Abstract
Hox homeobox genes play a crucial role in specifying the embryonic body pattern. However, a role for Hox genes in T-cell development has not been explored. The Hoxa-9 gene is expressed in normal adult and fetal thymuses. Fetal thymuses of mice homozygous for an interruption of the Hoxa-9 gene are one eighth normal size and have a 25-fold decrease in the number of primitive thymocytes expressing the interleukin-2 receptor (IL-2R, CD25). Progression to the double positive (CD4+CD8+) stage is dramatically retarded in fetal thymic organ cultures. This aberrant development is associated with decreased amounts of intracellular CD3 and T-cell receptor β (TCRβ) and reduced surface expression of IL-7R and E-cadherin. Mutant thymocytes show a significant increase in apoptotic cell death and premature downregulation of bcl-2 expression. A similar phenotype is seen in primitive thymocytes from adult Hoxa-9−/− mice and from mice transplanted with Hoxa-9−/−marrow. Hoxa-9 appears to play a previously unsuspected role in T-cell ontogeny by modulating cell survival of early thymocytes and by regulating their subsequent differentiation.
THE Hox HOMEOBOX genes play major roles in pattern formation and tissue identity during embryogenesis,1,2 and mutations in Hox genes can produce dramatic morphologic abnormalities involving many body structures.3,4 Recent data have implicated Hoxgenes in hematopoietic development.5Hox genes are expressed in stage- and lineage-specific patterns in primitive CD34+ blood cells, in lymphoid cell lines, in developing lymphocytes, and in activated mature T cells.6-9Furthermore, enforced overexpression of Hox genes in bone marrow cells is associated with a variety of hematologic defects affecting myeloid and lymphoid differentiation.10-13Recently, we reported defects in myeloid and B-lymphoid hematopoiesis in mice with a targeted disruption of the Hoxa-9gene.14 Because these animals also displayed reductions in thymic size, we evaluated them for possible defects in T-cell development.
Early thymic development is characterized by a complex series of ordered events (Figs 1C and 3A), including T-cell receptor (TCR) rearrangements and expression of many cell surface molecules, including CD3, CD4, CD8, CD44, and CD25, the α chain of the interleukin-2 receptor (IL-2R; reviewed in Nikolic-Zugic15). Well-defined control points in the T-cell developmental pathway insure that cells that do not complete key steps in the program are eliminated. A major control point involves an early proliferative stage that initiates differentiation of triple-negative (TN) thymocytes, a step requiring signaling through the IL-7 receptor (IL-7R).16 IL-7R signaling, which is mediated at least in part by bcl-2, permits rearrangement of the TCRβ gene17 and then assembly of the pre-TCR complex, which includes TCRβ and CD3.18 We demonstrate here that loss of Hoxa-9 function results in maturational defects at this early TN stage of T-cell development and increased death of fetal thymocytes.
MATERIALS AND METHODS
Mice.
Mice bearing a targeted disruption of Hoxa-9 were furnished by Dr Cynthia Peterson and Dr Mario Capecchi (University of Utah, Salt Lake City, UT). The details of engineering embryonic stem cells with a Hoxa-9 allele disrupted by a NeoR cassette have been described previously.14 BothHoxa-9+/− andHoxa-9−/− mice appear externally normal and are fertile. For most experiments, timed pregnancies between heterozygotes were performed to generate homozygotes with heterozygous and wild-type littermates as controls. Because, in all the assays performed, heterozygous animals were indistinguishable from wild-type mice, for some experiments Hoxa-9−/−mice were mated with heterozygotes to generate litters of homozygotes and heterozygotes as controls. Progeny of various intercrosses were genotyped by polymerase chain reaction (PCR) using DNA isolated from tail or toe clips of weanlings and a 3-primer system to distinguish wild-type from mutant Hoxa-9 alleles. The twoHoxa-9–specific primers were CGCTGGAACTGGAGAAGGAGTTTCTG and ATCCTGCGGTTCTGGAACC AGATC; the Neo-specific primer was TCTATCGCCTTCTTGACGAGTTC.
Fluorescence-activated cell sorting (FACS) analysis.
For FACS analysis, cell suspensions from mouse thymuses were washed in phosphate-buffered saline (PBS) with 1% fetal calf serum (FCS) and 0.01% NaN3 (FACS buffer). Fc receptors were blocked, when possible, by preincubation with neat mouse serum or hybridoma supernatant from 2.4G2 (anti-FcR clone). Cells were then stained with appropriately diluted fluorochrome-conjugated antibodies. Cells were analyzed on a Becton Dickinson FACScan using Cellquest software (Becton Dickinson, San Jose, CA). Depending on whether the source of thymic tissue was fetal or adult, 5 × 103 to 1 × 105 cells were routinely analyzed. Dead cells were excluded using forward and side scatter gating or propidium iodide staining where possible.
Antibodies.
Antibodies used for surface staining included anti-αβ TCR (H57)-phycoerythrin (PE), anti-CD4-fluorescein isothiocyanate (FITC), anti-CD8α-ΤriColor, anti-CD25-PE (Caltag, South San Francisco, CA), anti-CD44-FITC (Pharmingen, San Diego, CA), and monoclonal antibody ECCD-2 against E-cadherin (Zymed, South San Francisco, CA). Monoclonal antibody A7R34, directed against the murine IL-7R, was furnished by Dr T. Sudo (Toray Industries, Kamakura, Japan).19 Negative controls used were Streptavidin-FITC, Streptavidin-TriColor (Caltag), and Streptavidin-PE (Pharmingen). For the detection of intracytoplasmic proteins, thymocytes were first permeabilized with 0.03% saponin and then stained with specific antibody. Antibodies used for intracellular staining included anti-TCRβ, anti-CD3 (Caltag), hamster anti-bcl-2 (3F11; Pharmingen), and antihamster-PE (Caltag).
Reverse transcription-PCR (RT-PCR) analysis of Hoxa-9 expression in fetal mouse tissues.
RT-PCR analysis was performed following standard procedures, using an oligo (dT) primer at 42°C for 2 hours in a 20 μL reaction containing 10 U of SuperScript II reverse transcriptase (RT; GIBCO BRL, Grand Island, NY), manufacturer's RT buffer, 10 U RNasin (Promega, Madison, WI), 10 mmol/L dithiothreitol (DTT), and each dNTP at 1 mmol/L. Control reactions were performed without RT. Equal aliquots of RT reaction mixtures, normalized by independent PCR for β-actin, were amplified using primers derived from sequences 3′ to the homeodomain: 5′ CGCGGATCCGGACCGAGCAAAAGACG AGTG 3′ and 5′ CCGGAATTCTAGCTTCCACAATCAC 3′. Hoxa-9 and β-actin PCR were shown to be in the linear range. PCR products were analyzed by Southern blotting with a known Hoxa-9 probe.
Fetal thymic organ cultures.
Day-15.5 fetal thymuses were cultured in Dulbecco's modified Eagle's medium with 10% FCS and 2 mmol/L glutamine, according to methods of Robinson et al,20 with the exception that the filter was floated on the media instead of resting on a gelfoam sponge. Thymocytes were harvested after 6 or 10 days in culture by gently squeezing lobes under a coverslip and then stained and analyzed for FACS.
Assays for apoptosis.
To measure the percentage of hypodiploid cells, nuclei were isolated from freshly harvested day-15.5 fetal thymuses, incubated overnight with a hypotonic solution of propidium iodide, and analyzed on the FACScan at the low flow setting as previously described.21The average percentage of hypodiploid nuclei (those located left of the 2n DNA) from normal fetal thymuses was approximately 5%. To assay thymocytes for membrane changes consistent with apoptosis, annexin V staining was performed on fetal thymuses. Thymuses were first enzymatically digested into a single-cell suspension by incubating in 100 μL of 1 mg/mL type 1 collagenase in PBS for 30 minutes at 37°C. Cells were stained first with CD45-biotin (Pharmingen) followed by streptavidin-PE (Pharmingen) to select lymphoid cells from contaminating stromal cells. The suspension was then stained with annexin V according to published methods and analyzed by FACS.22
RESULTS
Reduced cellularity and TN thymocytes in adult and fetalHoxa-9−/− thymuses.
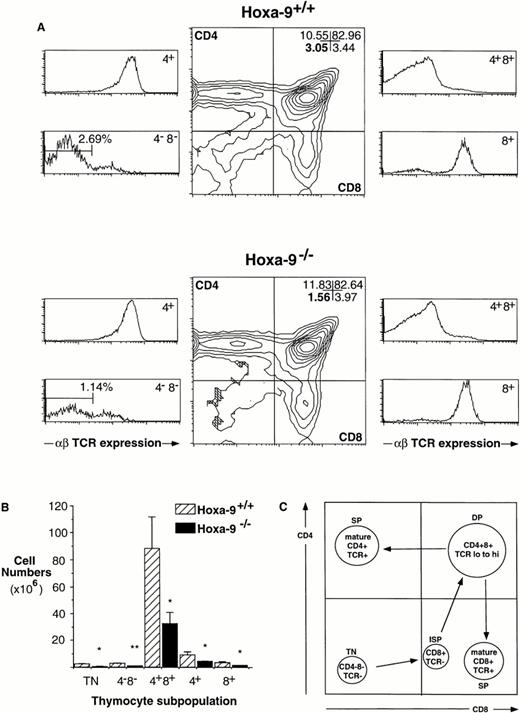
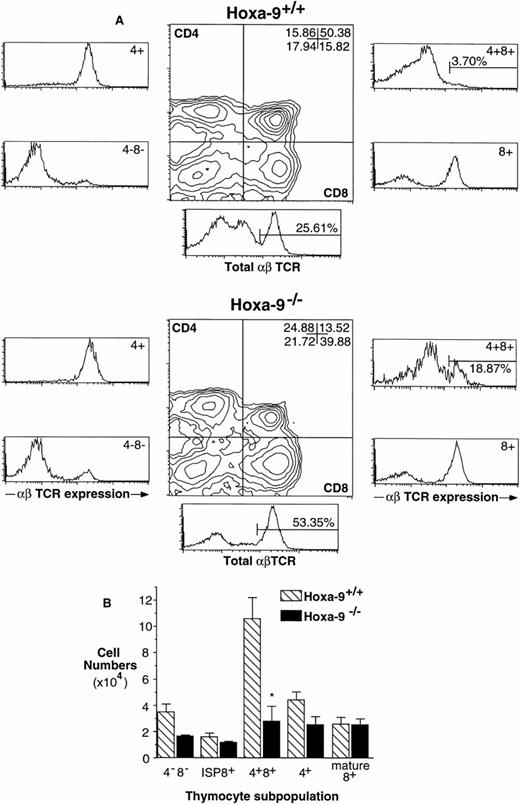
The thymuses of adult Hoxa-9−/− mice are 60% less cellular than the glands of wild-type littermates (mean cell number, 50.3 ± 14 × 106 forHoxa-9−/− animals [n = 8]v 120 ± 24 × 106 for wild-type mice [n = 7]; two-tailed P < .05). AdultHoxa-9−/− thymuses have normal ratios of DP and mature single-positive (SP) cells as defined by CD4 and CD8 expression (Fig 1A). However, using an anti-TCR antibody, a significant contraction in the TN subpopulation was evident (Fig 1A and B), with a twofold reduction in the percentage of TN cells. When absolute cell numbers are compared (Fig 1B), there is a 3.6-fold reduction in TN cells in mutant thymuses compared with wild-type littermates (0.67 ± 0.13 × 106 cellsv 2.41 ± 0.48 × 106; P < .01).
Defects in Hoxa-9−/− adult thymuses. (A) Hoxa-9−/− thymuses have reduced percentages of TN cells. FACS analysis using antibodies against CD4, CD8, and αβ TCR were performed. Hoxa-9−/−thymuses showed a twofold reduction in the percentage of CD4−CD8− cells (lower left quadrant, middle panels) and significant contraction of TN cells (lower left side panels), with no changes in the percentages of mature populations. The numbers in the CD4−CD8−panel represent the TN cells expressed as a percentage of total thymocytes. (B) Absolute numbers of T-cell subsets. Based on analyses of 5 litters, there were statistically significant decreases in cell numbers of TN, DN, DP, and SP populations of theHoxa-9−/− thymuses (*P < .05; **P < .01). (C) Schematic representation of normal T-cell development, correlated with FACScan profiles. Note that the CD4−CD8+ subset (lower right quadrant) includes both mature and immature (ISP) cells, which can be distinguished by their expression or lack of expression of TCR (A, lower right side panels).
Defects in Hoxa-9−/− adult thymuses. (A) Hoxa-9−/− thymuses have reduced percentages of TN cells. FACS analysis using antibodies against CD4, CD8, and αβ TCR were performed. Hoxa-9−/−thymuses showed a twofold reduction in the percentage of CD4−CD8− cells (lower left quadrant, middle panels) and significant contraction of TN cells (lower left side panels), with no changes in the percentages of mature populations. The numbers in the CD4−CD8−panel represent the TN cells expressed as a percentage of total thymocytes. (B) Absolute numbers of T-cell subsets. Based on analyses of 5 litters, there were statistically significant decreases in cell numbers of TN, DN, DP, and SP populations of theHoxa-9−/− thymuses (*P < .05; **P < .01). (C) Schematic representation of normal T-cell development, correlated with FACScan profiles. Note that the CD4−CD8+ subset (lower right quadrant) includes both mature and immature (ISP) cells, which can be distinguished by their expression or lack of expression of TCR (A, lower right side panels).
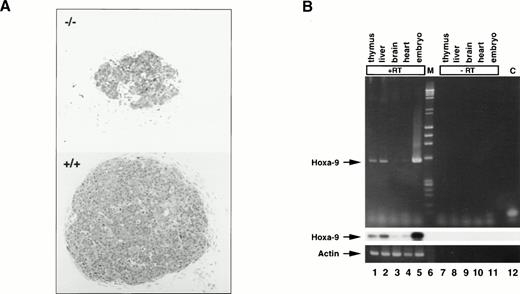
The thymic defect was much more severe in day-15.5 fetalHoxa-9−/− mice, whose thymuses had only one eighth the cellularity of those of normal littermates (0.18 ± 0.03 × 105 [n = 8] v 1.4 × 105 ± 0.02 [n = 7]; P < .001). Histologically, the mutant glands appeared much smaller and less cellular (Fig 2A). It should be noted that at this early stage in development the thymus is composed largely of TN thymocytes.
Hoxa-9 in day-15.5 fetal thymuses. (A) Microphotographs of sections of mutant (top) and normal (bottom) day-15.5 fetal thymuses stained with methylene blue (original magnification × 150). Sections were taken through the midportion of each gland. (B) Hoxa-9 gene expression in normal fetal thymus. RT-PCR using primers specific for Hoxa-9 was performed on day-15.5 fetal tissues and the amplified cDNAs probed for Hoxa-9by Southern blot analysis. Lanes 1 through 5 with RT; lane 1, thymus; lane 2, liver; lane 3, brain; lane 4, heart; lane 5, whole embryo; lane 6, markers (M); lanes 7 through 11, same tissues with no RT; lane 12, control (C) with no DNA. Strong signal is seen in lanes 1 and 2, representing fetal thymus and liver, respectively. (Upper panel) Ethidium bromide staining. (Middle panel) Southern blotting forHoxa-9. (Lower panel) Actin control.
Hoxa-9 in day-15.5 fetal thymuses. (A) Microphotographs of sections of mutant (top) and normal (bottom) day-15.5 fetal thymuses stained with methylene blue (original magnification × 150). Sections were taken through the midportion of each gland. (B) Hoxa-9 gene expression in normal fetal thymus. RT-PCR using primers specific for Hoxa-9 was performed on day-15.5 fetal tissues and the amplified cDNAs probed for Hoxa-9by Southern blot analysis. Lanes 1 through 5 with RT; lane 1, thymus; lane 2, liver; lane 3, brain; lane 4, heart; lane 5, whole embryo; lane 6, markers (M); lanes 7 through 11, same tissues with no RT; lane 12, control (C) with no DNA. Strong signal is seen in lanes 1 and 2, representing fetal thymus and liver, respectively. (Upper panel) Ethidium bromide staining. (Middle panel) Southern blotting forHoxa-9. (Lower panel) Actin control.
Expression of Hoxa-9 in murine fetal thymus.
Hoxa-9 expression in normal adult thymuses had been previously demonstrated.14 To determine if this gene was active during fetal thymic development, normal day-15.5 fetal tissues were subjected to RT-PCR analysis. Hoxa-9 mRNA is clearly expressed in thymus and liver, weakly detectable in heart, and virtually absent in brain (Fig 2B).
Reduced expression of CD25 (IL-2Rα chain) inHoxa-9−/− thymuses.
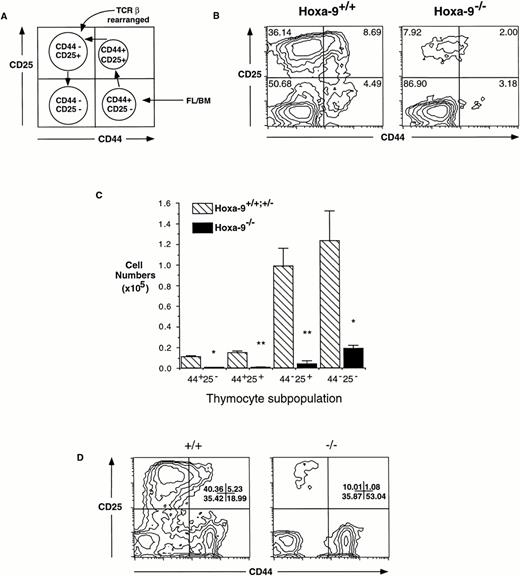
T-cell precursors entering the thymus from the fetal liver express high levels of the surface molecule CD44. These primitive TN T cells then proceed through an orderly developmental sequence (Fig 3A) that includes transient expression of the α chain of IL-2R (CD25), during which time active rearrangement of the TCRβ chain occurs, followed by downregulation of CD44.23 To map the defect in the TN population ofHoxa-9 mice more precisely, day-15.5 fetal thymocytes were analyzed using antibodies against CD44 and CD25. Figure 3B demonstrates a fourfold to fivefold contraction in the percentage of CD25+ cells in theHoxa-9−/− fetal thymus. When expressed in terms of absolute numbers, the CD25+ cells are reduced by approximately 25-fold (Fig 3C). It was interesting to note that the majority of mutant thymocytes appears to be CD25−CD44− cells (Fig 3B and C). In a normal thymus, CD25−CD44−thymocytes have completed TCRβ rearrangement; however, as shown below, this is not the case in the mutant fetal thymocytes. The possibility was considered that some of these cells in theHoxa-9−/− fetal thymus could represent other cell types that are also CD25−CD44−; however, FACS analysis showed no increase in myeloid, NK, or γδ Τ cells (data not shown).
Defective expression of CD25 in developingHoxa-9−/− T cells. (A) Schematic representation of normal differentiation stages within the TN population of T cells, correlated with FACS scan profiles. The curved arrow points to the stage at which TCRβ rearrangement is largely completed. (B) FACS analyses of day-15.5 fetal thymocytes was performed using antibodies against CD25 and CD44. A fourfold to fivefold reduction in the percentages of CD25+ cells (both CD44+ and CD44−) was seen in the mutant thymuses. (C) Absolute number of thymocyte subsets in fetal thymuses. Based on FACS analysis of 3 separate litters (representing 9 mutant and 12 control animals), there is an absolute decrease in all four major subsets of T cells inHoxa-9−/− thymuses, with a 25.9-fold decrease in the CD25+ compartments. (D) A representative FACS analysis of adult CD4−CD8− thymocytes. The percentage of CD25+ cells is decreased in the mutant thymus (right panel). Similar results were seen with 2 additionalHoxa-9−/− thymuses.
Defective expression of CD25 in developingHoxa-9−/− T cells. (A) Schematic representation of normal differentiation stages within the TN population of T cells, correlated with FACS scan profiles. The curved arrow points to the stage at which TCRβ rearrangement is largely completed. (B) FACS analyses of day-15.5 fetal thymocytes was performed using antibodies against CD25 and CD44. A fourfold to fivefold reduction in the percentages of CD25+ cells (both CD44+ and CD44−) was seen in the mutant thymuses. (C) Absolute number of thymocyte subsets in fetal thymuses. Based on FACS analysis of 3 separate litters (representing 9 mutant and 12 control animals), there is an absolute decrease in all four major subsets of T cells inHoxa-9−/− thymuses, with a 25.9-fold decrease in the CD25+ compartments. (D) A representative FACS analysis of adult CD4−CD8− thymocytes. The percentage of CD25+ cells is decreased in the mutant thymus (right panel). Similar results were seen with 2 additionalHoxa-9−/− thymuses.
To determine whether this defect in CD25 expression persisted into later life, adult CD4−CD8−thymocytes were also analyzed for expression of CD44 and CD25. TheHoxa-9−/− thymuses demonstrated a similar marked decrease in CD25 expression as that in the fetus (Fig3D), but with no reduction in CD44 expression. Furthermore, lethally irradiated mice reconstituted withHoxa-9−/− marrow also displayed CD25 downregulation in their CD4−CD8− T cells compared with controls (data not shown). This finding indicated that the defect was transplantable and therefore intrinsic to the hematopoietic cell.
The reduced expression of CD25 in primitiveHoxa-9−/− thymocytes did not represent a global inability to upregulate CD25, because, when activated by concanavalin A for 3 days, mature Thy-1+ T cells from adultHoxa-9−/− spleens express CD25 equally well as wild-type controls (data not shown). Additionally, the common γ chain of the IL-2R was expressed at normal levels inHoxa-9−/− fetal thymocytes (data not shown), indicating that other components of the IL-2 signaling pathway were not perturbed.
Retarded progression of Hoxa-9−/−thymocytes to DP T cells in fetal thymic organ cultures (FTOC).
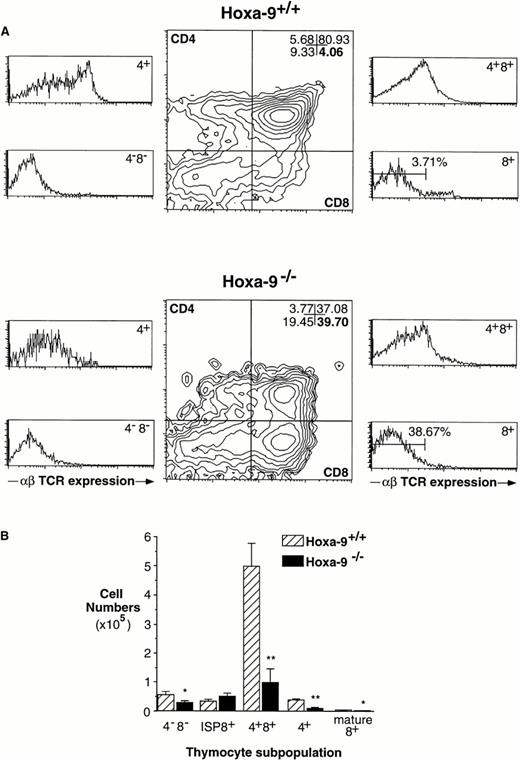
To examine the ability of TN cells inHoxa-9−/− mice to differentiate to DP cells, FTOC were performed. Individual day-15.5 fetal thymuses fromHoxa-9+/+ and Hoxa-9−/−mice were cultured for 6 days, and T-cell development was monitored by FACS analysis. There was a threefold decrease in total numbers of cells recovered from day-15.5 Hoxa-9−/− FTOCs compared with controls (1.99 ± 0.62 × 105 [n = 7] v 6.31 ± 0.95 × 105 [n = 7]; P < .005). In addition, there was a marked retardation in the progression of TN to DP, with a 10-fold increase (38.7% v 3.7%) in the percentage of CD8+ ISP cells and a twofold increase in the percentage of TN cells inHoxa-9−/− FTOCs (Fig 4A and B).
Abnormal development of Hoxa-9−/−thymocytes in 6-day FTOCs. (A) FACS analysis of thymocytes harvested from 6-day FTOCs using antibodies against CD4, CD8, and αβ TCR. There is a marked decrease in the number of DP cells (upper right quadrant of middle panels) in the mutant thymuses, associated with a 10-fold increase in the percentage of CD8+ cells (lower right quadrant). These latter cells are ISPs, ie, TCR−CD8+, as shown in the lower right side panels, where the numbers represent ISP cells as a percentage of total thymocytes. (B) Absolute numbers of T-cell subsets in 6-day FTOCs. Based on analyses from 4 separate FTOC experiments (representing 7 mutant and 8 wild-type thymuses), there is a delay in progression ofHoxa-9−/− thymocytes through the ISP stage and significant reductions in the numbers of more mature DP and SP cells.
Abnormal development of Hoxa-9−/−thymocytes in 6-day FTOCs. (A) FACS analysis of thymocytes harvested from 6-day FTOCs using antibodies against CD4, CD8, and αβ TCR. There is a marked decrease in the number of DP cells (upper right quadrant of middle panels) in the mutant thymuses, associated with a 10-fold increase in the percentage of CD8+ cells (lower right quadrant). These latter cells are ISPs, ie, TCR−CD8+, as shown in the lower right side panels, where the numbers represent ISP cells as a percentage of total thymocytes. (B) Absolute numbers of T-cell subsets in 6-day FTOCs. Based on analyses from 4 separate FTOC experiments (representing 7 mutant and 8 wild-type thymuses), there is a delay in progression ofHoxa-9−/− thymocytes through the ISP stage and significant reductions in the numbers of more mature DP and SP cells.
The marked relative increase in ISP cells in the day-6Hoxa-9−/− FTOCs could simply reflect selective loss of CD4+ CD8+ cells. To address this possibility, FTOCs were performed for a longer time period (10 days) to examine progression to DP and mature SP thymocytes. Surprisingly, the Hoxa-9−/− thymuses exhibited significantly increased percentages of mature SP CD4+ and CD8+ cells when compared with wild-type thymuses, indicating that there was no selective loss of DP cells but rather a compensatory enhancement of DP to SP conversion in the mutant thymocytes (Fig 5A). In addition, there was an fivefold increase in the percentage of αβTCRhi DP cells (representing cells undergoing active selection) in the Hoxa-9−/− FTOCs (Fig5A, upper right panels). This phenomenon is also apparent in the analysis of total cell numbers from the different thymocytes subsets (Fig 5B). It should be noted that, after 10 days in culture, the mutant thymuses now had only a twofold decrease in cellularity compared with wild-type controls.
Accelerated progression ofHoxa-9−/− thymocytes to SP cells in 10-day FTOCs. (A) FACS analysis of thymocytes harvested from 10-day FTOCs using antibodies against CD4, CD8, and αβ TCR. Most of theHoxa-9−/− cells have progressed through the DP stage to become mature SP cells. (B) Absolute numbers of T-cell subsets in 10-day FTOCs. Based on analyses from 3 separate FTOC experiments (representing 4 mutant and 6 wild-type thymuses), the numbers of SP mature cells in the mutant and normal FTOCs are not significantly different, but the number of Hoxa-9−/− DP is markedly reduced.
Accelerated progression ofHoxa-9−/− thymocytes to SP cells in 10-day FTOCs. (A) FACS analysis of thymocytes harvested from 10-day FTOCs using antibodies against CD4, CD8, and αβ TCR. Most of theHoxa-9−/− cells have progressed through the DP stage to become mature SP cells. (B) Absolute numbers of T-cell subsets in 10-day FTOCs. Based on analyses from 3 separate FTOC experiments (representing 4 mutant and 6 wild-type thymuses), the numbers of SP mature cells in the mutant and normal FTOCs are not significantly different, but the number of Hoxa-9−/− DP is markedly reduced.
Multiple defects in the TN check point of T-cell development inHoxa-9−/− thymuses.
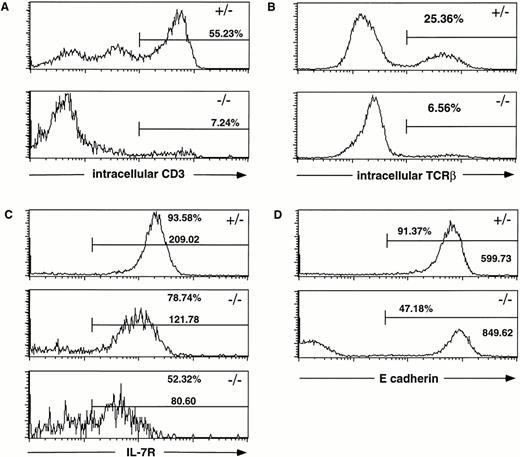
Because mutant mice displayed a major defect in early T-cell development, we analyzed the expression of proteins specifically required for the progression of TN cells to DP T cells. Because this step requires signaling through the pre-TCR/CD3 complex, day-15.5 and -16.5 fetal thymuses were analyzed for intracellular CD3 (Fig 6A) and TCRβ (Fig 6B) expression, respectively. Both were found to be markedly reduced inHoxa-9−/− thymocytes, indicating defective or retarded TCRβ rearrangement and assembly of a pre-TCR complex. Interestingly, the reduction of the levels of intracellular TCRβ was not associated with any measurable reduction in mRNA levels of RAG-1 and RAG-2 as assayed by semiquantitative RT-PCR (J. Taubenberger, personal communication, October 1997). Because previous studies had demonstrated a key role of IL-7R signaling for TCRβ gene rearrangement,24 IL-7R expression was also analyzed and found to be markedly downregulated compared with heterozygous controls (Fig 6C).
Multiple defects in the TN check point of T-cell development in Hoxa-9−/− thymuses. Day-15.5 or day-16.5 fetal thymocytes were subjected to FACS analysis using the specific antibodies indicated in each panel. (A) Intracellular CD3 expression. The percentage of mutant thymocytes expressing intracellular CD3 is markedly reduced. (B) Intracellular TCRβ expression. Approximately one fourth of the normal fetal thymocytes have successfully rearranged their TCRβ chain by day 16.5, whereas the extent of TCRβ rearrangement in theHoxa-9−/− T cells is diminished by fourfold. (C) IL-7R expression. The percentage of IL-7R+ thymocytes in Hoxa-9−/− thymuses is significantly decreased as is the mean fluorescence intensity. (D) E-cadherin expression on fetal thymocytes. The fraction ofHoxa-9−/− thymocytes expressing high levels of E-cadherin is diminished by half. All analyses were repeated with at least 3 separate mutant and control animals.
Multiple defects in the TN check point of T-cell development in Hoxa-9−/− thymuses. Day-15.5 or day-16.5 fetal thymocytes were subjected to FACS analysis using the specific antibodies indicated in each panel. (A) Intracellular CD3 expression. The percentage of mutant thymocytes expressing intracellular CD3 is markedly reduced. (B) Intracellular TCRβ expression. Approximately one fourth of the normal fetal thymocytes have successfully rearranged their TCRβ chain by day 16.5, whereas the extent of TCRβ rearrangement in theHoxa-9−/− T cells is diminished by fourfold. (C) IL-7R expression. The percentage of IL-7R+ thymocytes in Hoxa-9−/− thymuses is significantly decreased as is the mean fluorescence intensity. (D) E-cadherin expression on fetal thymocytes. The fraction ofHoxa-9−/− thymocytes expressing high levels of E-cadherin is diminished by half. All analyses were repeated with at least 3 separate mutant and control animals.
Abnormal expression of E-cadherin in mutant TN thymocytes.
It has been previously demonstrated that the adhesion molecule E-cadherin is expressed on fetal TN thymocytes as well as on thymic epithelial cells25,26 and that treating dispersed fetal thymuses with anti-E-cadherin antibody profoundly disturbs T-cell development.27 Given that E-cadherin has been proposed to be a target gene for certain Hox proteins,28 we wished to determine if E-cadherin expression was perturbed inHoxa-9−/− TN thymocytes. FACS analysis using an E-cadherin antibody demonstrated that fewer than half of the mutant thymocytes express E-cadherin, whereas approximately 90% of control TN T cells stained brightly (Fig 6D).
Increased apoptotic cell death in fetalHoxa-9−/− thymocytes.
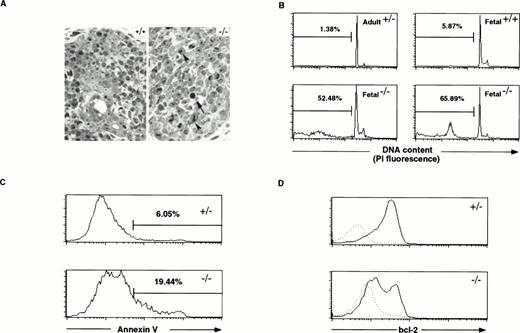
Because developing T cells need signaling through the IL-7R and the pre-TCR complex for their survival and because both of these receptors appear to be reduced in Hoxa-9−/−thymocytes, we investigated whether there was evidence of decreased cell survival in Hoxa-9−/− TN thymuses. Increased cell death was suspected based on the increased side scatter and reduced average size of viable fetalHoxa-9−/− thymocytes by forward scatter on FACS analysis and by a significant increase in cells staining with propidium iodide (data not shown). Histology of the mutant glands also showed increased numbers of pyknotic cells and nuclei with areas of condensed chromatin (Fig 7A).
Increased cell death in Hoxa-9−/−fetal thymuses. (A) Microphotographs of sections of mutant (right) and normal (left) day-15.5 fetal thymuses stained with methylene blue (original magnification × 560). The arrow points to a pyknotic nucleus and the arrow heads mark areas of chromatin condensation. (B) Day-15.5 Hoxa-9−/− thymocytes show a marked increase in hypodiploid nuclei by propidium iodide staining and FACS analysis. The percentage of hypodiploid nuclei in mutant thymuses is dramatically increased (lower two panels) as compared with control adult and fetal thymuses (upper two panels). (C) Annexin V staining of fetal thymocytes. Mutant thymuses (n = 7) show an approximate threefold increase in the percentage of cells binding high levels of annexin V as compared with control animals (n = 10), as well as significant increases in the fraction binding intermediate levels. (D) Bcl-2 protein levels in day-15.5 thymocytes. Control thymocytes (n = 12) show high levels in bcl-2 protein in nearly all cells, whereas half of the Hoxa-9−/− thymocytes (n = 4) show markedly reduced levels of bcl-2 (solid lines). Dotted lines depict staining with hamster IgG-PE as a negative control.
Increased cell death in Hoxa-9−/−fetal thymuses. (A) Microphotographs of sections of mutant (right) and normal (left) day-15.5 fetal thymuses stained with methylene blue (original magnification × 560). The arrow points to a pyknotic nucleus and the arrow heads mark areas of chromatin condensation. (B) Day-15.5 Hoxa-9−/− thymocytes show a marked increase in hypodiploid nuclei by propidium iodide staining and FACS analysis. The percentage of hypodiploid nuclei in mutant thymuses is dramatically increased (lower two panels) as compared with control adult and fetal thymuses (upper two panels). (C) Annexin V staining of fetal thymocytes. Mutant thymuses (n = 7) show an approximate threefold increase in the percentage of cells binding high levels of annexin V as compared with control animals (n = 10), as well as significant increases in the fraction binding intermediate levels. (D) Bcl-2 protein levels in day-15.5 thymocytes. Control thymocytes (n = 12) show high levels in bcl-2 protein in nearly all cells, whereas half of the Hoxa-9−/− thymocytes (n = 4) show markedly reduced levels of bcl-2 (solid lines). Dotted lines depict staining with hamster IgG-PE as a negative control.
To further analyze this phenomenon of increased cell death, cell suspensions from individual day-15.5 fetalHoxa-9+/+ andHoxa-9−/− thymuses were stained with a hypotonic propidium iodide solution and analyzed by FACS to measure ploidy. Figure 7B shows that greater than 50% of the fetalHoxa-9−/− thymocytes are hypodiploid and presumably apoptotic, compared with less than 10% hypodiploid cells in control fetal thymuses. In some of theHoxa-9−/− thymuses, the percentage of hypodiploid cells exceeded 75%. Finally, analyzing cells for Annexin V binding showed a marked increase in both high and intermediate levels of binding in the mutant T cells, indicating membrane changes seen in early apoptosis (Fig 7C).
The antiapoptotic protein bcl-2 is normally expressed at high level in TN thymocytes and is downregulated in most T cells as they progress to the DP stage.29 30 To ascertain whether bcl-2 expression was altered in primitive Hoxa-9−/− T cells, thymocytes from day-15.5 fetuses were stained intracellularly with anti-bcl-2 antibody. The majority of control thymocytes express high levels of bcl-2, whereas Hoxa-9−/−thymocytes show a distinctive biphasic pattern, with approximately half the cells showing very diminished levels of the protein (Fig7D).
DISCUSSION
In summary, Hoxa-9−/− mice have a major defect in fetal thymic development with severe reductions in immature thymocytes and blunted expression of several surface markers, including CD25, IL-7R, and E-cadherin, all accompanied by a marked downregulation of bcl-2 expression and an increase in cell death. These defects are intrinsic to the Hoxa-9−/−hematopoietic cells and not the thymic stroma, because mice transplanted with Hoxa-9−/− bone marrow display similar thymic abnormalities. The perturbations in T-cell development associated with loss of Hoxa-9 function are complex, because, in addition to the marked abnormalities at the TN stage, mutant thymocytes show delayed progression through the ISP stage and a curious acceleration from DP to mature single-positive T cells. It is not clear whether these later abnormalities reflect a true physiologic role for Hoxa-9 at the ISP and DP stages of T-cell differentiation or whether they represent the consequences and/or compensations for the defects seen at the TN stage. The fact that the block in T-cell development inHoxa-9−/− mice is not complete may indicate that paralogous Hox genes are partially compensating for the mutation. The T-cell defect is much more severe in fetal thymuses than in adults, perhaps because of the stress of rapid growth in the fetal thymus.
One interpretation of the immunophenotypic findings inHoxa-9−/− fetal thymuses is that early T-cell development is potentially accelerated, with an abnormally high proportion of the mutant cells having already arrived at the CD25−CD44− stage of TN differentiation by day 15.5 of gestation. However, normal CD25−CD44− TN thymocytes express significant levels of intracellular TCRβ and CD3, unlike a large majority of the Hoxa-9−/− fetal thymocytes. Thus, one explanation for the role of Hoxa-9 in early T-cell development is that it initiates and/or orchestrates certain molecular events that occur during the CD25+ step of TN differentiation. In this model, the absence of Hoxa-9−/− activity results in increased cell death in thymocytes that reach the CD25−CD44− stage, because few of them have assembled a surface pre-TCR complex through which to receive the required survival signals.
The precise genetic programs that are modulated by Hoxa-9function during T-cell development remain to be determined. The dramatic reduction in CD25+ TN cells suggests a defect in the IL-2 signaling pathway. Mice with a null mutation of the IL-2Rγ chain or a dominant-negative mutant IL-2Rγ chain show a similar slowing of the TN to DP transition.31,32 However, the common IL-2Rγ chain expression is not reduced inHoxa-9−/− thymuses, and the activity of the α chain, CD25, is not required for normal T-cell development.33 In addition, expression of CD25 in matureHoxa-9−/− T cells can still be activated normally by mitogens. CD44 expression is also diminished in the mutant fetal thymocytes, but not in the thymocytes of adultHoxa-9−/− mice. These data taken together suggest that the lack of CD25 and CD44 expression per se inHoxa-9−/− fetal thymuses does not account for the observed defects in early T-cell development, but is rather indicating a lack of thymocytes at that stage of TN differentiation in the mutant glands.
The T-cell defects in Hoxa-9−/− mice could also be a consequence of defective IL-7/IL-7R receptor signaling. T-cell precursors entering the thymus appear to be wholly dependent on IL-7 for their survival and for TCRβ rearrangement.16,34One expected consequence of blunted IL-7 signaling is decreased cell survival, and in fact Hoxa-9−/− fetal thymuses show a marked increase in cell death. It is important to recognize that the survival signal furnished via the IL-7/IL-7R pathway appears to be largely mediated by bcl-2. Indeed, a bcl-2 transgene can completely rescue the defect in T-cell development seen in animals with mutant IL-7 receptors.35 36 Thus, the defective expression of IL-7R and/or bcl-2 inHox-a9−/− thymuses could contribute to the observed defects in T-cell development.
Thymic epithelial cells are the predominant source of IL-7 in the thymus,37 and scanning electron microscopy has shown the intimate connection between developing T cells and the surrounding thymic epithelium.38 It is therefore conceivable that perturbations in the normally tight interactions between developing thymocytes and thymic epithelium underlie the defect inHoxa-9−/− animals. One of the adhesion molecules that appears to mediate these tight interactions is E-cadherin, and interfering with homotypic E-cadherin interactions between thymic epithelium and primitive thymocytes severely perturbs thymocyte development.27 Some data suggest that adhesion molecules, including N-CAM and certain cadherins, are transcriptionally regulated by Hox proteins.39-41 A Hoxa-1mutant mouse has been shown to have reduced cadherin-6 expression in the head folds of day-8.5 embryos.42 Evidence linkingHox proteins to the regulation of E-cadherin comes from work by Goomer et al,28 who demonstrated that Hoxd-9 could bind to DNA sequences in an enhancer in the E-cadherin gene. A reporter construct containing this enhancer element could be activated byHoxd-9 in transient transcription assays and overexpression ofHoxd-9 upregulated expression of the endogenous E-cadherin gene. These results are interesting, because Hoxd-9 has a high degree of homology (>90% identity at the amino acid level) toHoxa-9, and both proteins can bind to the same DNA target sequence (W.-F. Shen and C. Largman, unpublished observations).
Previous studies have furnished evidence for a link between homeobox genes and the regulation of cell proliferation. Kawabe et al43 reported that the orphan homeobox gene HOX11 could interact with genes involved in cell-cycle control and that misexpression of HOX11 in frog oocytes produced G2 arrest. It is noteworthy that we failed to demonstrate any significant alteration in cell cycling in the Hoxa-9−/−thymocytes (D. Izon, data not shown). Alternatively, there is evidence that homeobox genes may regulate cell survival and death. Expression of the homeobox fusion gene E2A-PBX1 in transgenic mice severely perturbs lymphoid development, with severe lymphopenia and high levels of apoptosis.44 Other investigators used antisense oligonucleotides to block the expression of PAX-type homeobox genes and observed apoptotic cell death.45
Homeobox genes are vital for cell lineage commitment decisions during embryogenesis and later development in such diverse species as flies and mammals. Hox genes are active in hematopoietic stem cells6 and have been implicated in leukemogenesis.11 46 However, until recently, there has been little definitive evidence to suggest that they function in normal blood cell development. The results presented here clearly demonstrate that Hoxa-9 is required for full and efficient T-cell maturation and that loss of Hoxa-9 function perturbs the chronology of early T-cell differentiation. These experiments suggest a novel and unexpected role for a Hox gene in T-cell development, modulating the death and differentiative potential of early thymocytes.
ACKNOWLEDGMENT
The authors are grateful to Drs C. Peterson and M. Capecchi for furnishing the Hoxa-9−/− mice and to Dr T. Sudo for supplying antibody A7R34. We also thank Drs R.K. Humphries, G. Sauvageau, and A. Kruisbeek for their critical review of the data and Drs J. Taubenberger, S. Boehme, and J.M. McCune for many helpful comments.
Supported in part by National Institutes of Health Grants No. RO1 DK 48642 (H.J.L.) and N44 DK 3-2219 (C.L.) and grants from the Department of Veterans Affairs (H.J.L. and C.L.). H.J.L. is a recipient of a VA Career Development Award.
Address reprint requests to H. Jeffrey Lawrence, MD, Hematology Research (151H), Veterans Affairs Medical Center, 4150 Clement St, San Francisco, CA 94121; e-mail: lawrencej@vacom.ucsf.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal