Abstract
Growing evidence supports a pathophysiological role for platelets during the manifestation of postischemic reperfusion injury; in the current study, we investigated the nature and the molecular determinants of platelet-endothelial cell interactions induced by ischemia/reperfusion (I/R). Platelet-endothelium and leukocyte-endothelium interactions after 1 hour of ischemia were monitored in vivo within mouse small intestine. By intravital fluorescence microscopy, we observed that platelets, like leukocytes, roll along or firmly adhere to postischemic microvascular endothelial cells. In contrast, few leukocyte-endothelial cell interactions were detected in sham-operated controls. Monoclonal antibodies against P-selectin significantly attenuated platelet rolling and adherence in response to I/R. To identify whether platelet or endothelial P-selectin plays the major role in mediating postischemic platelet-endothelial cell interactions, P-selectin-deficient or wild-type platelets were transfused into wild-type or P-selectin-deficient mice, respectively. Whereas platelets lacking P-selectin rolled along or adhered to postischemic wild-type endothelium, interactions between wild-type platelets with mutant endothelium were nearly absent, indicating that I/R-induced platelet-endothelium interactions are dependent on the expression of P-selectin by endothelial cells. Concomitantly, P-selectin expression in the intestinal microvasculature was enhanced in response to I/R, whereas no upregulation of P-selectin was observed on circulating platelets. In summary, we provide first in vivo evidence that platelets accumulate in the postischemic microvasculature early after reperfusion via P-selectin-ligand interactions. Platelet recruitment and subsequent activation might play an important role in the pathogenesis of I/R injury.
RESTORATION OF BLOOD flow after prolonged ischemia initiates a series of events that may accumulate in microvascular and parenchymal cell injury. Leukocyte adhesion to the endothelium and subsequent leukocyte emigration to the interstitial compartments are hallmarks of ischemia/reperfusion (I/R) injury.1-4 Once adherent or emigrated, leukocytes release toxic oxygen products and proteases, thus contributing to the manifestation of I/R-induced vascular and tissue damage.5Whereas many of the mechanisms underlying the I/R-induced inflammatory response still remain unknown, growing evidence suggests a role for platelets in the pathogenesis of postischemic reperfusion injury. Ischemia leads to the accumulation and activation of platelets within vascular beds early after reperfusion.6 Correspondingly, several studies have demonstrated beneficial effects of platelet depletion during I/R.7,8 Although platelets are anuclear, they possess a cellular machinery comparable to leukocytes in many aspects. Platelets have a cytoskeleton that allows cell motion. Upon activation, they generate oxygen radicals9-11 and release proinflammatory mediators such as thromboxane A2, leukotrienes, serotonine, platelet factor 4, and platelet-derived growth factor (PDGF).12-17 In addition, platelets have the potential to modulate leukocyte functional responses.18 19Hence, the adhesion and activation of platelets to postischemic endothelial cells might significantly aggravate endothelial cell damage and contribute to leukocyte activation and recruitment at the site of injury. However, the exact role of platelets in the manifestation of I/R injury, in particular the behavior of platelets during I/R in vivo, and the molecular mechanisms whereby platelets accumulate in postischemic tissues have not been identified so far.
Platelets carry several adhesion molecules required for cell-cell interaction, such as P-selectin, a putative P-selectin ligand (CD 162 ?), PECAM-1, and several integrins (LFA-1, glycoprotein IIb/IIIa), which play a key role in mediating platelet adhesion to subendothelial matrix proteins.20,21 P-selectin, which is stored in α-granules of platelets and Weibel-Palade bodies of endothelial cells, is rapidly expressed on the cell surface upon activation, eg, by hypoxia/reoxygenation.22 P-selectin is a key mediator of leukocyte rolling and subsequent extravasation during I/R.4,23 Concomitantly, there is growing evidence that platelets also interact with stimulated endothelial cells via this particular selectin.24 This finding, together with a rapid expression upon stimulation, makes P-selectin a likely candidate involved in mediating platelet-endothelial cell interactions during initial postischemic reperfusion in which the endothelial barrier is not necessarily destroyed. However, the role of endothelial/platelet P-selectin for postischemic thrombocyte adhesion has not been investigated thus far.
The aim of the present study, therefore, was to characterize the nature of platelet-endothelial cell interactions in vivo under physiologic conditions and to assess whether platelet interactions with the microvascular endothelium are altered during intestinal I/R. The role of P-selectin as a molecular determinant of I/R-induced platelet-endothelial cell interactions was assessed by in vivo immunoinhibition with anti-P-selectin monoclonal antibodies (MoAbs). Because both endothelial cells and platelets express P-selectin, the contribution of P-selectin from either platelets or endothelial cells was established by the use of P-selectin-deficient mice.
MATERIALS AND METHODS
Animals.
Female Balb/c mice (Charles River, Sulzfeld, Germany) and C57BL/6J/129Sv mice (wild-type and P-selectin-deficient25), aged 5 to 7 weeks, were used (50 experimental animals and 36 platelet donors). P-selectin-deficient mice were generated and kindly provided by Prof A.L. Beaudet's group (Houston, TX).25 All experimental procedures performed on mice were approved by the German legislation on protection of animals.
Surgical procedure.
The animals were anesthetized by inhalation of isoflurane-N2O (FiO2 0.35, 0.015 L/L isoflurane; Forene; Abbott GmbH, Wiesbaden, Germany) and placed in supine position on a heating pad for maintenance of body temperature between 36°C and 37°C. Polyethylene catheters (PE 50, ID 0.28 mm; Portex, Hythe, UK) were inserted into the left carotid artery and jugular vein for recording of mean arterial and central venous blood pressure, for blood sampling, for injection of fluorescent dye, and for infusion of fluorescent platelets, respectively. After a transverse laparotomy, a segment of the jejunum was gently exteriorized. Segmental intestinal ischemia was induced by isolating and clamping the arterial vessels supplying the jejunum for 1 hour. During the entire experimental procedure, the tissue was constantly superfused with 37°C Ringer's lactate to avoid temperature changes and exposure to ambient air.
Blood sampling and platelet preparation.
Blood from P-selectin-deficient or wild-type mice was harvested by cardiac puncture and collected in 1-mL polypropylene tubes containing 0.1 mL volume of 38 mmol/L citric acid/75 mmol/L trisodium citrate/100 mmol/L dextrose. Rhodamine-6G (0.05%; 50 μL/mL whole blood, MW 479; Sigma, St Louis, MO) was added to label platelets in vitro, and the blood was centrifuged at 250g for 10 minutes. Platelet-rich plasma was gently transferred to a fresh tube, and the centrifugation was repeated at 2,000g for 10 minutes. The platelet pellet was resuspended in 0.5 mL phosphate-buffered saline (PBS; Seromed, Berlin, Germany). Fluorescent platelets (50 × 106) were infused intravenously (IV) over 10 minutes. The purity of the platelet suspension was confirmed before infusion using a Coulter ACT Counter (Coulter Corp, Miami, FL).
Intravital fluorescence microscopy.
As described previously (Massberg et al),25athe exposed segment was scanned from the oral to the aboral section starting 5 minutes after the onset of reperfusion. Five nonoverlapping regions of interest were selected randomly. Within these regions, 1 to 2 second order (A2) arterioles and postcapillary venules were visualized in the intestinal submucosa using a modified Leitz-Orthoplan microscope with a 100W HBO mercury lamp, attached to a Ploemo-Pak illuminator (Leitz, Wetzlar, Germany) for epi-illumination.26 With a 25× water immersion objective (W 25x/0.6; Leitz), the magnification on the video screen (PVM-1442 QM, diagonal 33 cm; Sony, Munich, Germany) was 450×. The microscopic images were recorded by a CCD video camera (FK 6990, Cohu; Prospective Measurements, San Diego, CA) and transferred to a video system for off-line evaluation. Leukocytes were stained in vivo by intravenous injection of 0.05% acridine orange (0.05 mL IV, MW 302; Merck, Darmstadt, Germany). Platelets (50 × 106) labeled with rhodamine-6G ex vivo were infused via the jugular vein with a syringe pump (S250i Pump; WPI, Sarasota, FL) for 10 minutes starting 5 minutes before reperfusion. Both platelet- and leukocyte-endothelial cell interactions were visualized in the same animal by the use of two different excitation and emission filter sets (Leitz).26
Video analysis.
Quantitative assessment of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Vessel diameters were determined by a computer-assisted image analysis system (CAP IMAGE; Dr H. Zeintl, Heidelberg, Germany).27 Platelet-endothelial cell and leukocyte-endothelial cell interactions were analyzed within A2-arterioles (mean diameter, 40 μm) and postcapillary venules (mean diameter, 60 μm) per animal, respectively. Platelets and leukocytes were classified according to their interaction with the endothelial cell lining as free flowing, rolling, and adherent cells. Rolling platelets or leukocytes were defined as cells crossing an imaginary perpendicular through the vessel at a velocity significantly lower than the centerline velocity in the microvessel; their numbers are given as cells per second per vessel diameter. Adherent cells were defined in each vessel segment as cells that did not move or detach from the endothelial lining within an observation period of 30 seconds. Adherence is quantified as number of cells per square millimeter of endothelial surface, calculated from the diameter and length of the vessel segment observed, assuming cylindrical geometry. In every experimental animal, platelet/leukocyte rolling and adhesion were analyzed within 5 to 7 arterioles and venules. Video sequences of 30 seconds were recorded of each vessel using the two different excitation and emission filter sets. Twenty-five minutes were required to complete intravital microscopy.
Experimental protocol.
To investigate the effects of I/R on platelet-endothelial cell interaction, the mouse small intestine was subjected to 1 hour of ischemia (group B, n = 6). Intravital microscopy was performed 5 to 30 minutes after the onset of reperfusion. Sham-operated animals without intestinal I/R served as controls (group A, n = 6). In group C, antimouse-P-selectin antibody (2 mg/kg body weight IV, n = 6) was administered before reperfusion to study the role of P-selectin for I/R-induced platelet-endothelial cell interactions. A separate group of animals (group D, n = 6) received the equivalent dose of isotype-matched control antibody before the onset of reperfusion (ChromPure rat IgG; Dianova, Hamburg, Germany). To assess the role of P-selectin from either platelets or endothelium in mediating I/R-induced platelet-endothelial cell interactions, interaction of wild-type platelets with P-selectin-deficient endothelium (group E, n = 6) and interaction of P-selectin-deficient platelets with wild-type endothelium (group F, n = 6) were investigated by intravital fluorescence microscopy. For each experiment, 1 separate platelet donor was required.
Histology and immunostaining for P-selectin and von Willebrand factor.
Tissue samples from the intestinal segment studied were taken 60 minutes after the onset of reperfusion. The biopsies were frozen in liquid nitrogen and stored at −80°C. Acetone-fixed cryostat sections (6 μm) were stained for P-selectin and von Willebrand factor (vWF), respectively, by applying the peroxidase technique in a three-stage procedure. Rabbit antimouse vWF and P-selectin MoAbs and commercially available immunohistochemistry kits (Vectastain; Camon, Wiesbaden, Germany) were used. Control sections were incubated with an isotype-matched primary antibody. Endogenous peroxidase activity was blocked with methanol-H2O2 for 10 minutes at room temperature. An easily detectable reddish-brown-colored end product was obtained by development in H2O2/3-amino-9-ethylcarbazol. The sections were counter-stained with Mayer's hemalaun.
Electron microscopy.
Tissue was excised for electron microscopy from sham-operated control and I/R-experiments (groups A and B). After sampling for light microscopy, a perfusion with 10 mL Ringer's lactate followed by Karnovsky's solution (0.04 mL/g body weight) was performed via the carotid artery for 5 minutes. After this perfusion, small, approximately 1 × 2 mm pieces were taken from the jejunal segment and further fixed in Karnovsky's solution for 2 to 3 days. The samples were postfixed with 2% osmium tetroxide in Sörensen buffer (pH 7.4) at room temperature and then dehydrated and embedded in Araldite monomer solution for 24 hours at 60°C. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and examined under a Zeiss EM 900 transmission electron microscope operating at 80 kV.
Flow cytometry.
The expression of CD11b and L-selectin on circulating granulocytes and P-selectin on circulating platelets during I/R was determined ex vivo in a separate set of experiments. The left carotid artery was cannulated, a transverse laparotomy was performed, and a segmental intestinal ischemia (1 hour) was induced as described above (n = 7). Sham-operated animals without I/R served as control (n = 7). Blood samples were drawn from the carotid artery before ischemia as well as 30 and 60 minutes after the onset of reperfusion. Expression of CD11b, L-selectin (CD62L) on leukocytes, and P-selectin (CD62P) on platelets was assessed by direct immunofluorescence using rat antimouse phycoerythrin- or fluorescein isothiocyanate (FITC)-coupled monoclonal IgG (Pharmingen, Hamburg, Germany). Fluorochrome-coupled IgG1 and IgG2 isotype-matched control antibodies were used to exclude unspecific binding. The cells were incubated with saturating amounts of MoAb for 30 minutes at 4°C. After incubation, cells were washed twice with PBS. A commercially available lysing solution (FACS Lysing solution; Becton Dickinson, Heidelberg, Germany) was added for the removal of erythrocytes and fixation of cells. For determination of platelet P-selectin expression, lysis and fixation were omitted. Analysis of 10,000 events was performed on a FACSort flow cytometer (Becton Dickinson). Granulocytes and platelets were selectively analyzed for their fluorescence properties using a Lysis II data handling program (Becton Dickinson). The relative fluorescence intensity of a given sample was calculated by subtracting the signal obtained when cells were incubated with the corresponding isotype control from the signal generated by cells incubated with the test antibody.
MoAbs.
Unlabeled rat antimouse-P-selectin antibodies for in vivo immunoinhibition studies were generated as described by Weller et al.28 Isotype-matched control IgG was obtained from Dianova. For flow cytometry, commercially available FITC- and phycoerythrin (PE)-labeled rat antimouse CD11b, L-selectin, P-selectin MoAb, and isotype- matched rat IgG1 and IgG2were used (Pharmingen). Immunohistology was performed using monoclonal rat antimouse P-selectin and vWF antibodies, respectively, purchased from Pharmingen.
Statistics.
Data analysis was performed with a statistical software package (SigmaStat for Windows; Jandel Scientific, Erkrath, Germany). The Kruskal-Wallis test followed by Dunn's method was used for the estimation of stochastic probability in intergroup comparisons. Mean values ± standard error of the mean (SEM) are given. Pvalues less than .05 were considered significant.
RESULTS
Platelet preparation for intravital fluorescence microscopy.
The purity and fluorescent labeling of the platelet sample was ascertained before infusion by flow cytometry. Platelet separation by differential centrifugation yielded a platelet suspension with negligible amounts of other cellular components. Less than 5 leukocytes/106 platelets were found in the platelet preparations used for the present study. P-selectin expression (mean fluorescence intensity) after blood sampling was not increased when additional differential centrifugation was performed (P < .788), indicating that significant platelet activation during the preparation procedure did not occur. However, in response to thrombin (1 U/mL), a 10-fold increase of P-selectin expression on identically treated platelets was observed (P < .05). A concomitant increase in the forward scatter light signal showed that the cells sustained the ability to form aggregates (data not shown). No adverse effects on heart rate or blood pressure were observed upon IV infusion of 50 × 106 labeled platelets.
Leukocyte-endothelial cell interactions in vivo.
Leukocyte behavior in the intestinal microcirculation was investigated in control animals and after segmental I/R. Rolling and firm adhesion of leukocytes were never observed in arterioles and only rarely in postcapillary venules of the intestinal submucosa of sham-operated wild-type mice (Table 1). In contrast, I/R markedly enhanced leukocyte-endothelial cell interactions in postcapillary venules. Both leukocyte rolling and firm adherence were significantly increased 15 minutes after the onset of reperfusion. Immunoinhibition by anti-P-selectin MoAb almost completely prevented rolling and attenuated firm adhesion of leukocytes. Concomitantly, microvascular leukocyte accumulation in response to I/R was nearly absent in mice deficient in P-selectin (data not shown), indicating a key role of P-selectin for early postischemic leukocyte recruitment at the site of injury.
Leukocyte-Endothelial Cell Interactions in Response to Intestinal Ischemia/Reperfusion
| . | Sham . | I/R + Vehicle . | I/R + Control-IgG . | I/R + Anti-CD62P MoAb . |
|---|---|---|---|---|
| No. of rolling leukocytes [1/s/mm] | 6.0 ± 1.1 | 24.3 ± 5.6-150 | 35.7 ± 8.0-150 | 0.8 ± 0.4 |
| No. of adherent leukocytes [mm−2] | 2.0 ± 1.6 | 212.9 ± 21.7-150,-151 | 178.6 ± 37.3-151 | 52.9 ± 12.7 |
| . | Sham . | I/R + Vehicle . | I/R + Control-IgG . | I/R + Anti-CD62P MoAb . |
|---|---|---|---|---|
| No. of rolling leukocytes [1/s/mm] | 6.0 ± 1.1 | 24.3 ± 5.6-150 | 35.7 ± 8.0-150 | 0.8 ± 0.4 |
| No. of adherent leukocytes [mm−2] | 2.0 ± 1.6 | 212.9 ± 21.7-150,-151 | 178.6 ± 37.3-151 | 52.9 ± 12.7 |
Leukocyte interactions with the microvascular endothelium of postcapillary venules were visualized using intravital fluorescence microscopy. Mean ± SEM, n = 6 animals per group.
P < .01 versus CD62P MoAb, Dunn's Method.
P < .01 versus sham.
Platelet-endothelial cell interactions in vivo.
Platelet interactions with the microvascular endothelium showed striking parallels to leukocyte-endothelial cell interactions; both intermittent adhesion (rolling) and firm adhesion of platelets were seen. However, platelet-endothelial cell interactions were not confined to postcapillary venules, but were also prominent in smaller arterioles (mean diameter, 40 μm) within the intestinal submucosa (Figs 1 and2). Similar to leukocytes, platelets rarely interacted with the microvascular endothelium in sham-operated wild-type mice. Only a few platelets were encountered rolling along the endothelial cell lining of arterioles and postcapillary venules, respectively. Firm adherence was 60.0 ± 25.7 and 23.8 ± 8.8 mm-2 in arterioles and venules, respectively, indicating that platelet-endothelial cell interactions play a minor role under physiologic conditions. In contrast, I/R induced platelet interactions with the endothelial cell surface already 5 minutes after the onset of reoxygenation without further increase throughout the remaining observation period. There were 26.9 ± 3.2 and 42.0 ± 5.8 platelets/sec/mm vessel diameter rolling along the arteriolar and venular vessel wall, respectively. In parallel, the number of firmly adherent platelets increased 10-fold, compared with sham-operated animals. Frequently aggregated leukocytes coated with rhodamine-labeled platelets were observed. Anti-P-selectin MoAb almost completely prevented platelet rolling and firm adhesion in arterioles and venules in response to I/R, indicating that P-selectin is the molecular determinant of postischemic platelet-endothelial cell interactions during initial postischemic reperfusion. Moreover, anti-P-selectin MoAb inhibited the interaction of platelets with leukocytes. These effects were specific inasmuch as an isotype-matched control MoAb did not attenuate I/R-induced platelet interactions with either endothelium or leukocytes.
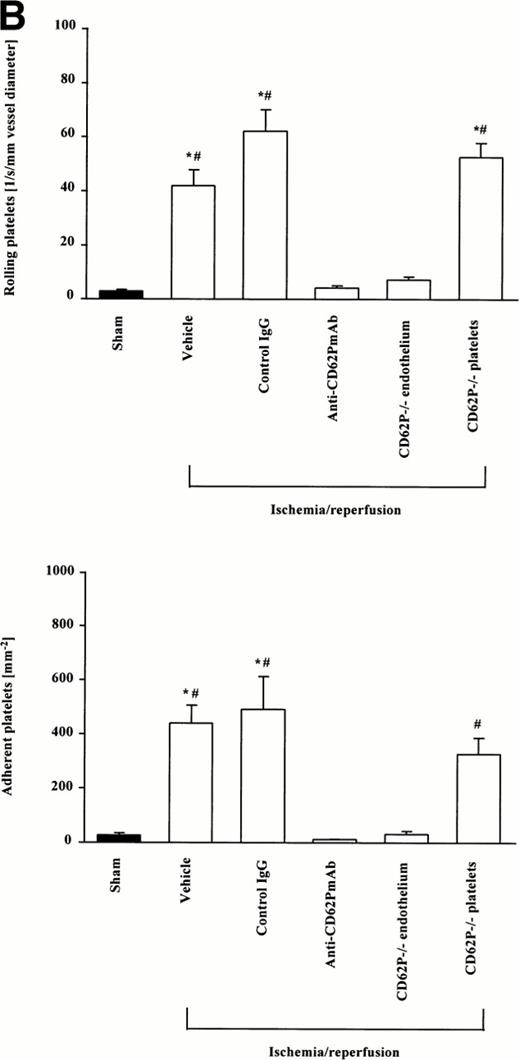
Platelet-endothelial cell interactions during I/R of the small intestine in vivo. Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using intravital fluorescence microscopy. Sham-operated animals served as controls. According to their interaction with the endothelial cell lining, platelets were classified into rolling or firmly adherent cells. Rolling platelets (upper panels) are presented as the number of cells per second and millimeters of vessel diameter; adherent platelets (lower panels) are given per square millimeter of vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01 versus sham, #P < .01 versus anti-CD62P MoAb, Dunn's method.
Platelet-endothelial cell interactions during I/R of the small intestine in vivo. Platelet-endothelial cell interactions were investigated in arterioles (A) and venules (B) using intravital fluorescence microscopy. Sham-operated animals served as controls. According to their interaction with the endothelial cell lining, platelets were classified into rolling or firmly adherent cells. Rolling platelets (upper panels) are presented as the number of cells per second and millimeters of vessel diameter; adherent platelets (lower panels) are given per square millimeter of vessel surface. Mean ± SEM, n = 6 experimental animals per group. *P < .01 versus sham, #P < .01 versus anti-CD62P MoAb, Dunn's method.
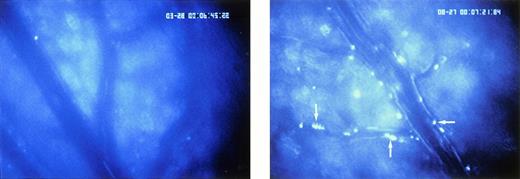
I/R-induced platelet-endothelial cell interactions in vivo. Rhodamine-6G-labeled platelets are visualized within postcapillary venules using intravital fluorescence microscopy. Whereas few platelets adhere to the venular endothelium in sham-operated animals (group B, left panel), platelet attachment to the endothelium is significantly enhanced in response to I/R already 5 minutes after the onset of reoxygenation (group A, right panel, arrow). Original monitor magnification × 450.
I/R-induced platelet-endothelial cell interactions in vivo. Rhodamine-6G-labeled platelets are visualized within postcapillary venules using intravital fluorescence microscopy. Whereas few platelets adhere to the venular endothelium in sham-operated animals (group B, left panel), platelet attachment to the endothelium is significantly enhanced in response to I/R already 5 minutes after the onset of reoxygenation (group A, right panel, arrow). Original monitor magnification × 450.
Because both platelets and endothelial cells express P-selectin, it was important to clarify whether endothelial or platelet P-selectin was required for I/R-dependent platelet-endothelial cell interactions. To study this, wild-type or P-selectin-deficient platelets were separated and transfused into P-selectin-deficient or wild-type mice, respectively. During postischemic reperfusion, P-selectin-deficient platelets interacted similarly with wild-type endothelium as described above for wild-type platelets. In contrast, I/R-induced platelet rolling and firm adhesion was almost absent when wild-type platelets were transfused into P-selectin-deficient mice (Fig 1). These results provide evidence that endothelial P-selectin interacting with a putative P-selectin ligand on the platelet is the key mediator for postischemic platelet-endothelial cell interaction. Because aggregation of mutant platelets with wild-type leukocytes was not observed, our results support the notion that platelet P-selectin interacting with a ligand on myeloid cells, presumably PSGL-1, is responsible for platelet-leukocyte interaction in response to I/R.
Electron microscopy.
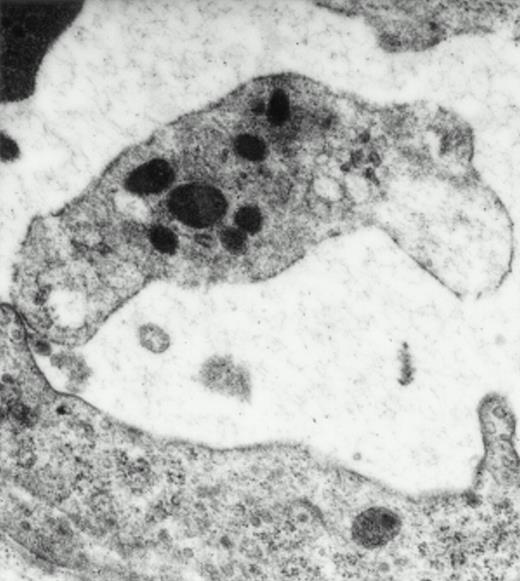
Electron microscopy confirmed the presence of platelets adherent to endothelial cells in response to I/R. Platelets in various states of activation were frequently observed attached to the postischemic microvascular endothelium (Fig 3). Adherent, single, or aggregated platelets appeared degranulated extending pseudopodia to the endothelial cell surfaces. In most instances, platelets adhered directly to endothelial cells, and obvious endothelial denudation was not detected. Occasionally, neutrophils carrying activated platelets adherent on the surface could be demonstrated (Fig 3). In animals not exposed to I/R, few platelets with direct contact with the endothelium were seen. Platelets in these animals generally retained a discoid shape with prominent α-granules.
Platelets in postischemic microvasculature visualized by electron microscopy. Electron microscopy demonstrated platelets attached to both endothelial cells (arrowhead) and neutrophils (arrows) in response to I/R (upper panel, original magnification × 3,000). Platelets bind directly to endothelial cells (lower panel, original magnification × 20,000).
Platelets in postischemic microvasculature visualized by electron microscopy. Electron microscopy demonstrated platelets attached to both endothelial cells (arrowhead) and neutrophils (arrows) in response to I/R (upper panel, original magnification × 3,000). Platelets bind directly to endothelial cells (lower panel, original magnification × 20,000).
Immunohistochemistry.
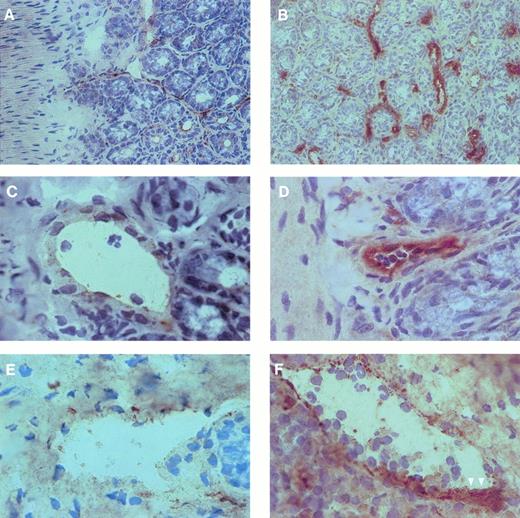
To investigate the expression of P-selectin within the postischemic microvasculature, immunohistochemistry was performed on cryostat sections using MoAb against P-selectin. In addition, vWF immunoreactivity was investigated to identify vascular structures within the serial sections. In sham-operated wild-type mice, P-selectin was only weakly expressed by vascular endothelium of venules and to a lesser extent of arterioles (Fig 4). I/R markedly enhanced immunoreactivity for P-selectin in both arterioles and venules (Fig 4). Because of the large increase in platelet adhesion to the postischemic endothelium, we were not able to clearly distinguish by light microscopy whether the enhanced P-selectin expression was indeed present on the endothelial cells or whether it was due to platelets attached to the vessel wall. Formation of platelet aggregates, identified by an intense staining for P-selectin, was a prominent phenomenon after I/R. In contrast, platelet aggregates, which in some instances contained polymorphonuclear leukocytes, were never seen in sham-operated control animals or in animals treated with anti-P-selectin MoAb. Similarly, in P-selectin-deficient mice, immunoreactivity for P-selectin was never detected, neither after sham-operation nor after I/R.
P-selectin expression in arterioles and venules of mouse small intestine. Cryostat sections of intestinal specimens were incubated with a MoAb against P-selectin and stained using the peroxidase-antiperoxidase technique as described in the Materials and Methods. Counterstaining was performed using Mayer's hemalaun. In control animals (A, C, and E), P-selectin was weakly expressed by vascular endothelium of arterioles (C) and venules (E). In contrast, I/R (B, D, and F) markedly enhanced immunoreactivity for P-selectin in both arterioles (D) and venules (F). Formation of platelet aggregates, identified by an intense staining for P-selectin, was a prominent phenomenon after I/R (arrowheads). The magnification of the objective was 40× in (A) and (B) and 100× in (C) through (F), respectively.
P-selectin expression in arterioles and venules of mouse small intestine. Cryostat sections of intestinal specimens were incubated with a MoAb against P-selectin and stained using the peroxidase-antiperoxidase technique as described in the Materials and Methods. Counterstaining was performed using Mayer's hemalaun. In control animals (A, C, and E), P-selectin was weakly expressed by vascular endothelium of arterioles (C) and venules (E). In contrast, I/R (B, D, and F) markedly enhanced immunoreactivity for P-selectin in both arterioles (D) and venules (F). Formation of platelet aggregates, identified by an intense staining for P-selectin, was a prominent phenomenon after I/R (arrowheads). The magnification of the objective was 40× in (A) and (B) and 100× in (C) through (F), respectively.
Flow cytometry.
Based on our in vivo results, endothelial cell activation with upregulation of P-selectin by exocytosis of Weibel-Palade bodies appears to be the primary mechanism for postischemic platelet-endothelial cell interaction. Nonetheless, it was important to determine whether I/R would lead to an activation of platelets. To study this, P-selectin expression on circulating platelets in response to intestinal I/R was evaluated by flow cytometry. No change in platelet P-selectin immunoreactivity was observed during postischemic reperfusion; 25.3% ± 4.2%, 22.2% ± 6.8%, and 24.6% ± 5.8% of all platelets stained positive for P-selectin before ischemia as well as 30 and 60 minutes after the onset of reperfusion, respectively. Furthermore, there was no shift in mean fluorescence intensity when compared with baseline conditions or to sham-operated control animals. In contrast, I/R was associated with activation of circulating leukocytes, as indicated by a 1.5-fold enhanced CD11b expression 30 and 60 minutes after the onset of reperfusion (data not shown). Shedding of L-selectin was not observed (data not shown).
DISCUSSION
Platelets might play an important role during the manifestation of I/R injury. Platelets, like leukocytes, accumulate in postischemic/reperfused tissues and contribute to endothelial and parenchymal cell injury.6-8 Whereas several distinct adhesive interactions involved in the process of I/R-induced leukocyte accumulation have been described,4 the mechanisms that initiate platelet accumulation in the postischemic microvasculature have not been identified to date. The present study, therefore, has investigated the in vivo interactions of platelets with the microvascular endothelium in response to intestinal I/R. We found that platelets similar to leukocytes roll along and firmly adhere to the endothelial surface of microvessels during postischemic reperfusion. Local accumulation of platelets was observed reaching a maximum within minutes after the onset of reperfusion, indicating that platelets are among the first cells recruited to the site of injury.
Because P-selectin, which is stored in intracellular granules of endothelial cells and platelets, can be rapidly mobilized to the cell surface in response to various stimuli independent of de novo mRNA or protein synthesis,29,30 we evaluated the role of this lectin-like adhesion molecule in mediating initial postischemic platelet-endothelial cell interactions. Our results provide strong evidence that P-selectin expressed by the endothelium is the molecular determinant of I/R-induced platelet-endothelial cell interactions: An anti-P-selectin MoAb attenuated rolling and subsequent firm adherence of platelets. Moreover, rolling and adherence of wild-type platelets were nearly absent in mice with P-selectin-deficient endothelium, whereas P-selectin-deficient and wild-type platelets interacted similarly with postischemic wild-type endothelium. No increase in the P-selectin expression was found on circulating wild-type platelets during postischemic reperfusion. In contrast, I/R clearly enhanced P-selectin immunoreactivity in the postischemic microvasculature. This is likely due either to endothelial platelet adhesion or the result of a redistribution of the P-selectin to the endothelial cell surface. Although we were not able to demonstrate P-selectin mobilization to the endothelial cell surface by immunohistochemistry studies, both in vitro and ex vivo experiments have previously documented that hypoxia/reoxygenation22 or thrombin31,32 that is generated during I/R33induces rapid mobilization of P-selectin onto the endothelial cell surface. At the same time, it has been demonstrated that the expression of P-selectin on the endothelium upon endothelial activation induces platelet-endothelial cell interactions in vivo.24 Hence, in line with our findings, this indicates that the P-selectin present on the endothelium mediates platelet adhesion to stimulated/injured endothelial cells in response to I/R. This is in contrast to platelet adhesion to the exposed subendothelial matrix via integrins such as the GPIIb/IIIa complex (CD41/CD61) and via the leucin-rich glycoprotein complex GPIb-X (CD42a/CD42b), which occurs when the endothelium is denuded.21
The interactions between platelets and endothelium in contrast to leukocyte-endothelial cell interactions were not restricted to venules, but were also prominent in arterioles. Several reasons may explain the presence of platelet-endothelial cell interactions in arterioles. It has been shown in vivo that near the vessel wall the amount of platelets per unit volume is significantly higher in arterioles compared with venules.34 Based on estimates of wall shear rates in vivo,35 platelets near the vessel wall are exposed to much lower shear forces as compared with the leukocytes due to their smaller diameter (2 μm). This means that even weak receptor-ligand interactions may be sufficient to allow platelet-endothelial cell interactions in arterioles. Differences in the properties of the P-selectin ligand expressed on platelets and leukocytes could also contribute to the different behavior of these cell types. However, there have been no studies that have addressed this question, and the exact nature of the ligand on platelets that binds to endothelial P-selectin remains unclear. PSGL-1, a glycoprotein that avidly binds to P-selectin, is expressed predominantly on myeloid cells and mediates leukocyte-endothelium interactions and leukocyte-platelet interactions in vitro and in vivo.12,36-39 Although PSGL-1 might also be involved in platelet-endothelial cell interactions, the presence of this mucin-like glycoprotein on the platelet membrane has not yet been documented.40 Hence, platelets might also carry a different, undefined P-selectin ligand. This ligand would be most likely a sialylated carbohydrate determinant, such as sialyl Lewisx.41
Although platelet P-selectin appears to play a minor role for I/R-induced platelet-endothelial cell interactions, the complex adhesive apparatus expressed on the platelet cell surface allows them to cooperate and synergize with leukocytes during I/R. Firm adherence of circulating leukocytes to inflamed vascular endothelium is an essential component of a cascade that results in the adhesion and eventual emigration of leukocytes through the vessel wall. Platelets and leukocytes colocalize in areas of infarction,42indicating that platelets might significantly contribute to I/R-induced inflammatory responses. By adhesion to endothelial cells or subendothelial structures, platelets might occupy a position analogous to endothelium with respect to leukocyte accumulation and emigration. P-selectin expressed by surface adherent, activated platelets mediates rolling of leukocytes under flow in vitro and is involved in leukocyte attachment to artificial vessel surfaces in vivo.43-47Rolling leukocytes may sequentially develop firm adhesion and transmigrate across surface-adherent platelets via the β2-integrin CD11b/CD18,46 indicating that platelets are actually involved in the multistep process of I/R-induced leukocyte accumulation and extravasation. However, leukocytes do not attach solely to surface adherent platelets. As our results demonstrate, circulating leukocyte-platelet aggregates are formed in vivo after exposure to I/R, and neutralization of P-selectin diminishes these interactions. Similarly, aggregates are absent in P-selectin-deficient mice, providing further evidence that platelet-leukocyte aggregation involves platelet P-selectin interacting with a functional ligand on the leukocyte, presumably PSGL-1.11
P-selectin-mediated platelet interactions with leukocytes or endothelial cells may modify and directly promote functional responses. Adhesion of activated platelets to leukocytes induces nuclear translocation of NF-κB, and enhances expression of CD11b/CD18 and generation of monocyte chemotactic protein (MCP)-1 and superoxide anions.12,44,48 In all instances, binding of platelet P-selectin to its ligand on the leukocyte is required. Although similar studies have not yet been performed with regard to platelet-endothelial cell-interactions, platelet attachment to postischemic endothelial cells might modulate endothelial cell function. Platelets release proinflammatory and chemotactic mediators such as PAF, epithelial-derived neutrophil activating factor-78 (ENA-78), neutrophil-activating peptide-2 (NAP-2), RANTES, platelet factor 4, serotonin, interleukin-8, and monocyte chemotactic protein-3 (MCP-3),12-17,49 whereby they may activate both endothelium and leukocytes. Hence, it is conceivable that platelet-endothelium interaction via P-selectin facilitates juxtacrine activation of the cells by mediating close cell-to-cell contact.50 Whether P-selectin-ligand interactions may also directly initiate intracellular signaling pathways that effect further endothelial cell functional responses has not been elucidated so far.
In conclusion, we have demonstrated that platelets, similarly to leukocytes, roll along and firmly adhere to microvascular endothelium during postischemic reperfusion. I/R-induced platelet-endothelial cell interactions are mediated via endothelial P-selectin, whereas platelet P-selectin promotes platelet interactions with leukocytes. Because platelets release potent proinflammatory chemokines and modulate leukocyte function, platelet accumulation in the postischemic microvasculature might significantly contribute to the manifestation of I/R injury.
ACKNOWLEDGMENT
The authors thank Sylvia Münzing, Elke Schütze, Katrin Baltzer, and Bärbel Lorenz for their excellent and skillful technical assistance.
Supported by Research Grant Biomed 2 Contract No. BMH4-CT95-0875 (DG12-SSMA).
Address reprint requests to Steffen Massberg, MD, Institute for Surgical Research, Ludwig-Maximilians-University, Klinikum Grosshadern, Marchioninistrasse 15, D-81366 Munich, Germany; e-mail: massberg@icf.med.uni-muenchen.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal