ly5.1. C57Bl/6-Ly5.2 recipient mice were lethally irradiated with 1,050 rads as a single dose from a 137Cs source. Freshly sorted stem cells or progenitor cells cultured on stromal cells were injected into the tail vein along with 1 × 106 whole bone marrow cells from an Ly5.2 congenic source for radioprotection. Groups of 6 to 8 mice were used for each cell type injected. For analysis of reconstitution, mice were bled from the retro-orbital sinus and assayed for the presence of Ly5.1+ cells at 4 weeks or 8 weeks after transplantation. Peripheral blood was collected in phosphate buffered saline with 1 mmol/L EDTA and 10 U/mL heparin (Sigma). Red cells were removed by sedimentation in 1% Dextran T-500 (Pharmacia Biotech, Piscataway, NJ) and lysing in cold 0.15 mol/L NH4Cl. Cells were stained with anti-Ly5.1 antibody (104; Pharmingen) and then stained with PE-labeled streptavidin (Jackson ImmunoResearch).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of cell line transcripts.
Total RNA was prepared from each stromal cell line using RNA isolation reagent (STAT 60; TEL-TEST “B” Inc, Friendswood, TX). The Perkin-Elmer (Foster City, CA) RT-PCR kit was used as described previously.23 For each reaction, 1 μg of total RNA was used for a total of 35 cycles in a Perkin-Elmer thermocycler using the following conditions: 1 minute at 95°C, 1 minute at 56°C, and 2 minutes at 72°C. PCR reactions were electrophoresed on 1.5% agarose gels, stained with ethidium bromide, and examined. The PCR primers were as follows: stem cell factor (SCF)-5′: 5′-TCTTCCAAATGACTATATGATAACCCTC-3′, SCF-3′: 5′-ATTCCTAAGGGAGCTGGCTGCAACAGGG-3′; FLK2 ligand (FLK2/FLT3 L)-5′: 5′-CTGCTGTTGCTGCTGCTGAGTCCTTGC-3′, FLK2/FLT3L-3′: 5′-GTCCGGCTGGCACTGCACCTCCAGGC-3′; vascular endothelial growth factor (VEGF)-5′: 5′-GTGATCAAGTTCATGGACGTCTACCAGCG-3′, VEGF-3′: 5′-TACGTTCGTTTAACTCAAGCTGCCTCGC-3′; macrophage colony-stimulating factor (M-CSF)-5′: 5′-CAATGCTAACGCCACCGAGAGGCTCCAGG-3′, M-CSF-3′: 5′-GGTACTCCTGGGTGGTCGCTGCTTGGC-3′; granulocyte colony-stimulating factor (G-CSF)-5′: 5′-AGTGACATATGGTCAGGACGAGAGGC-3′, G-CSF-3′:5′-GGGCCACCCCTAGGTTTTCCATCTGCT-3′; transforming growth factor β-1 (TGFβ-1)-5′: 5′-CCTTGCTGCTGCCTGTAGATGGGACTGAC-3′, TGFβ-1–3′: 5′-GGTGCCCTGCCAGAAGACATGGCCTCC-3′; CD34-5′: 5′-CTACCACGGAGACTTCTACACAAGG-3′, CD34-3′:5′-CCAACCTCACTTCTCGGATTCCAGAGC-3′; CD31-5′: 5′-TGCGATGGTGTATAACGTCACCTCCA-3′, CD31-3′: 5′-GCTTGGCAGCGAAACACTAACACGTG-3′; IL-3–5′: 5′-GTGGCCGGGATACCCACCGTTTAAC-3′, IL-3–3′: 5′-GAGACGGAGCCAGATGCGGGCTGAGGTGG-3′; IL-6–5′: 5′-ATACCACTCCCAACAGACCTGTCTATACC-3′, IL-6–3′: 5′-TGTGACTCCAGCTTATCTGTTAGGAGAGC-3′; thrombopoietin (TPO)-5′: 5′-CTTTGTCTATCCCTGTTCTGCTGCCTGCTG-3′, TPO-3′: 5′-TGAGAAGTACTGCTTGGGACAGCTGTGG-3′; EPO-5′: 5′-GAACGTCCCACCCTGCTGCTTTTACTCTCC-3′, EPO-3′: 5′-CCCAGTACCCGAAGCAGTGAAGTGAGGC-3′; granulocyte-macrophage colony-stimulating factor (GM-CSF)-5′: 5′-TCTACAGCCTCTCAGCACCCACCCGCTCA-3′, GM-CSF–3′: 5′-GGCTGTCTATGAAATCCGCATAGGTGG-3′; IL-1–5′: 5′-TGTCCAGATGAGAGCATCCAGCTTC-3′, IL-1–3′: 5′-GATTCTTTCCTTTGAGGCCCAAGGCCA-3′; IL-11–5′: 5′-AGATCTGGACAGCGCTGTTCTCTCCTAA-3′, IL-11–3′: 5′-AGTCGAGTCTTTAACAACAGCAGGCC-3′; LIF-5′: 5′-TCTCTTCATTTCCTATTACACAGCTC-3′, LIF-3′: 5′-AGAAGGCCTGGACCACCACACTTAT-3′; von Willebrand factor (vWF)-5′: 5′-ATGATGGAGAGGTTACACATCTCTCAG-3′, vWF-3′: 5′-CCAGCTCATCCACCCCACTGAGCAG-3′; TIE-2–5′: 5′-GAGAACATGTGAGAAAGCTTGTGAG-3′, TIE-2–3′: 5′-CTAGGGTCATTTCTTCACTAGT-3′; FLK1-5′: 5′-CTTAGGTGCCTCCCCATACCCTGGG-3′, FLK1-3′: 5′-TGGCCGGCTCTTTCGCTTACTGTTC-3′; FLT-1–5′: 5′-ATGATGCCAGCAAGTGGGAGTTTGC-3′, FLT-1–3′: 5′-GGTTTCCATATTTGCAGTATTC-3′; delta-like protein (dlk)-5′: 5′-GAACCATGGCAGTGCATCTGCAAGGA-3′, dlk-3′: 5′-TTGCACAGACACTCGAAGCTCACCTG-3′.
Analysis of fetal liver stem cell division on DAS104-4 cells.
Sorted lin−CD34+Sca-1+c-Kit+fetal liver stem cells were stained with 8 × 10−6 mol/L PKH67 (Sigma)29 for 3 minutes at room temperature. Cells were washed three times and fluorescence intensity of PKH67-GL was analyzed by FACS. A total of 5 × 103 PKH67+ cells were plated onto irradiated DAS104-4 cells in 10-cm dishes and cultured for 3 days, 4 days, or 5 days. Cells were obtained by 2% collagenase H treatment, and cell aggregates were dissociated by gentle passage through syringe with a 22-G needle. Cells were suspended in L-15/5% FBS and incubated with CD16/CD32 antibody for 15 minutes on ice. Subsequently, cells were stained with CD45 (Ly-5, 30-F11) antibody conjugated with PE (Pharmingen) for 30 minutes on ice. After washing twice, cells were suspended in L-15/5% FBS with 1 μg/mL PI and passed through a nylon mesh screen. Cells were gated for live cells (PI−) and CD45 (PE+ hematopoietic cells), and PKH67-GL fluorescence intensity was analyzed by FACS. Fluorescence intensity of stem cells in PKH67+ cells was obtained by gating cells for high expression of PKH67. Mean ± SD was evaluated from triplicate dishes. Cells in PKH67 high or low gates were isolated by cell sorting and used for reconstitution as described above.
RESULTS
Generation of AGM-derived CD34+ endothelial cell lines.
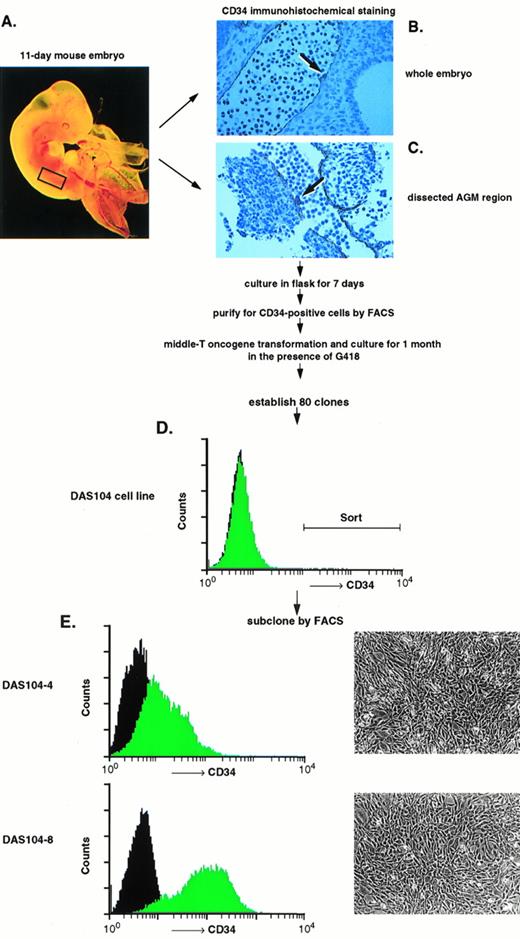
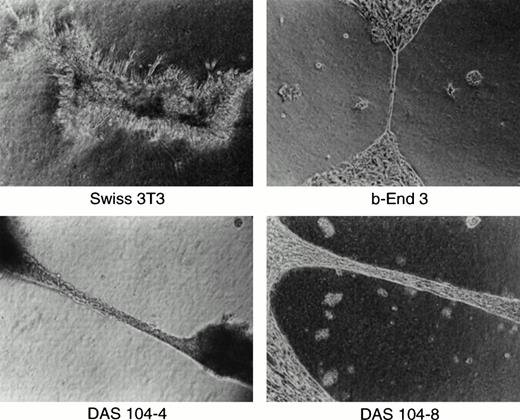
We and others have previously shown the utility of the anti-CD34 polyclonal antibody for the isolation of both embryonic endothelium as well as hematopoietic progenitor cells.5,16,23,30 This antibody is particularly useful for endothelial cell isolation and characterization, because global analyses of the adult and embryo revealed that only hematopoietic progenitor cells, endothelial cells, and a small subset of neurons express this antigen.20,21,28Other groups have found clusters of CD34+ hematopoietic progenitor cells attached to the ventral endothelium of the dorsal aorta using anti-CD34 antibodies to stain the AGM region in both human and murine embryos, and these results are confirmed in Fig 1.18,19 At least a portion of these CD34+ hematopoietic cells are stem cells capable of repopulating irradiated bone marrow, consistent with the proposal that AGM-derived endothelium supports hematopoietic stem cells.16,31 To obtain these endothelial cells, the AGM region of day-11 murine embryos was isolated by dissection (Fig 1A). Staining of this isolated tissue with anti-CD34 antibody showed positively reacting blast-like hematopoietic progenitor cells, a subset of which were presumably stem cells, and elongated, endothelial-like cells that also expressed CD34 (Fig 1C). Because endothelial cells are adherent and the numbers of cells obtained were low, isolated AGMs were enzymatically dissociated and the adherent cells were expanded in tissue culture for 7 days. CD34+ adherent cells, presumably of endothelial origin, were isolated by FACS, and the resultant cells were transformed using a polyoma virus middle-T–expressing retrovirus. Earlier we and others had shown that this oncogene appears to transform endothelial cells and maintain these cells in an endothelial-like state.23,27 32 CD34+ (ie, endothelial), middle-T–transformed, G418-resistant cells were isolated by FACS, and 80 cell lines were established. Thirty lines were tested for their ability to mediate the expansion of differentiated hematopoietic cells, the morphologies of hematopoietic cell colonies, and the expansion of high proliferative potential mixed colonies (HPP-CFC) in methyl cellulose. One cell line, termed DAS 104, was chosen for its ability to mediate high-level expansion of differentiated hematopoietic cells as well as HPP-CFC, and this line was sorted again for CD34 expression by FACS (Fig 1D). Two morphologically similar CD34+ adherent cell lines, DAS 104-4 and DAS 104-8 (Fig 1E), were selected for further analysis. The endothelial cell nature of these lines was additionally tested using RT-PCR. As Table 1 shows, a number of endothelial cell markers, including CD34, FLT1, and FLK1 (both VEGF receptors), vWF, and CD31 (platelet endothelial cell adhesion molecule [PECAM]), were found to be expressed by both cell lines. Finally, both the DAS 104-4 and DAS 104-8 cell lines efficiently formed capillary-like tubes when placed in Matrigel (Fig 2). Together, these data are consistent with the conclusion that these two lines represent endothelium derived from the AGM region.
Isolation and characterization of AGM-derived endothelial cells. (A) A day-11 murine embryo highlighting (rectangle) the AGM region that was dissected and used as a source for CD34+endothelial cells. (B) Transverse section through 11-day murine embryo AGM region stained with anti-CD34 antibody. Note the positively stained endothelium and adherent hematopoietic progenitor cells attached to the dorsal aorta (arrow), similar to those previously observed in the blood islands of the yolk sac and the AGM region of murine and human embryos.18-21 (C) Dissected AGM region stained with anti-CD34 antibody. Note the positively stained endothelial cells and adherent hematopoietic progenitor cells attached to the dorsal aorta (arrow). (D) A progenitor DAS 104 cell line was generated by polyoma virus middle-T transformation and was stained with anti-CD34 antibody. The CD34+ cells were isolated by FACS sorting. (E) DAS 104-4 and DAS 104-8 cell lines were stained with anti-CD34 antibody and analyzed by FACS. Note that both cell lines are positive for the CD34 antigen. Morphology of DAS 104-4 and DAS 104-8 cell lines showing typical flattented, endothelial-like structures.
Isolation and characterization of AGM-derived endothelial cells. (A) A day-11 murine embryo highlighting (rectangle) the AGM region that was dissected and used as a source for CD34+endothelial cells. (B) Transverse section through 11-day murine embryo AGM region stained with anti-CD34 antibody. Note the positively stained endothelium and adherent hematopoietic progenitor cells attached to the dorsal aorta (arrow), similar to those previously observed in the blood islands of the yolk sac and the AGM region of murine and human embryos.18-21 (C) Dissected AGM region stained with anti-CD34 antibody. Note the positively stained endothelial cells and adherent hematopoietic progenitor cells attached to the dorsal aorta (arrow). (D) A progenitor DAS 104 cell line was generated by polyoma virus middle-T transformation and was stained with anti-CD34 antibody. The CD34+ cells were isolated by FACS sorting. (E) DAS 104-4 and DAS 104-8 cell lines were stained with anti-CD34 antibody and analyzed by FACS. Note that both cell lines are positive for the CD34 antigen. Morphology of DAS 104-4 and DAS 104-8 cell lines showing typical flattented, endothelial-like structures.
Transcript Analysis of AGM-Derived Endothelial Cell Lines
| Transcript . | DAS104-4 . | DAS104-8 . |
|---|---|---|
| Endothelial markers | ||
| VEGF | ++ | ++ |
| CD34 | ++ | ++ |
| vWF | ++ | ++ |
| FLK1 | ++ | + |
| FLT1 | ++ | ++ |
| CD31 | ++ | + |
| TIE-2 | +/− | +/− |
| Hematopoietic cytokines | ||
| IL-1 | + | + |
| IL-3 | − | − |
| IL-6 | ++ | ++ |
| IL-11 | ++ | ++ |
| EPO | − | − |
| TPO | + | + |
| LIF | ++ | ++ |
| TGF-β | + | + |
| FLT3 | ++ | ++ |
| SCF | ++ | ++ |
| M-CSF | + | + |
| G-CSF | − | − |
| GM-CSF | ++ | + |
| DLK | ++ | ++ |
| Transcript . | DAS104-4 . | DAS104-8 . |
|---|---|---|
| Endothelial markers | ||
| VEGF | ++ | ++ |
| CD34 | ++ | ++ |
| vWF | ++ | ++ |
| FLK1 | ++ | + |
| FLT1 | ++ | ++ |
| CD31 | ++ | + |
| TIE-2 | +/− | +/− |
| Hematopoietic cytokines | ||
| IL-1 | + | + |
| IL-3 | − | − |
| IL-6 | ++ | ++ |
| IL-11 | ++ | ++ |
| EPO | − | − |
| TPO | + | + |
| LIF | ++ | ++ |
| TGF-β | + | + |
| FLT3 | ++ | ++ |
| SCF | ++ | ++ |
| M-CSF | + | + |
| G-CSF | − | − |
| GM-CSF | ++ | + |
| DLK | ++ | ++ |
Total RNA was isolated from the DAS104-4 and DAS104-8 cell lines and treated with DNAase. Transcripts were measured using specific oligonucleotide primers and 35 cycles of PCR under standard conditions. The resultant reactions were analyzed by agarose gel electrophoresis and ethidium bromide staining.
Abbreviations: ++, strong ethidium bromide stained band; +, weak ethidium bromide stained band; +/−, barely detectable ethidium bromide stained band; −, undetectable ethidium bromide stained band. All positive bands migrated with the appropriate molecular weight.
Capillary formation in vitro. Murine 3T3 fibroblasts, brain-derived capillary endothelial cell line b-End-3, and DAS 104-4 or DAS 104-8 endothelial cell lines were plated in Matrigel, grown for 2 days, and photographed.
Capillary formation in vitro. Murine 3T3 fibroblasts, brain-derived capillary endothelial cell line b-End-3, and DAS 104-4 or DAS 104-8 endothelial cell lines were plated in Matrigel, grown for 2 days, and photographed.
Expansion of fetal liver stem cells on AGM-derived cell lines.
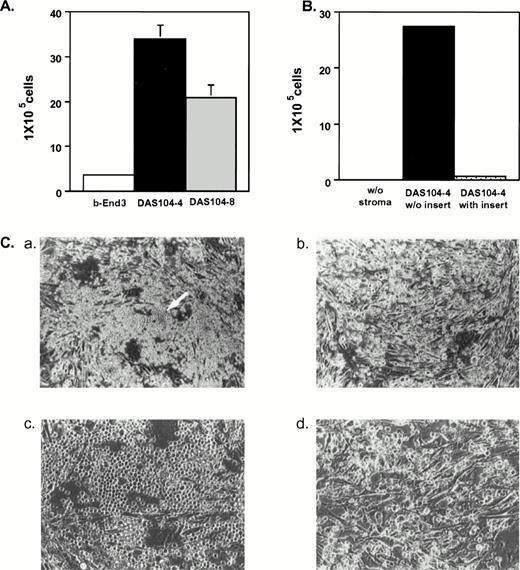
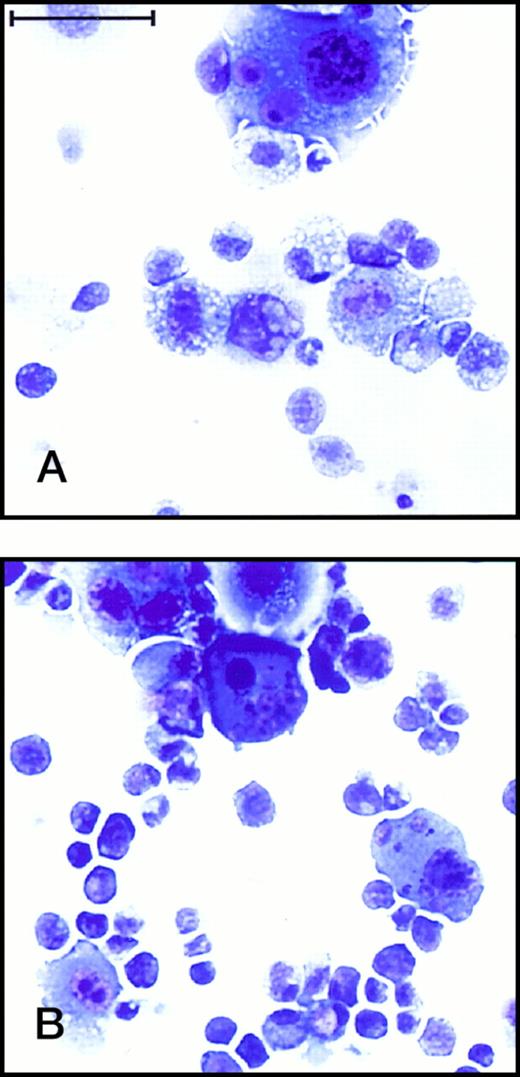
Initial studies were done to examine the ability of each AGM-derived cell line to mediate the expansion and differentiation of lin−CD34+Sca-1+c-Kit+hematopoietic stem cells isolated from fetal liver.33Figure 3A illustrates that DAS 104-4 and DAS 104-8 cells were capable of inducing several thousand-fold increases in hematopoietic cell number after only 7 days of coculture, whereas the polyoma middle-T–transformed endothelial cell line, b-End 3,27 showed only modest expansion of these progenitor cells. Examination of expanded hematopoietic cells by Wright-Giemsa staining revealed that the amplified cells appeared to be less mature when grown on DAS 104-4 versus DAS 104-8 (Fig 4). However, the cells expanded on both cell lines appeared to have a predominately myeloid appearance. Rare blast-like cells were also observed in the cytospin analyses of expanded cells, and examination of the colony morphology of hematopoietic cells incubated on either AGM-derived cell line revealed both large macrophage-like cells and small blast-like cells in “cobblestone”-type hematopoietic colonies (Fig 3C). In addition, c-Kit+ cells isolated from both the AGM and lin−CD34loSca-1+c-Kit+cells from adult bone marrow are also dramatically expanded on both endothelial cell lines (data not shown). These data suggested that both AGM-derived endothelial cell lines were capable of mediating the dramatic and rapid expansion and differentiation of fetal liver, AGM, and adult bone marrow hematopoietic stem cells along at least the myeloid pathway.
Expansion of fetal liver hematopoietic stem cells on AGM-derived endothelial cell lines. (A) Three thousand fetal liver stem cells (lin−CD34+Sca-1+c-Kit+) were plated on each stromal cell line and cultured for 7 days. Cells were trypsinized, and nonadherent cells were collected and counted. (B) Cell contact is required for efficient expansion of fetal liver stem cells on DAS 104-4. One thousand fetal liver stem cells (lin−−CD34+Sca-1+c-Kit+) were plated either directly onto the DAS 104-4 cell line or in a transwell (insert) immersed in media conditioned by this cell line. After 1 week, nonadherent hematopoietic cells were isolated and counted. (C) Hematopoietic colony morphologies of fetal liver stem cells plated on the DAS 104-4 cell lines after 5 days in culture. Panels a (20×) and c (200×) show a “cobblestone”-type structure containing blast-like cells (arrow in panel a). Panels b (20×)and d (200×) show macrophage-like cells that predominate in the cultures.
Expansion of fetal liver hematopoietic stem cells on AGM-derived endothelial cell lines. (A) Three thousand fetal liver stem cells (lin−CD34+Sca-1+c-Kit+) were plated on each stromal cell line and cultured for 7 days. Cells were trypsinized, and nonadherent cells were collected and counted. (B) Cell contact is required for efficient expansion of fetal liver stem cells on DAS 104-4. One thousand fetal liver stem cells (lin−−CD34+Sca-1+c-Kit+) were plated either directly onto the DAS 104-4 cell line or in a transwell (insert) immersed in media conditioned by this cell line. After 1 week, nonadherent hematopoietic cells were isolated and counted. (C) Hematopoietic colony morphologies of fetal liver stem cells plated on the DAS 104-4 cell lines after 5 days in culture. Panels a (20×) and c (200×) show a “cobblestone”-type structure containing blast-like cells (arrow in panel a). Panels b (20×)and d (200×) show macrophage-like cells that predominate in the cultures.
Wright-Giemsa staining of hematopoietic cells expanded on DAS 104-4 and DAS 104-8. (A) Hematopoietic cells expanded for 7 days on DAS 104-4. The large cells are stromal cells and the small cells are hematopoietic cells. Note the relatively immature morphology of the hematopoietic cells. (B) Hematopoietic cells expanded for 7 days on DAS 104-8. Note the mature macrophage-like appearance of the smaller hematopoietic cells. The bar corresponds to 100 μm.
Wright-Giemsa staining of hematopoietic cells expanded on DAS 104-4 and DAS 104-8. (A) Hematopoietic cells expanded for 7 days on DAS 104-4. The large cells are stromal cells and the small cells are hematopoietic cells. Note the relatively immature morphology of the hematopoietic cells. (B) Hematopoietic cells expanded for 7 days on DAS 104-8. Note the mature macrophage-like appearance of the smaller hematopoietic cells. The bar corresponds to 100 μm.
Fetal liver stem cell expansion requires direct contact.
Because morphological analyses of embryos showed direct contact between CD34 hematopoietic progenitor cells and the yolk sac and AGM endothelium,18-20 we investigated if direct contact was required for hematopoietic cell amplification by the AGM-derived stromal cells. Fetal liver stem cells (lin−CD34+Sca-1+c-Kit+) were incubated with the DAS 104-4 cell line either directly or using a transwell apparatus that inhibited physical contact between the hematopoietic and stromal cells. Nonadherent (hematopoietic) cell numbers were determined 1 week after inoculation of the cultures with the fetal liver stem cells. As Fig 3B illustrates, cells that were in direct contact with the stromal cell line were amplified approximately 2,600-fold, while cells separated from the stromal cells by the transwell apparatus showed only an approximately 70-fold amplification. Hematopoietic reconstitution assays of lethally irradiated animals (see below) revealed that hematopoietic cells derived from transwell cultures were incapable of mediating bone marrow reconstitution, whereas cells derived from direct contact cultures mediated long-term reconstitution (data not shown). These data show that direct physical contact between the stromal cells and the hematopoietic progenitors is required for maximal amplification and differentiation, consistent with the in vivo morphological analyses showing clusters of CD34+ hematopoietic cells attached to the endothelium of the dorsal aorta.18 19
Differentiation of fetal liver stem cells along erythroid and B-lymphoid pathways.
Previous analysis of yolk-sac–derived endothelial cell lines showed that the addition of EPO to cultures containing yolk-sac CD34+ hematopoietic progenitor cells induced a fraction of these cells to differentiate along the erythroid pathway.23Other studies showed the ability of a different yolk-sac–derived endothelial cell line to induce B-cell formation.24 To examine the ability of AGM-derived endothelial cells to induce both erythroid and B-lymphoid differentiation, fetal liver stem cells were incubated with each cell line in the presence of EPO or IL-7, respectively. Expanded cells were then analyzed for the erythroid cell surface marker, TER-119, or the B-cell marker, B 220, using FACS. As Table 2 illustrates, the majority of cells expanded in the absence of any exogenous differentiation factors expressed the myeloid-specific markers Mac-1 and Gr-1, consistent with the cytospin analysis described above. In the presence of EPO, the DAS 104-8 cell line induced fetal liver stem cells to differentiate predominately down the erythroid lineage pathway, with approximately 72% of the cells expressing the TER-119 marker. Even more striking were results obtained with IL-7 on the DAS 104-8 cell line, where approximately 93% of the mature cells expressed the B220 B cell marker. These data together suggested that the DAS 104-8 cell line was capable of efficiently inducing differentiation of fetal liver stem cells down the myeloid, erythroid, and B-lymphoid pathways. In contrast to DAS 104-8, DAS 104-4 appeared to be far less effective at provoking differentiation down the erythroid and B-lymphoid pathways. Thus, this cell line induced only approximately 24% of differentiated cells down the erythroid path in the presence of EPO and was essentially incapable of mediating the formation of B cells in the presence of IL-7 (Table2). These data argue that, although the AGM-derived endothelial cell lines are heterogeneous in their effects on the growth and differentiation of hematopoietic stem cells, they are capable of efficiently inducing the diverse differentiation of fetal liver stem cells.
Differentiation of Fetal Liver Stem Cells on AGM-Derived Cell Lines
| Antibody . | % Lineage Positive Cells . | |
|---|---|---|
| DAS104-4 . | DAS104-8 . | |
| Mac-1 | 96.9 ± 0.7 | 93.8 ± 0.9 |
| Gr-1 | 76.0 ± 4.8 | 67.4 ± 4.9 |
| TER-119 | 13.8 ± 2.6 | 14.2 ± 4.0 |
| B220 | 2.6 ± 0.4 | 3.3 ± 4.0 |
| TER-119 (+EPO) | 24.4 ± 0.9 | 71.7 ± 2.7 |
| B220 (+IL-7) | 1.8 ± 0.8 | 92.5 ± 1.6 |
| Antibody . | % Lineage Positive Cells . | |
|---|---|---|
| DAS104-4 . | DAS104-8 . | |
| Mac-1 | 96.9 ± 0.7 | 93.8 ± 0.9 |
| Gr-1 | 76.0 ± 4.8 | 67.4 ± 4.9 |
| TER-119 | 13.8 ± 2.6 | 14.2 ± 4.0 |
| B220 | 2.6 ± 0.4 | 3.3 ± 4.0 |
| TER-119 (+EPO) | 24.4 ± 0.9 | 71.7 ± 2.7 |
| B220 (+IL-7) | 1.8 ± 0.8 | 92.5 ± 1.6 |
Day-14 fetal liver stem cells (lin−CD34+Sca-1+c-Kit+) were plated on each DAS cell line and cultured for 11 to 12 days with or without the indicated added cytokine. Nonadherent cells were obtained by vigorous pipetting and stained with PE-conjugated antibodies as indicated. The frequency of lineage positive cells was determined by FACS in triplicate wells (mean ± SD). TER-119+ cells were analyzed using the red cell gate by FACS.
Stem cell maintenance on AGM-derived endothelial cells.
Although the above data indicated that one of the AGM-derived endothelial cell lines was capable of inducing differentiation along three mature hematopoietic lineages, they did not address the ability of these endothelial cells to maintain or expand hematopoietic stem cells. This question was particularly important in light of in vitro studies that suggested that isolated AGM regions appeared to expand repopulating hematopoietic stem cells when placed in organ culture for 3 days.17 To investigate this question, we purified lin−CD34+Sca-1+c-Kit+hematopoietic stem cells from fetal liver using FACS and examined whether DAS 104-4, DAS 104-8, and a previously described yolk-sac–derived endothelial cell line, YS CL72,23 could maintain these cells in an undifferentiated state in culture. Table 3 illustrates that the DAS 104-4 cell line expanded this population of fetal liver stem cells after 7 days of incubation in two separate experiments, whereas the DAS 104-8 cell line was unable to mediate expansion of these progenitor cells. Although these data suggest that DAS 104-4 may support fetal liver stem cells more efficiently than DAS 104-8, the ability of such progenitor cells to mediate reconstitution of the bone marrow of lethally irradiated animals must be shown to prove this conjecture.34 35 To explore this question, competitive repopulation assays were performed with lin−CD34+Sca-1+c-Kit+ fetal liver stem cells. Table 4 shows that as few as 1,000 uncultured lin−CD34+Sca-1+c-Kit+fetal liver stem cells could substantially contribute (66% to 72%) to circulating hematopoietic cells, including mature T-cell, B-cell, and myeloid lineages, at 15 weeks after transfer to lethally irradiated animals using the competitive repopulation assay. Animals transplanted with the equivalent of 1,000 lin−CD34+Sca-1+c-Kit+fetal liver stem cells cultured for 7 days on the DAS 104-8 cell lines showed only approximately 1% contribution of these cultured cells to mature hematopoietic lineages, consistent with the supposition that this cell line is incapable of maintaining these stem cells in an undifferentiated state. In sharp contrast to these results, Table 4shows that incubation of these fetal liver stem cells on DAS 104-4 for 7 days resulted in little apparent loss of hematopoietic reconstituting ability. Thus, the equivalent of 1,000 fetal liver stem cells incubated on this cell line for 7 days gave similar levels of hematopoietic reconstitution (approximately 63% to 85%) at 15 weeks post transplantation as 1,000 primary fetal liver stem cells, suggesting that this cell line is able to maintain these hematopoietic cells in an undifferentiated state in spite of the fact that these progenitor cells are also dramatically expanded down the myeloid pathway of differentiation on this cell line. Finally, fetal liver stem cells incubated on the YS CL 72 cell line showed a modest degree of stem cell maintenance (approximately 4% to 30% reconstitution) as compared with the AGM-derived DAS 104-4 cell line. These data thus show that the DAS 104-4 cell line provides an efficient supportive environment for the maintenance or self-renewal of fetal liver–derived hematopoietic stem cells. In addition, the results are consistent with the conclusion that the endothelium of the AGM region provides a milieu in vivo for both the support and differentiation of these pluripotent progenitor cells as well.
Expansion of Fetal Liver lin−CD34+Sca-1+c-Kit+ Stem Cells on AGM-Derived Cell Lines
| Experiment . | DAS104-4 . | DAS104-8 . |
|---|---|---|
| 1 | 3.61-fold | 1.32-fold |
| 2 | 5.58-fold | 0.96-fold |
| Experiment . | DAS104-4 . | DAS104-8 . |
|---|---|---|
| 1 | 3.61-fold | 1.32-fold |
| 2 | 5.58-fold | 0.96-fold |
Day-14 fetal liver lin−CD34+Sca-1+c-Kit+stem cells were plated onto each stromal cell line and cultured for 7 days. Cell were trypsinized and the frequency of lin−CD34+Sca-1+c-Kit+cells was evaluated by FACS.
Lineage Reconstitution in Lethally Irradiated Mice Transplanted With Uncultured or Cultured Fetal Liver Stem Cells
| Transplanted Cells . | Total % Ly5.1 Cells3-150 . | % Lineage (+) Ly5.1 Cells3-151 . | ||||
|---|---|---|---|---|---|---|
| 4 wk . | 8 wk . | 15 wk . | T Cells . | B Cells . | Myeloid Cells . | |
| Fetal liver cells3-152 (1 × 103cells) | 63.2 59.5 59.6 64.0 | 66.3 70.1 72.8 70.5 | 73.9 77.1 80.0 77.8 | 7.6 5.8 10.4 5.6 | 52.5 56.7 57.1 63.2 | 11.5 12.8 9.5 5.8 |
| Cultured on DAS104-43-153 ( of culture) | 58.1 62.3 51.0 47.2 | 61.3 73.1 63.8 57.2 | 62.9 84.4 69.8 70.6 | 3.9 8.5 5.5 3.3 | 51.3 66.1 54.9 57.1 | 5.8 7.8 7.4 9.0 |
| Cultured on DAS104-8 ( of culture) | 5.3 2.1 2.2 | 1.8 1.7 1.2 | 1.0 0.8 0.7 | ND ND ND | ND ND ND | ND ND ND |
| Cultured on YS CL 72 (of culture) | 13.0 9.7 14.5 | 6.9 25.0 18.8 | 4.7 30.2 11.9 | 0.2 0.8 3.0 | 4.1 25.1 5.9 | 0.1 2.9 2.7 |
| Transplanted Cells . | Total % Ly5.1 Cells3-150 . | % Lineage (+) Ly5.1 Cells3-151 . | ||||
|---|---|---|---|---|---|---|
| 4 wk . | 8 wk . | 15 wk . | T Cells . | B Cells . | Myeloid Cells . | |
| Fetal liver cells3-152 (1 × 103cells) | 63.2 59.5 59.6 64.0 | 66.3 70.1 72.8 70.5 | 73.9 77.1 80.0 77.8 | 7.6 5.8 10.4 5.6 | 52.5 56.7 57.1 63.2 | 11.5 12.8 9.5 5.8 |
| Cultured on DAS104-43-153 ( of culture) | 58.1 62.3 51.0 47.2 | 61.3 73.1 63.8 57.2 | 62.9 84.4 69.8 70.6 | 3.9 8.5 5.5 3.3 | 51.3 66.1 54.9 57.1 | 5.8 7.8 7.4 9.0 |
| Cultured on DAS104-8 ( of culture) | 5.3 2.1 2.2 | 1.8 1.7 1.2 | 1.0 0.8 0.7 | ND ND ND | ND ND ND | ND ND ND |
| Cultured on YS CL 72 (of culture) | 13.0 9.7 14.5 | 6.9 25.0 18.8 | 4.7 30.2 11.9 | 0.2 0.8 3.0 | 4.1 25.1 5.9 | 0.1 2.9 2.7 |
Abbreviation: ND, not determined.
The expression of the donor-type Ly5.1 marker on peripheral blood cells was determined at 4 weeks, 8 weeks, and 15 weeks post transplantation.
The expression of both Ly5.1 donor marker and lineage markers CD4 and CD8 (T cells), B220 (B cells), and Mac-1 and Gr-1 (myeloid cells) was determined on peripheral blood cells at 15 weeks post transplantation.
Day-14 fetal liver cells (Ly5.1) were purified for lin−CD34+Sca-1+c-Kit+stem cells. Purified fetal liver stem cells (1 × 103) were transplanted with 1 × 106 Ly5.2 competitor bone marrow cells into lethally irradiated Ly5.2 mice. Shown are data from individual mice.
3 × 103 fetal liver stem cells were plated onto DAS104-4 and cultured for 7 days. One third of culture (corresponding to 1 × 103 stem cells) was transplanted with 1 × 106 Ly5.2 competitor bone marrow cells into lethally irradiated Ly5.2 mice.
Stem cell self-renewal on DAS 104-4 stromal cells.
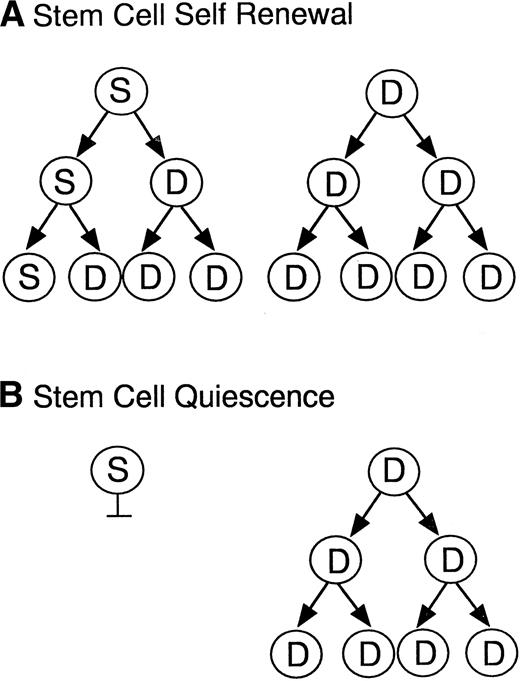
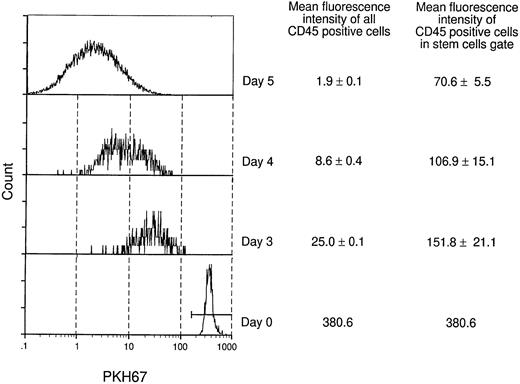
As the two models in Fig 5 illustrate, the maintenance of hematopoietic stem cells on the DAS 104-4 cell line could be explained by either the self-renewal of the stem cell after each cell division (model A) or the induction of a quiescent state without division of the stem cell (model B). To examine which of these two possible modes of stem cell maintenance was occurring in this in vitro system, highly purified stem cells were labeled with PKH67, a highly fluorescent dye that becomes impermeable after stem cell uptake. If model A is correct then the level of fluorescence in the entire stem cell population should decrease with time in culture, and there should be virtually no highly fluorescent cells remaining after 7 days in vitro. Alternatively, if model B is correct then a fraction of the initial stem cells, which should be significant in view of the purity of the starting population, should maintain a high level of fluorescence, whereas the remaining fraction of more differentiated cells, which divide and ultimately give rise to the mature hematopoietic cells, should show a decreasing level of fluorescence with time in culture. As Fig 6 illustrates, the vast majority of fetal liver–derived stem cells labeled with the fluorescent dye PKH67-GL29 rapidly lose fluorescence as they are cultured on the DAS 104-4 cell line, consistent with the self-renewal model A. While it might be argued that only a very small fraction of the initial lin−CD34+Sca-1+c-Kit+were actual stem cells, analysis of the extremely small numbers of cells (<0.5%) remaining in the highly fluorescent gate after 3, 5, or 7 days of culture on DAS 104-4 reveals that these cells also lose fluorescence with increasing time in culture (Fig 6). To further address the question of self-renewal versus quiescence, we examined the reconstituting ability of cells in the PKH67-low versus -high gates. As Fig 7 shows, all of the reconsituting activity was found in the PKH67-high region, where the cells showed a mean fluorescence intensity of 8.45 versus 279.4 for the starting population. These data suggest that the stem cells divide and self-renew on the DAS 104-4 cell line, but their proliferation rate is less than that for the committed cell population. Together with the hematopoietic reconstitution experiments in Table 4, these data argue for a self-renewal process as the mechanism for stem cell maintenance in this system.
Models of stem cell maintenance. Illustrated are two models for stem cell maintenance. In both models, the starting population is assumed to contain both stem cells (S) and more differentiated progenitor cells (D). Model A illustrates a self-renewal process where the stem cell divides asymmetrically to give rise to a committed and a pluripotent daughter cell, thus maintaining stem cell numbers at each generation. Model B illustrates a quiescence process where stem cells are maintained in a nonproliferative state and the committed progenitors divide to give rise to mature lineages.
Models of stem cell maintenance. Illustrated are two models for stem cell maintenance. In both models, the starting population is assumed to contain both stem cells (S) and more differentiated progenitor cells (D). Model A illustrates a self-renewal process where the stem cell divides asymmetrically to give rise to a committed and a pluripotent daughter cell, thus maintaining stem cell numbers at each generation. Model B illustrates a quiescence process where stem cells are maintained in a nonproliferative state and the committed progenitors divide to give rise to mature lineages.
Analysis of fluorescently labeled stem cells in vitro. lin−CD34+Sca-1+c-Kit+fetal liver stem cells were stained with the fluorescent dye PKH67.29 PKH67+ cells were plated onto DAS104-4 cells and cultured for 3, 4, or 5 days. Cells were obtained, gated for live cells (PI−) and CD45 (hematopoietic cells), and PKH67-GL fluorescence intensity was analyzed by FACS. Fluorescence intensity of stem cells in the PKH67highregion was obtained by gating cells for high expression of PKH67 and analyzing the fluorescence intensity of these cells. Note that the fluorescence intensity of cells in the PKH67high region goes down with time, although at a slower rate than the bulk of cells.
Analysis of fluorescently labeled stem cells in vitro. lin−CD34+Sca-1+c-Kit+fetal liver stem cells were stained with the fluorescent dye PKH67.29 PKH67+ cells were plated onto DAS104-4 cells and cultured for 3, 4, or 5 days. Cells were obtained, gated for live cells (PI−) and CD45 (hematopoietic cells), and PKH67-GL fluorescence intensity was analyzed by FACS. Fluorescence intensity of stem cells in the PKH67highregion was obtained by gating cells for high expression of PKH67 and analyzing the fluorescence intensity of these cells. Note that the fluorescence intensity of cells in the PKH67high region goes down with time, although at a slower rate than the bulk of cells.
Reconstituting activity of cells in the PHK67-low versus PHK67-high region. PKH67-labeled cells were either directly injected into lethally irradiated animals or incubated for 7 days on DAS 104-4, sorted for PKH-67-low versus -high fluorescence, and then injected into lethally irradiated animals. As can be seen, all of the reconstituting activity is found in the PKH67-high fraction with a mean fluorescence of 8.45 versus a starting fluorescence of 279.4, consistent with self-renewal and maintenance of stem cell phenotype of hematopoietic cells proliferating on DAS 104-4.
Reconstituting activity of cells in the PHK67-low versus PHK67-high region. PKH67-labeled cells were either directly injected into lethally irradiated animals or incubated for 7 days on DAS 104-4, sorted for PKH-67-low versus -high fluorescence, and then injected into lethally irradiated animals. As can be seen, all of the reconstituting activity is found in the PKH67-high fraction with a mean fluorescence of 8.45 versus a starting fluorescence of 279.4, consistent with self-renewal and maintenance of stem cell phenotype of hematopoietic cells proliferating on DAS 104-4.
Cytokine profiles in the DAS 104-4 and 104-8 cell lines.
Cytokine and growth factor expression were compared in the DAS 104-4 and DAS 104-8 cell lines using RT-PCR to examine if differences in synthesis of known factors could account for the differential ability of these lines to maintain repopulating stem cells, although it should be noted that a caveat to this procedure is its lack of quantitation. Table 1 illustrates that both cell lines expressed the same diversity of hematopoietic regulatory molecules. This includes dlk, the recently described delta-like molecule that was proposed to mediate stem cell self-renewal or maintenance in a fetal liver–derived stromal cell line.26,36 In addition, both cell lines express IL-11, a cytokine previously proposed to maintain stem cells in culture when used together with either stem cell factor or FLK2 ligand.37 Because DAS 104-8 does not maintain stem cells yet appears to express the same types of hematopoietic factors as DAS 104-4, these data argue for the possibility that a novel protein(s) expressed by the DAS 104-4 cell line may be involved with the self-renewal of fetal liver stem cells.
DISCUSSION
The hematopoietic microenvironment must provide both for the differentiation as well as the self-renewal of the hematopoietic stem cell.1,3,38 A great deal of data have accumulated that suggest that these events are accomplished by physical interactions between hematopoietic stem/progenitor cells and stromal elements in the various hematopoietic sites.25,26,39 Although the bone marrow and fetal liver are commonly used as sources for these stromal elements, the morphological complexity of these tissues makes the isolation of the appropriate hematopoietic-supportive stromal cell type formidable. In addition, it is not clear if hematopoietic stem cells are expanded in adult bone marrow, because the majority of these cells seem to be in a nondividing state.40 In contrast, the morphologies of both the yolk-sac and the AGM region of the early mammalian embryo are relatively simple, especially with respect to hematopoietic elements. In both cases, these regions show clusters of CD34+ hematopoietic progenitor cells attached to the endothelium of either the yolk-sac blood islands or the AGM-localized dorsal aorta.18-20 In addition, it appears that both of these regions contain CD34+ hematopoietic progenitor cells that are capable of repopulating the bone marrow of irradiated recipients, although the yolk-sac–derived stem cells may be deficient in trafficking to the adult bone marrow compartment.16,31,41 Perhaps most important is the finding that hematopoietic stem cells in the AGM region are capable of a modest level of expansion in vitro, suggesting that if these cells are the ones observed to be attached to the endothelium, this endothelial site may produce a factor(s) capable of stem cell expansion.17Together, these results suggest that AGM-derived endothelium may be an important supportive cell for hematopoietic development and stem cell maintenance in the early embryo, and the data reported here are in large part consistent with this hypothesis.
Because the major conclusion of this paper is that the endothelium of the AGM region, like that of the yolk sac,23 appears to be involved with the regulation of hematopoietic stem cell self-renewal and growth, it is important to emphasize the methodologies and results that indicate that the DAS cell lines are in fact endothelial cells. A number of laboratories have conclusively shown that the CD34+ cells in the embryo and adult appear to be predominately either endothelial or hematopoietic in origin.19-21 In addition, endothelial cells, but not hematopoietic progenitor cells, are adherent under tissue culture conditions. Thus, the combination of using anti-CD34 antibody to purify cells from the AGM region, together with the use of a tissue culture incubation step to isolate adherent CD34+ cells, should result in an enriched population of endothelial cells. In addition, the polyoma middle-T oncogene has been shown to be relatively specific for endothelial cells,32 and earlier studies showed that inoculation of retroviruses expressing this oncogene into murine embryos resulted in the production of only endothelial cell tumors that could be used as a source for endothelial cell lines.27Thus, the use of this oncogene to transform the isolated AGM-derived endothelial cells should result in cell lines that maintain at least a fraction of their endothelial characteristics. This latter hypothesis has been shown in the case of the DAS cell lines that express the endothelial markers CD34, FLT1, FLK1, vWF, and CD31 (PECAM). In addition, these cells produce the endothelial growth factor VEGF, which may allow for autocrine-mediated proliferation of these cell lines by activation of the FLT1 or FLK1 receptors. The formation of capillary-like structures when these cells are grown in Matrigel also strongly supports the contention that DAS 104-4 and 104-8 are endothelial cells. Together, these data argue that these cell lines are endothelial in origin and phenotype.
The endothelial cell lines decribed here fullfill a number of criteria that would be expected from an in vitro representation of hematopoiesis in the AGM region. For example, both cell lines are capable of mediating the dramatic expansion of progenitor cells along the myeloid pathway, and the DAS 104-8 line is also capable of expansion along the erythroid and B-lymphoid pathways in the presence of EPO or IL-7, respectively. In addition, direct physical contact is required for this high level of expansion and differentiation, suggesting that the growth factor(s) involved with hematopoietic expansion in this system is likely to be immobilized on the stromal cell surface. Both this ability to expand progenitor cells along all three mature hematopoietic lineages as well as the requirement for direct cell contact are likely to represent phenomena found in the intraembryonic hematopoietic site (Fig 1).18,19 In addition, the DAS 104-4 cell line is also capable of maintaining fetal liver stem cells in a pluripotent state in spite of the fact that this line contains transcripts for a diversity of growth and differentiation factors (Table 1). This latter result partially mimics the in vitro organ culture of isolated AGM regions,17 where hematopoietic stem cells are not only maintained in an undifferentiated state but are also modestly expanded. The reasons for the apparent lack of amplification of these pluripotent cells in our in vitro system are not apparent but may be due to changes in the AGM-derived endothelial cells during oncogenic transformation or prolonged cell culture. An additional possibility is that other, non-endothelial cell-type interactions may be required for amplification, but not maintenance, of the pluripotent stem cell. Alternatively, the amplification observed in the AGM organ culture studies17 may be conversion of a constant number of stem cells to a state that allows them to more efficiently home to the bone marrow, a modulation that may not occur under our culture conditions. In spite of this lack of stem cell expansion, the DAS 104-4 cell still remains one of the few stromal cell lines capable of maintaining competitively repopulating progenitor cells for several days in vitro,25 26 and this result thus makes this cell line a potentially interesting source for a factor(s) involved with stem cell maintenance.
The heterogeneous hematopoietic activity of the DAS 104-4 and 104-8 cell lines may have relevance with respect to the choice between stem cell maintenance and differentiation.39 To recapitulate, the DAS 104-8 cell line was highly effective at inducing stem cells to differentiate along all three hematopoietic pathways, but the line was ineffective at mediating stem cell self-renewal. In contrast, the DAS 104-4 cell line was poor at inducing, especially, the differentiation of cells along the lymphoid pathway, but was highly effective at maintaining repopulating stem cells. Interestingly, morphological examination of hematopoietic cells expanded on these two stromal lines showed a less mature population derived from DAS 104-4 versus DAS 104-8. These data are consistent with a hypothesis suggesting that maintenance of the stem cell phenotype on the DAS 104-4 cell line may be due to the lack of a factor that is necessary to induce these pluripotent cells to become responsive to differentiation factors such as EPO or, especially, IL-7. This conjecture seems unlikely, however, because myeloid differentiation seems to readily occur on this cell line. Alternatively, the DAS 104-8 cell line may lack a factor, expressed by the DAS 104-4 line, which instructs the stem cell to maintain a pluripotent state in the presence of these differentiation factors. Of interest is the fact that repopulating stem cells are maintained on the DAS 104-4 cell line despite the significant expansion down the myeloid pathway of development. This suggests either that a fraction of the initial cells are maintained in a quiescent, yet pluripotent, state or that the initial undifferentiated cells produce both committed and pluripotent cells after they divide. However, if the fetal liver stem cells are maintained in a quiescent state on DAS 104-4, then this state must be induced, because stem cells from this embryonic site are thought to be an actively dividing population.35,40 This has important implications for the mechanisms of stem cell maintenance,42 and the system described here may allow for an analysis of this important question.
Analysis of hematopoietic stem cell proliferation in the DAS 104-4 culture system indicated that these cells divided yet maintained a pluripotent state (Table 4; Figs 6 and 7). This result is compatible with the self-renewal model (model A) illustrated in Fig 5, and it suggests that stem cell division produces a pluripotent as well as a differentiated progenitor offspring. These data are thus consistent with two recent reports,29,43 which both showed that bone marrow stem cells appeared to maintain a pluripotent state in spite of the fact that they were in a nonquiescent (ie, cycling) situation. The potential mechanism that might allow for the maintenance of pluripotentiality after cell division can only be speculated upon at this point, but it seems likely that some type of asymmetric distribution of cell components is involved. Recent data in the Drosophila system indicate that an asymmetric distribution of the Prospero transcription factor by the Miranda protein is involved with stem cell maintenance.44 It will be interesting to determine if a similar mechanism is involved with the stem cell maintenance observed here.
Examination of hematopoietic cytokine expression by RT-PCR revealed that a number of these factors were significantly expressed in both AGM-derived endothelial lines. Thus, both lines showed clear expression of transcripts for stem cell factor, FLK2 ligand, M-CSF, IL-6, IL-11, TPO, and LIF, consistent with the suggestion that these factors alone may not be involved with stem cell maintenance on the DAS 104-4 cell line, although the RT-PCR method does not detect quantitative differences between expression of these factors. Although many studies have attempted to use a diversity of known hematopoietic growth factors to maintain repopulating stem cells in culture, only a recent study by Ogawa and colleagues has shown, using the stringent competitive repopulation assay, that stem cells incubated with either stem cell factor or FLK2 ligand plus IL-11 are maintained in a pluripotent state.37 Although these data suggest that these cytokine combinations might explain the DAS 104-4 stromal cell maintenance of repopulating stem cells, the fact that both the DAS 104-4 and 104-8 lines make all of these factors argues for a more novel combination of factors to explain the differing stem cell maintenance abilities of these two stromal cell lines. In support of this conjecture are data showing that the cytokine transcripts found in yolk-sac–derived endothelial cell lines, including YS CL72, which modestly maintains repopulating stem cells, show a remarkably similar pattern to those found for the two AGM-derived cell lines.23 The same argument can be made for the delta-like protein, dlk. This epidermal growth factor repeat–containing protein was shown to be expressed in a fetal liver–derived stromal line that was capable of maintaining competitively repopulating stem cells for several months.26,36 In addition, it was shown that expression of this protein in a stromal cell line incapable of mediating in vitro hematopoiesis endowed the line with the ability to expand cobblestone areas and maintain colony-forming activity and a low level of repopulating stem cells. These data were thus consistent with the suggestion that dlk was involved with the maintenance of early progenitor cells, including stem cells.36 Although the data reported here are not inconsistent with the ability of this factor to maintain progenitor cell colony-forming activity in vitro, the fact that both the DAS 104-4 and DAS 104-8 cell lines express the transcript encoding this factor is inconsistent with the possibility that dlk maintains repopulating stem cells, assuming the dlk transcript is translated in both cell lines. The RT-PCR transcript analysis reported here is thus consistent with the possibility that a novel factor(s) is involved with the maintenance of competitively repopulating stem cells by the DAS 104-4 cell line.
In summary, the results reported here show that endothelium derived from the AGM region of day-11 murine embryos is capable of mediating hematopoietic expansion along erythroid, myeloid, and lymphoid pathways as well as maintaining competitively repopulating hematopoietic stem cells, apparently by a self-renewal mechanism. These data concur with morphological studies suggesting a role for the endothelium of the dorsal aorta in regulating hematopoiesis in the AGM region, and they are consistent with previous analyses showing a similar role for the endothelium of the yolk-sac blood islands. It is thus likely, at least in the case of embryonic hematopoiesis, that blood cell development requires an interaction between hematopoietic progenitor cells and a subset of the endothelium in a diversity of hematopoietic sites. The cell lines described here may now provide for an opportunity to dissect the molecules involved with the maintenance and differentiation of hematopoietic stem cells during embryonic blood cell formation.
ACKNOWLEDGMENT
We thank Charles Hoffman for help with figures.
Address reprint requests to Laurence A. Lasky, PhD, Department of Molecular Oncology, Genentech, Inc, 460 Pt San Bruno Blvd, South San Francisco, CA 94080; e-mail: lal@gene.com.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal