Abstract
Endoglin (CD105) is a cell surface component of the transforming growth factor-β (TGF-β) receptor complex highly expressed by endothelial cells. Mutations in the endoglin gene are responsible for the hereditary hemorrhagic telangiectasia type 1 (HHT1), also known as Osler-Weber-Rendu syndrome (OMIM 187300). This is an autosomal dominant vascular disorder probably caused by a haploinsufficiency mechanism displaying low levels of the normal protein. To understand the mechanisms underlying the regulated expression of endoglin, a genomic DNA clone containing 3.3 kb of the 5′-flanking sequence of the human endoglin gene has been isolated. The 5′-flanking region of the endoglin gene lacks consensus TATA and CAAT boxes, but contains two GC-rich regions and consensus motifs for Sp1, ets, GATA, AP-2, NFκB, and Mad, as well as TGF-β–, glucocorticoid-, vitamin D-, and estrogen-responsive elements. As determined by primer extension and 5′ RACE experiments, a cluster of transcriptional start sites was found to be located 350 bp upstream from the translation initiation codon. To analyze the endoglin promoter activity, the upstream −400/+341 fragment was fused to the luciferase gene and transient transfections were conducted in several cell types. This construct displayed a tissue-specific activity in human and bovine endothelial cells. Analysis of various deletion constructs showed the existence of a basal promoter region within the −81/+350 fragment as well as major transcriptional regulatory elements within the −400/−141 fragment. Electrophoretic mobility shift assays demonstrated the specific interaction of a member of the ets family with a consensus motif located at position −68. A promoter construct mutated at this ets sequence showed a much reduced activity as compared with the wild-type construct, supporting the involvement of this ets motif in the basal activity of the promoter. The endoglin promoter exhibited inducibility in the presence of TGF-β1, suggesting possible therapeutic treatments in HHT1 patients, in which the expression level of the normal endoglin allele might not reach the threshold required for its function. Isolation and characterization of the human endoglin promoter represents an initial step in elucidating the controlled expression of the endoglin gene.
ENDOGLIN, ALSO KNOWN as CD105, is a 180-kD homodimeric membrane glycoprotein strongly expressed by human endothelial cells.1 The gene encoding endoglin has been identified as the target gene for the autosomal dominant vascular disorder known as hereditary hemorrhagic telangiectasia type 1 (HHT1).2 HHT is a highly penetrant autosomal dominant vascular dysplasia associated with frequent epistaxis, gastrointestinal bleedings, telangiectases, and arteriovenous malformations in brain, lung, and liver.3 Telangiectases of HHT patients show enlargement of the postcapillary venules and eventual direct connection with arterioles, thus bypassing the capillary network. Most mutations of the endoglin gene identified thus far in HHT1 involve truncations of the extracellular domain of the protein.2,4,5 However, a number of missense mutations have now been described,6,7including a start codon mutation that is predicted to lead to a null allele.7 These data, in combination with analysis of the levels of transcript and protein from the mutant allele, suggest the possibility of a haploinsufficiency model for HHT1.5,7,8The specific function of endoglin responsible for the vascular dysplasia in HHT1 is not known, but it is most likely related to the transforming growth factor-β (TGF-β) system,9 because endoglin is a functional component of the membrane TGF-β receptor complex. Thus, endoglin binds TGF-β1 and TGF-β3 with high affinity (KD = 50 pmol/L) in human endothelial cells10; the heteromeric association between endoglin and the TGF-β signaling receptors I and II has been suggested by coimmunoprecipitation experiments11,12; and it has been demonstrated that endoglin expression is capable of modulating cellular responses to TGF-β.13 TGF-β regulates several processes of endothelial cells, including proliferation, migration, and adhesion; and perturbation of one or more of these processes may cause the vascular dysplasia observed in HHT1 patients.
The gene encoding endoglin has been localized to human chromosome 914 and it contains 15 exons,2,4 13 of which code for the extracellular domain of the protein. Molecular cloning of the human endoglin cDNA demonstrated the existence of at least two different isoforms, probably arising by alternative splicing.15,16 Endoglin homologues have been also isolated in mouse and pig, displaying greater than 70% identity with the human protein sequence.11,17,18 Although the major site of expression of endoglin is on endothelial cells, it is also present, to a lesser extent, on macrophages,19,20proerythroblasts,21 early B-lineage precursors,22 syncytiotrophoblasts of term placenta,23 and stromal cells.18,22 Endoglin is absent from peripheral monocytes, but it is expressed by in vitro differentiated monocytes, monocytic cells treated with TGF-β, and after phorbol ester treatment of monocytic cell lines.13 19As a step toward understanding the regulation of endoglin expression, we have isolated and characterized a genomic clone containing endoglin promoter activity.
MATERIALS AND METHODS
Screening of genomic libraries.
Endoglin genomic clones were isolated from cosmid and λ EMBL3 libraries. A human chromosome 9 cosmid library was initially screened with an endoglin cDNA probe lacking a complete 5′ end. Seventeen positive clones were screened again with a secondary oligo probe to the 5′ end of the endoglin cDNA. Three positive clones were identified and clone 149H12 was selected for further characterization. The 3.3-kbSacII/PvuII fragment (Fig1) of the endoglin promoter was subcloned into pBlueScript (Stratagene, La Jolla, CA) and sequenced in both directions on an ABI310 Prism DNA sequencer (PE Applied Biosystems, Foster City, CA). Also, a human leukocyte genomic library in phage λ EMBL3 was screened by plaque hybridization with a 3.1-kb EcoRI fragment of S-endoglin cDNA in pUC13.16 After four rounds of screenings, positive clones were isolated and one of them, containing a 14-kb genomic insert, was selected for further analysis. This clone was subjected to restriction enzyme digestions and hybridization with a synthetic oligonucleotide PE#4 (R) (5′-CAGGAGAAGTGGACAC-3′) corresponding to the 5′ region of endoglin cDNA. Additional digestions and hybridizations of this construct with the PE#4 oligonucleotide allowed the isolation of a 741-bp BamHI/BbrPI fragment (Fig 1) that encodes the 5′ untranslated region of endoglin mRNA. This fragment was cloned into a pUC19 plasmid and sequenced by the dideoxynucleotide chain-termination method (Pharmacia, Upsala, Sweden) with plasmid- and endoglin-specific primers.
Partial restriction enzyme map of the 5′-flanking region of the endoglin gene. The top bar represents the 3.3-kbSacII/PvuII fragment containing the untranslated region (▨), the signal peptide encoding region (░), and part of the first intron (▪). The 741-bpBamHI/BbrPI fragment was cloned into the luciferase reporter vector pXP2. The schematic representation is approximately to scale.
Partial restriction enzyme map of the 5′-flanking region of the endoglin gene. The top bar represents the 3.3-kbSacII/PvuII fragment containing the untranslated region (▨), the signal peptide encoding region (░), and part of the first intron (▪). The 741-bpBamHI/BbrPI fragment was cloned into the luciferase reporter vector pXP2. The schematic representation is approximately to scale.
Plasmid constructions.
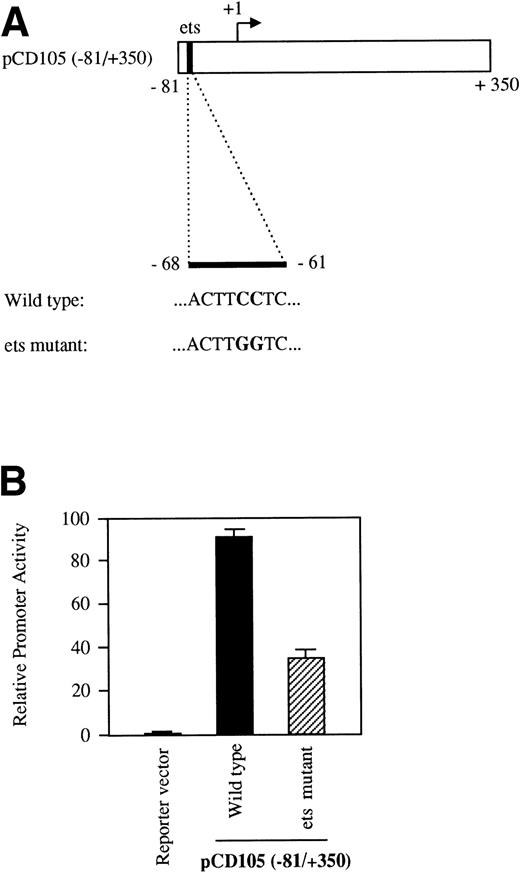
To assay for promoter activity, genomic fragments were inserted into a reporter vector containing the promoterless firefly luciferase gene.24 To generate deletions of the endoglin promoter, we used either unique restriction sites or polymerase chain reaction (PCR) amplification reactions. Reporter plasmids containing the following fragments (in parenthesis) were constructed: pCD105 (−851/+350), pCD105 (−851/−400), pCD105 (−400/+341), pCD105 (−224/+341), pCD105 (−141/+341), and pCD105 (−81/+350). Plasmid pCD105 (−81/+350)-Mut was constructed by PCR using pCD105 (−81/+350) as a template and contains a mutation at the ets site located at −68 (ACTTGGTC instead of ACTTCCTC). Constructs were numbered taking as a reference the transcription initiation site (+1). Correct orientation and sequence were verified in each construct.
Cells.
K562 (human erythro-leukemia), U-937 (human promonocytic), and HepG2 (human hepatoma) cell lines were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, penicillin (100 U/mL), and gentamycin (25 μg/mL) in a 5% CO2 atmosphere at 37°C. Bovine aortic endothelial cells (BAEC) and human umbilical vein endothelial cells (HUVEC) were isolated from cannulated vessels incubated in the presence of collagenase for 20 minutes at 37°C. Detached cells were plated in gelatin-coated flasks and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS. Keratinocytes, kindly provided by Dr F. Larcher (CIEMAT, Madrid, Spain), were obtained by trypsinization of porcine skin followed by coculturing in the presence of lethally irradiated fibroblasts as described.25The human microvascular endothelial cell line (HMEC-1) was generated by transfection with a pBR-322–based plasmid containing the coding region for the SV40 A gene product and large T antigen and was kindly provided by Dr Edwin Ades (Centers for Disease Control and Prevention, Atlanta, GA).26 Flow cytometry analyses showed that BAEC, HUVEC, U-937, and HMEC-1 are positive for endoglin expression, whereas keratinocytes, K562, and HepG2 lack endoglin expression1,19 27 (data not shown).
Transfection.
Transfections of HUVEC, BAEC, and HMEC-1 were performed following the calcium phosphate technique. Briefly, cells were plated at 1 × 105 cells/35-mm culture dish and the following day were transfected with calcium phosphate precipitates containing 1 μg of plasmid CMVβgal mixed with 2 μg of the test plasmid. After transfection, cultures were washed and replenished with complete media. Cells were harvested 48 hours after transfection and the enzymatic activities were determined. When required, BAEC were treated 24 hours after transfection with 0.1 to 10 ng/mL of TGF-β1 (R&D Systems, Minneapolis, MN). Transfections on keratinocytes, HepG2, and K562 cells were performed using lipofectin (GIBCO BRL, Grand Island, NY). Briefly, 2 μg of the test plasmid and 1 μg of CMVβgal were mixed with 5 μg Lipofectin in RPMI 1640 and the mixture was incubated with the cells for 24 hours. Then, RPMI 1640 containing 10% FCS was added, the cells were incubated for an additional period of 24 hours, and the corresponding enzymatic activities were assayed.
Luciferase activity was measured using a TD-20/20 Luminometer (Promega, Madison, WI). Internal normalization was performed by cotransfection of the test plasmids with CMVβgal, a β-galactosidase expression vector driven by the cytomegalovirus promoter. After normalization, the activity of the reporter constructs in each cell line was referred to the activity of the pGL2-SV40, a control vector containing the SV40 promoter and enhancer linked to the luciferase gene. The promoterless plasmid pXP2 containing the luciferase gene was used as a negative control. Plasmid pxp-200 containing the promoter region of P-cadherin28 and the TGF-β–inducible reporter construct p3TP-Lux29 were used as controls in TGF-β stimulation experiments.
Primer extension analysis.
Primer extension assays were performed using a commercial kit (AMV Reverse Transcriptase Primer Extension System; Promega). Briefly, the synthetic oligonucleotide PE#2 (R) (5′-GGGTGCTGGGCTCCAATGGATG-3′), corresponding to the 5′ end of the endoglin cDNA, was labeled with [γ-32P] ATP and T4 polynucleotide kinase. About 100 fmol of the 5′-end-labeled primer was hybridized with 1 μg of poly (A)+ RNA from either human placenta or human lung (Clontech, Palo Alto, CA) in 11 μL of 50 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 10 mmol/L MgCl2, 10 mmol/L dithiothreitol (DTT), 1 mmol/L dNTPs, and 0.5 mmol/L spermidine (AMV Primer Extension Buffer 1×) for 20 minutes at 65°C and subsequently cooled down for 10 minutes at room temperature. The reverse transcription reaction was performed at 42°C for 30 minutes after the addition of 9 μL of Reverse Transcriptase Mix (5 μL of AMV Primer Extension Buffer 2×, 1.4 μL of 40 mmol/L sodium pyrophosphate, 1 μL [1U] of AMV reverse transcriptase, and nuclease-free water to 9 μL). Extension products were separated by an 8% polyacrylamide-7 mol/L urea gel, in parallel with the sequence of the promoter DNA performed with the same primer as used in the reverse transcription reaction.
Rapid amplification of 5′ cDNA ends (5′-RACE).
The 5′ RACE experiments were performed to map the transcriptional start site using a commercial kit (Marathon cDNA Amplification Kit; Clontech). Briefly, cDNA was synthesized using total RNA from PMA-treated U937 cells or poly (A)+ RNA from human placenta and then a DNA adaptor was ligated to both ends of the cDNA using T4 DNA ligase. PCR reactions were performed in a Perkin Elmer DNA Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT) using three different oligonucleotides as primers: a 27-mer adaptor primer (AP1), complementary to the cDNA adaptor ligated to the ends of the cDNA, and two 5′-endoglin cDNA-specific oligonucleotides, PE#4 (R) and PE#2 (R). A first set of reactions was performed with primers AP1 and PE#4 under the following conditions: 1 cycle of 1 minute at 94°C and 40 cycles of 30 seconds at 94°C (denaturing), 30 seconds at 50°C (annealing), and 1 minute at 68°C (extension), using a polymerase mixture of Taq and Pwo DNA polymerases (Expand Long Template PCR System; Boehringer Mannheim, Mannheim, Germany). The products of these reactions were amplified with a second set of PCR reactions using AP1 and PE#2 primers, the same polymerase mixture, and the following conditions: 1 cycle of 1 minute at 94°C and 30 cycles of 30 seconds at 94°C (denaturing) and 1 minute at 68°C (annealing and extension). As final products, DNA fragments of approximately 100 to 200 bp were obtained, which were subsequently cloned into pCR II vector (Invitrogen, Leek, The Netherlands) and sequenced.
Electrophoretic mobility shift assays (EMSAs).
The probes for the EMSAs were prepared by annealing complementary synthetic oligonucleotides followed by end labeling with [α32P]dCTP and Klenow fragment. Nuclear extracts from BAEC or human promonocytic U937 cells were obtained as described.30 When required, pretreatment of the cells with TGF-β1 was performed at 10 ng/mL for 1 day. The binding reaction was performed by preincubating 10 μg of nuclear proteins with 2.5 μg of poly (dl-dC) in a buffer containing 70 mmol/L KCl, 5 mmol/L MgCl2, 0.1 mmol/L ZnCl2, 0.5 mmol/L DTT, 0.05% (wt/vol) NP40, 12% glycerol, 1 mg/mL bovine serum albumin (BSA), and 20 mmol/L HEPES, pH 7.5, on ice for 10 minutes. Amounts of 0.5 to 2 ng of end-labeled double-stranded probe (30,000 to 50,000 cpm) were added to the reaction mixture containing the nuclear extract and incubated for 30 minutes at 4°C. Samples were electrophoresed on a 5% polyacrylamide gel in 0.5× TBE (89 mmol/L Tris-borate and 2 mmol/L EDTA) buffer at 150 V at room temperature for 3 hours. For the competition experiments, 100-fold excess unlabeled oligonucleotides were incubated with the reaction mixture for 20 minutes before the addition of the radiolabeled probe. Similarly, antibodies against ets-2 (generous gift of Dr Arun Seth, Women’s College Hospital, Toronto, Canada) and fos/jun (Santa Cruz Biotechnology, Santa Cruz, CA) were incubated with the nuclear extracts before the radiolabeled probe was added to the reaction mixture. The sequences of the oligonucleotide probe and competitors used in this study were as follows: probe −88/−59-WT 5′-CCAGTGACAAAGCCCGTGGCACTTCCTCTA-3′ (nucleotides −88 to −59 from the endoglin genomic sequence); probe −88/−59-Mut 5′-CCAGTGACAAAGCCCGTGGCACTTGGTCTA-3′ (probe −88/−59 mutated at the ets site); and probe CD11c-PU.1, 5′-ACTTGCTTCCTCAGTACCTTG-3′. The sequence of CD11c-PU.1 corresponds to the CD11c promoter and contains a consensus PU.1 site of the ets family.31 Probe BR1 was a 198-bp fragment of theChironomus BR1 promoter used here as a negative control.32
RESULTS
Sequence and transcription initiation analysis of the 5′-flanking region of endoglin gene.
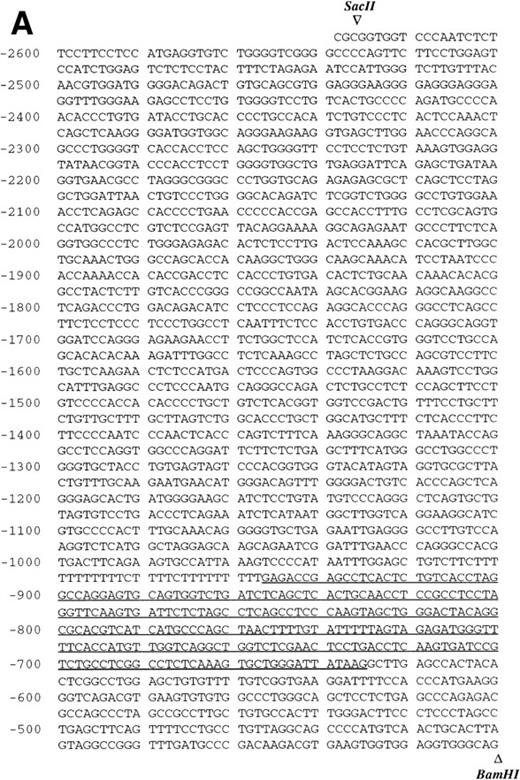
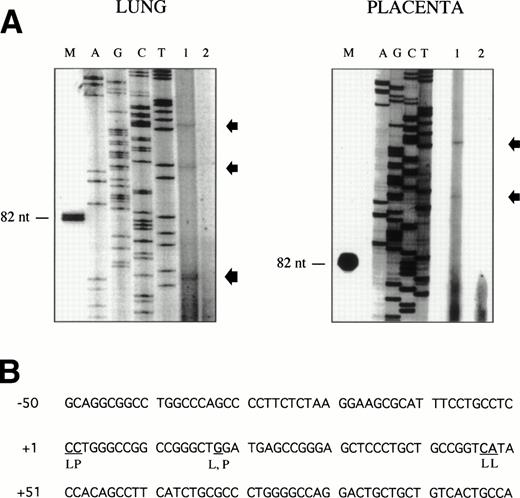
Sequence analysis of the 5′-flanking region (Fig2) included 3 kb corresponding to untranslated sequence. The 281 bp immediately upstream from the translation initiation codon ATG were found to be identical to the sequence deduced by cDNA cloning.16 In addition, we were able to extend the sequence 2.7 kb along the 5′ region, described here for the first time. To identify the transcriptional start site, we performed primer extension and 5′ RACE analysis. The oligonucleotide PE#2 (R), corresponding to the sequence starting at 231 bp upstream from the translation initiation codon, was used as a primer for the reverse transcription of poly (A)+ RNA from either human placenta or human lung. The result of this experiment (Fig 3) shows major transcription initiation sites at cytosines numbers as +1 (lung) and +2 (placenta), located 350 bp upstream from the ATG initiation codon. Minor start sites were also identified at guanine +18 (placenta and lung), cytosine +47 (lung), and adenosine +48 (lung). Further information regarding the transcription initiation was obtained by 5′ RACE experiments (Fig 4). Primer PE#4 (R) was used for cDNA synthesis of mRNA from either PMA-stimulated U-937 cells or human placenta. An anchor oligonucleotide was then ligated to the 3′ end of the synthesized cDNA. Nested primer PE#2 (R) and the anchor complementary oligonucleotide were used to amplify the 5′-region of endoglin mRNA. Electrophoretic analysis indicated that the PCR amplification resulted in four distinct bands of sizes ranging between 100 and 200 bp (Fig4A), suggesting the existence of several transcription initiation sites. The bands were recovered from the gel and cloned for sequencing. As shown in Fig 4B, sequencing of these bands showed transcription initiation at a guanine at +9 (placenta), at a cytosine at position +39 (U937 cells), and at a cytosine at position +78 (U937 cells and placenta). Taken together, the primer extension and 5′ RACE experiments indicated multiple initiation sites within a 78-bp fragment (positions +1, +2, +9, +18, +39, +47, +48, and +78). In the immediate upstream region from these transcriptional start sites, no consensus TATA or CAAT boxes were found. However, two GC-rich sequences, including an Sp1 binding site, were observed (−5 and −47; Fig 2B). In addition, the 5′-flanking sequence of endoglin gene contains several putative regulatory elements also present in other endothelial genes. These consensus elements include 18 AP-2 sites, 5 motifs of the ets family of transcription factors, 1 GATA site, 2 sites for NFκB, and 5 Sp1 sites (Fig 2B and Table 1). Furthermore, potential TGF-β–responsive elements were also identified, including two sites for the TGF-β–related signaling protein Mad, 10 TGF-β activation elements (TAE), 6 TGF-β control elements (TCE), and 2 TGF-β–inhibitory elements (TIE; Table2). Also, various steroid-responsive motifs could be identified, such as three estrogen response elements (ERE), one glucocorticoid response element (GRE), and five vitamin D response elements (VDRE). An Alu sequence, often located upstream of regular transcription units, was found to expand from −666 to −927 (Fig2A).
Nucleotide sequence of the 5′-flanking region of the endoglin gene. Numbers at the left margin refer to the transcription start site (+1) determined by primer extension analysis in this report. Arrowheads indicate consensus sites for specific restriction enzymes. (A) Sequence of the upstreamSacII/BamHI fragment (−2616 to −401). An Alu sequence located between −927 and −666 is underlined. (B) Sequence of the downstream BamHI/PvuII region (−400 to +672). Solid arrows indicate the transcription start sites reported here by primer extension: +1, +18, +47, and +48 (lung); +2 and +18 (placenta). Open arrows indicate the transcription start sites reported here by 5′ RACE: +9, and +78 (placenta); +39 and +78 (U937). The endoglin cDNA sequence previously described (Bellón et al16) is underlined with a dashed line. Consensus sequences for putative regulatory motifs include AP-2, Ets, GATA, NFκB, and Sp1. The location of the oligonucleotides PE#2 and PE#4 used for hybridization studies are indicated with a dotted overline. The derived aminoacid sequence of the signal peptide is shown underneath the coding region of the first exon using the three letter code. The nucleotide sequences of the 3.3-kb SacII/PvuII and 0.74-kbBamHI/BbrPI fragments have been assigned the EMBL/GeneBank accession nos. AF035753 and Y11653, respectively.
Nucleotide sequence of the 5′-flanking region of the endoglin gene. Numbers at the left margin refer to the transcription start site (+1) determined by primer extension analysis in this report. Arrowheads indicate consensus sites for specific restriction enzymes. (A) Sequence of the upstreamSacII/BamHI fragment (−2616 to −401). An Alu sequence located between −927 and −666 is underlined. (B) Sequence of the downstream BamHI/PvuII region (−400 to +672). Solid arrows indicate the transcription start sites reported here by primer extension: +1, +18, +47, and +48 (lung); +2 and +18 (placenta). Open arrows indicate the transcription start sites reported here by 5′ RACE: +9, and +78 (placenta); +39 and +78 (U937). The endoglin cDNA sequence previously described (Bellón et al16) is underlined with a dashed line. Consensus sequences for putative regulatory motifs include AP-2, Ets, GATA, NFκB, and Sp1. The location of the oligonucleotides PE#2 and PE#4 used for hybridization studies are indicated with a dotted overline. The derived aminoacid sequence of the signal peptide is shown underneath the coding region of the first exon using the three letter code. The nucleotide sequences of the 3.3-kb SacII/PvuII and 0.74-kbBamHI/BbrPI fragments have been assigned the EMBL/GeneBank accession nos. AF035753 and Y11653, respectively.
Mapping of the endoglin transcriptional start site by primer extension. The oligonucleotide PE#2 corresponding to the 5′ end of the endoglin cDNA was hybridized to poly (A)+ RNA from human placenta or lung. The products of annealing served as templates for reverse transcriptase. The extension products were separated on a denaturing polyacrylamide gel alongside a Sanger sequence primed on a plasmid DNA template using the same primer as that used in the reverse transcription reaction (A). Lane 1 contains the poly (A)+ RNA from lung or placenta, as indicated; lane 2 contains a negative control without RNA; and lane M is a size marker band in nucleotides. Extended products are denoted by arrows. The nucleotides identified at the transcription initiation are underlined (B). L, lung; P, placenta.
Mapping of the endoglin transcriptional start site by primer extension. The oligonucleotide PE#2 corresponding to the 5′ end of the endoglin cDNA was hybridized to poly (A)+ RNA from human placenta or lung. The products of annealing served as templates for reverse transcriptase. The extension products were separated on a denaturing polyacrylamide gel alongside a Sanger sequence primed on a plasmid DNA template using the same primer as that used in the reverse transcription reaction (A). Lane 1 contains the poly (A)+ RNA from lung or placenta, as indicated; lane 2 contains a negative control without RNA; and lane M is a size marker band in nucleotides. Extended products are denoted by arrows. The nucleotides identified at the transcription initiation are underlined (B). L, lung; P, placenta.
5′ RACE analysis of the transcriptional start site of the endoglin gene. (A) Electrophoresis analysis of nested PCR amplification reactions. cDNA was synthesized from total RNA of phorbol ester-stimulated U-937 cells or poly (A)+ RNA from human placenta using reverse transcriptase. An adaptor was then ligated to both ends of cDNA and two sets of succesive PCR amplifications were performed: first with the anchor oligonucleotide AP1 in the presence of the oligonucleotide PE#4, and second with AP1 and PE#2 oligonucleotides. PCR products were analyzed by electrophoresis in a 3% agarose gel. Bands corresponding to U-937 (a and b) or placenta (c and d) were purified for cloning purposes and analyzed in a different gel. Lane M is a size ladder marker. Bands were visualized by ethidium bromide staining. (B) Nucleotide sequence of the cloned 5′-RACE products. PCR products (bands a through d from A) were isolated from the gel, cloned into plasmids, and sequenced. The complementary sequences for the anchor and nested primers used for the PCR reaction are underlined. The sequence of the commercial adaptor linked to the endoglin cDNA is overlined.
5′ RACE analysis of the transcriptional start site of the endoglin gene. (A) Electrophoresis analysis of nested PCR amplification reactions. cDNA was synthesized from total RNA of phorbol ester-stimulated U-937 cells or poly (A)+ RNA from human placenta using reverse transcriptase. An adaptor was then ligated to both ends of cDNA and two sets of succesive PCR amplifications were performed: first with the anchor oligonucleotide AP1 in the presence of the oligonucleotide PE#4, and second with AP1 and PE#2 oligonucleotides. PCR products were analyzed by electrophoresis in a 3% agarose gel. Bands corresponding to U-937 (a and b) or placenta (c and d) were purified for cloning purposes and analyzed in a different gel. Lane M is a size ladder marker. Bands were visualized by ethidium bromide staining. (B) Nucleotide sequence of the cloned 5′-RACE products. PCR products (bands a through d from A) were isolated from the gel, cloned into plasmids, and sequenced. The complementary sequences for the anchor and nested primers used for the PCR reaction are underlined. The sequence of the commercial adaptor linked to the endoglin cDNA is overlined.
Consensus Sequences for Putative Transcription Factors Found in Endoglin and Other Endothelial Genes Promoters
| Factor . | Consensus Sequence . | Endoglin Sequence (position)* . | Endothelial Genes . | Reference . | |
|---|---|---|---|---|---|
| AP-2 | CCC(A/C)N(G/C)(G/C)(G/C) | CCCAGCGC CCCCTCGG GGGATGGG GGGTGGGG CCCACCCC | (−397) (−256) (−176) R (−167) R (−126) | GPIIb, vWF, PF4, P-selectin, E-selectin, VCAM-1, ICAM-1 | 33 |
| CCCATCCC | (−108) | ||||
| CCCAGGGC | (−103) | ||||
| CCCCACCC | (−94) | ||||
| CCCAGCCC | (−37) | ||||
| GCCCTGGG | (+68) R | ||||
| CCCTGGGG | (+69) R | ||||
| CCCCTCCC | (+119) | ||||
| CCCCGCCC | (+124) | ||||
| CCCAGCCC | (+154) | ||||
| CCCCTCGG | (+188) | ||||
| CCCCTCGG | (+247) | ||||
| CCCACGCC | (+268) | ||||
| CCCAGCGC | (+322) | ||||
| Ets | (G/C)(A/C)GGA(A/T)G(T/C) | GtTTCCTC CAGGAAcT ACTTCCTC AGGAAGC AtTTCCTG | (−347) R (−294) (−68) R (−21) (−12) R | GPIIb, vWF, PF4, P-selectin, E-selectin, VLA-4, ICAM-1 | 34 |
| GATA | (A/T)GATA(A/G) | GATAA | (+315) | GPIIb, vWF, PF4, Endothelin-1, VLA-4, PECAM-1 | 35 |
| NFκB | GGG(A/G)N2(C/T)(C/T)CC | GGGGCTCCC GGGAGCTCCC | (−218) D/R (+27) D/R | E-selectin, VCAM-1, ICAM-1, PECAM-1 | 36 |
| Sp1 | (T/G)(A/G)GGC(T/G)(A/G)(A/G)(T/G) | ACCAGCCT CCCAGCCCC CCCCGCCCA CCAGCCCA CCCAGCCCC | (−194) R (−37) R (+124) R (+150) R (+154) R | GPIIb, vWF, PF4, Endothelin-1, P-selectin, E-selectin, ICAM-1 | 37 |
| Factor . | Consensus Sequence . | Endoglin Sequence (position)* . | Endothelial Genes . | Reference . | |
|---|---|---|---|---|---|
| AP-2 | CCC(A/C)N(G/C)(G/C)(G/C) | CCCAGCGC CCCCTCGG GGGATGGG GGGTGGGG CCCACCCC | (−397) (−256) (−176) R (−167) R (−126) | GPIIb, vWF, PF4, P-selectin, E-selectin, VCAM-1, ICAM-1 | 33 |
| CCCATCCC | (−108) | ||||
| CCCAGGGC | (−103) | ||||
| CCCCACCC | (−94) | ||||
| CCCAGCCC | (−37) | ||||
| GCCCTGGG | (+68) R | ||||
| CCCTGGGG | (+69) R | ||||
| CCCCTCCC | (+119) | ||||
| CCCCGCCC | (+124) | ||||
| CCCAGCCC | (+154) | ||||
| CCCCTCGG | (+188) | ||||
| CCCCTCGG | (+247) | ||||
| CCCACGCC | (+268) | ||||
| CCCAGCGC | (+322) | ||||
| Ets | (G/C)(A/C)GGA(A/T)G(T/C) | GtTTCCTC CAGGAAcT ACTTCCTC AGGAAGC AtTTCCTG | (−347) R (−294) (−68) R (−21) (−12) R | GPIIb, vWF, PF4, P-selectin, E-selectin, VLA-4, ICAM-1 | 34 |
| GATA | (A/T)GATA(A/G) | GATAA | (+315) | GPIIb, vWF, PF4, Endothelin-1, VLA-4, PECAM-1 | 35 |
| NFκB | GGG(A/G)N2(C/T)(C/T)CC | GGGGCTCCC GGGAGCTCCC | (−218) D/R (+27) D/R | E-selectin, VCAM-1, ICAM-1, PECAM-1 | 36 |
| Sp1 | (T/G)(A/G)GGC(T/G)(A/G)(A/G)(T/G) | ACCAGCCT CCCAGCCCC CCCCGCCCA CCAGCCCA CCCAGCCCC | (−194) R (−37) R (+124) R (+150) R (+154) R | GPIIb, vWF, PF4, Endothelin-1, P-selectin, E-selectin, ICAM-1 | 37 |
Abbreviations: GPIIb, integrin subunit β3; vWF, von Willebrand factor; PF4, platelet factor 4; VCAM-1, vascular cellular adhesion molecule 1; ICAM-1, intercellular adhesion molecule 1; VLA-4, integrin subunit α4; PECAM-1, platelet-endothelial cell adhesion molecule 1.
Putative TGF-β and Steroid Responsive Elements Found on the Endoglin Promoter
| Response Element . | Consensus Sequence . | Endoglin Sequence (position)* . | Reference . | |
|---|---|---|---|---|
| Mad | GCCGNCGC | GCCGTCGC | (+225) | 40 |
| CGCCGGC | (+272) R | |||
| TAE | TCGN5GCCAAG | CTTTAGCCAAG) | (−285) | 41 |
| TGGCACTTCC | (−72) R | |||
| TTGGCAGGCG | (−53) R | |||
| CGGCCTGGCC | (−45) | |||
| TGGCCCAGCC | (−40) R | |||
| GTCACTGCCA | (+91) | |||
| TCGCTCCGGCC | (+229) | |||
| CGTGCGAGCC | (+260) | |||
| CGCACAGGCC | (+327) | |||
| GGCCCCCACG | (+333) R | |||
| TCE | GCGTGGGGA | GTGGGG | (−220) | 42 |
| GgGTGGGG | (−167) | |||
| GCtTGGGGA | (−158) | |||
| CCCACGC | (+268) R | |||
| CCCCACG | (+336) R | |||
| GgGTGGGGA | (+437) | |||
| TIE | GN2TTGGTGA | TagCCAAGCC | (−282) R | 43 |
| GCCTTGGaGA | (−190) | |||
| ERE | AGGTCAN3TGACCT | GGTCACAA | (−308) D/R | 44 |
| GGTCATAC | (+44) D/R | |||
| TCCTGACC | (+182) D/R | |||
| GRE | GGTACAN3TGTTCT | ACAGCAT | (+346) D/R | 44 |
| VDRE | (A/G)GGT(G/C)AN2(A/G)(A/G)GGNCA | GGGTCACAAA GCCCCTTCTC CTCCTGACCC GAGCAGGGAC GGCCCCGGTG | (−309) (−33) R (+181) R (+215) (+276) R | 45 |
| Response Element . | Consensus Sequence . | Endoglin Sequence (position)* . | Reference . | |
|---|---|---|---|---|
| Mad | GCCGNCGC | GCCGTCGC | (+225) | 40 |
| CGCCGGC | (+272) R | |||
| TAE | TCGN5GCCAAG | CTTTAGCCAAG) | (−285) | 41 |
| TGGCACTTCC | (−72) R | |||
| TTGGCAGGCG | (−53) R | |||
| CGGCCTGGCC | (−45) | |||
| TGGCCCAGCC | (−40) R | |||
| GTCACTGCCA | (+91) | |||
| TCGCTCCGGCC | (+229) | |||
| CGTGCGAGCC | (+260) | |||
| CGCACAGGCC | (+327) | |||
| GGCCCCCACG | (+333) R | |||
| TCE | GCGTGGGGA | GTGGGG | (−220) | 42 |
| GgGTGGGG | (−167) | |||
| GCtTGGGGA | (−158) | |||
| CCCACGC | (+268) R | |||
| CCCCACG | (+336) R | |||
| GgGTGGGGA | (+437) | |||
| TIE | GN2TTGGTGA | TagCCAAGCC | (−282) R | 43 |
| GCCTTGGaGA | (−190) | |||
| ERE | AGGTCAN3TGACCT | GGTCACAA | (−308) D/R | 44 |
| GGTCATAC | (+44) D/R | |||
| TCCTGACC | (+182) D/R | |||
| GRE | GGTACAN3TGTTCT | ACAGCAT | (+346) D/R | 44 |
| VDRE | (A/G)GGT(G/C)AN2(A/G)(A/G)GGNCA | GGGTCACAAA GCCCCTTCTC CTCCTGACCC GAGCAGGGAC GGCCCCGGTG | (−309) (−33) R (+181) R (+215) (+276) R | 45 |
Abbreviations: Mad, mothers against decapentaplegic complex; TAE, TGF-β activating element; TCE, TGF-β control element; TIE, TGF-β inhibitory element; ERE, estrogen response element; GRE, glucocorticoid response element; VDRE, vitamin D response element.
The 5′-flanking region of the endoglin gene displays tissue-specific promoter activity.
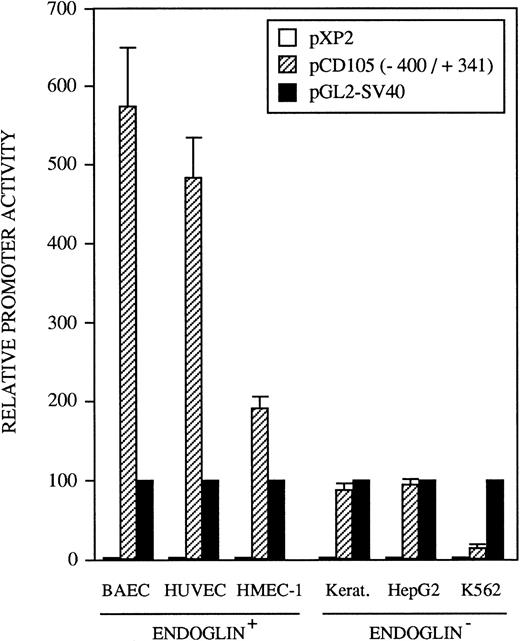
The identification of the transcription initiation sites suggested that the promoter activity was contained within the upstream 400-bp region. To determine whether the 5′-flanking region of the endoglin gene functions to direct transcription in a cell-type–dependent manner, the 741-bp BamHI/BbrPI genomic fragment spanning from −400 to +341 was placed upstream of the luciferase gene (Fig 1). The promoter activity of the complete 741-bp fragment was determined by transient transfections into HUVEC (endoglin+ human umbilical vein endothelial cells), BAEC (endoglin+ bovine aortic endothelial cells), HMEC-1 (endoglin+ human microvascular endothelial cells), porcine keratinocytes (endoglin−), K562 (endoglin− human erythro-leukemia), and HepG2 (endoglin− human hepatoma) cells. Figure 5 shows that luciferase activity in these cell types is indeed reflective of their endoglin expression. The promoter construct displayed the greatest activity in BAEC and HUVEC cells, followed by a significant activity in HMEC-1 cells. By contrast, the endoglin promoter displayed a much lower luciferase activity in the endoglin− keratinocytes, HepG2, and K562 cells. Thus, relative to K562 cells, the promoter activity was found to be approximately 40-fold in BAEC, 32-fold in HUVEC, and 12-fold in HMEC-1 cells. The promoter activity of the pXP2 basic vector was at background levels, whereas luciferase activity from the vector pGL2-SV40 containing the SV40 promoter and enhancer regions (a positive control for expression) was arbitrarily considered as 100.
Tissue-specific expression of the endoglin promoter. Endoglin+ (BAEC, HUVEC, and HMEC-1) and endoglin− (K562, HepG2, and keratinocytes) cells were transiently transfected with the pCD105 (−400/+341) endoglin promoter construct. Luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with a β-galactosidase expression vector. In addition, cells were transfected in parallel with the pGL2-SV40 vector, which contains the SV40 viral promoter activity, as a positive control. The promoterless pXP2 plasmid was also included as a negative control. This is a representative experiment of four separate experiments.
Tissue-specific expression of the endoglin promoter. Endoglin+ (BAEC, HUVEC, and HMEC-1) and endoglin− (K562, HepG2, and keratinocytes) cells were transiently transfected with the pCD105 (−400/+341) endoglin promoter construct. Luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with a β-galactosidase expression vector. In addition, cells were transfected in parallel with the pGL2-SV40 vector, which contains the SV40 viral promoter activity, as a positive control. The promoterless pXP2 plasmid was also included as a negative control. This is a representative experiment of four separate experiments.
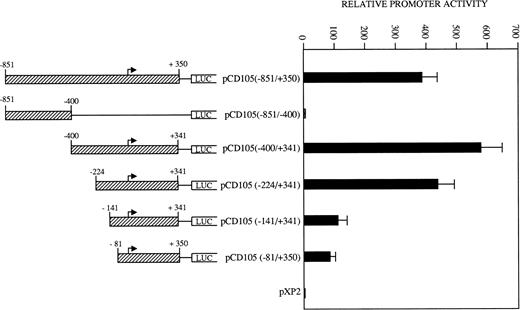
To determine the relevant elements that participate in endoglin transcription, the activities of a set of endoglin promoter fragments were compared (Fig 6). The largest construct pCD105 (−851/+350) and their pCD105 (−400/+341) and pCD105 (−224/+341) derivatives displayed similar activities, an average of 700-fold increase respect to the promoterless pXP2 vector in BAEC. By contrast, the plasmid pCD105 (−851/−400) showed background levels of promoter with respect to that of the other endoglin constructs, indicating that the critical elements of the promoter are located within the −400/+341 fragment. Constructs −141/+341 and −81/+350 displayed a decreased promoter activity in respect to pCD105 (−400/+341) and pCD105 (−224/+341) plasmids and similar to that of an SV40 promoter based construct. Because the −81/+350 construct contains only 81 bp upstream from the transcription initiation start site, these data indicate that this small region is capable of acting as a basal promoter, and additional regulatory elements contained within the immediate upstream 319-bp fragment allow for the optimal endoglin transcription.
Identification of critical elements within the endoglin promoter. A set of deletion constructs were generated and inserted into a reporter vector containing the luciferase gene. BAEC were transiently transfected with the indicated promoter constructs and luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors. This is a representative experiment of four separate experiments.
Identification of critical elements within the endoglin promoter. A set of deletion constructs were generated and inserted into a reporter vector containing the luciferase gene. BAEC were transiently transfected with the indicated promoter constructs and luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors. This is a representative experiment of four separate experiments.
The ets element at −68 is required for basal activity of the endoglin promoter.
As shown in Table 1, the region from −347 to −12 contains several potential ets elements that might regulate endoglin expression. The ets sequence at position −68 of the endoglin promoter is of interest, because similarly located motifs have been reported to mediate ets binding and transactivation of the von Willebrand factor and the vascular endothelial growth factor receptor 1 gene promoters.46,47 To determine whether this ets element functioned as a positive regulatory element in the endoglin gene, we mutated the wild-type sequence ACTTCCTCT to ACTTGGTCT in the pCD105 (−81/+350) construct (Fig 7A) and examined its effect on transcription. As shown in Fig 7B, when this construct was transfected into BAEC, luciferase activity was reduced by approximately 65% compared with that of the wild-type construct. To further corroborate these results and confirm that the ets element at −68 is able to interact with a member of the ets family of transcription factors, we performed EMSA experiments using as probes the oligonucleotides −88/−59-WT and −88/−59-Mut. Analysis of nuclear extracts from TGF-β–treated or untreated U937 cells and BAEC showed specific DNA binding proteins not affected by the presence of TGF-β, using as a probe the −88/−59 oligonucleotide (Fig 8A and B). Several lines of evidence indicated that the protein able to interact with the −88/−59 probe is a member of the ets family of transcription factors. First, the DNA-protein complex completely disappeared upon incubation with an antibody specific for ets-2. Second, the DNA-protein complex formation was markedly reduced when using the −88/−59-Mut probe that contains a mutation within the ets consensus sequence. Third, the oligonucleotide CD11c-PU.1, which contains a consensus motif for PU.1, a member of the ets family of transcription factors, partially competed with the −88/−59 probe. The partial competition obtained with the PU.1 oligonucleotide could be explained by the heterogeneity of the ets family of transcription factors, each recognizing a purine-rich sequence motif GGA/T, but differing from each other in specific flanking sequences.31 34 Together, these results suggest that a member of the ets family, likely ets-2, binds to and regulates endoglin gene expression.
The ets element at position −68 contributes to the transcriptional activity of the endoglin promoter. BAEC were transiently transfected with the pCD105 (−81/+350) (wild-type) or pCD105 (−81/+350)-Mut (ets mutant), which contains a mutation at the ets site (A). The luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors, used as positive and negative controls, respectively. This is a representative experiment of three separate experiments.
The ets element at position −68 contributes to the transcriptional activity of the endoglin promoter. BAEC were transiently transfected with the pCD105 (−81/+350) (wild-type) or pCD105 (−81/+350)-Mut (ets mutant), which contains a mutation at the ets site (A). The luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors, used as positive and negative controls, respectively. This is a representative experiment of three separate experiments.
Identification of an ets motif within the −88/−59 fragment. Nuclear extracts from TGF-β–treated or untreated U937 cells and BAEC were incubated with the [32P]-labeled −88/−59WT (A and B) or −88/−59Mut fragments (A), in the absence or in the presence of competitor oligonucleotides or specific antibodies as indicated. Excess of unlabeled −88/−59 probe was used as a control for specificity (A and B). The oligonucleotide CD11c-PU.1, containing a consensus binding site for the PU.1 member of the ets family of transcription factors, and fragment BR1, a negative control, were used in competition experiments (B). Specific antibodies to ets-2 or fos/jun were incubated with the nuclear extracts, using a preimmune serum as a negative control (B). Samples were electrophoresed on a 5% polyacrylamide gel and autoradiographed. The presence of specific complexes is indicated by an arrow at the margins. The open arrowheads indicate complexes formed by proteolytic products of ets as previously described.66
Identification of an ets motif within the −88/−59 fragment. Nuclear extracts from TGF-β–treated or untreated U937 cells and BAEC were incubated with the [32P]-labeled −88/−59WT (A and B) or −88/−59Mut fragments (A), in the absence or in the presence of competitor oligonucleotides or specific antibodies as indicated. Excess of unlabeled −88/−59 probe was used as a control for specificity (A and B). The oligonucleotide CD11c-PU.1, containing a consensus binding site for the PU.1 member of the ets family of transcription factors, and fragment BR1, a negative control, were used in competition experiments (B). Specific antibodies to ets-2 or fos/jun were incubated with the nuclear extracts, using a preimmune serum as a negative control (B). Samples were electrophoresed on a 5% polyacrylamide gel and autoradiographed. The presence of specific complexes is indicated by an arrow at the margins. The open arrowheads indicate complexes formed by proteolytic products of ets as previously described.66
Effect of TGF-β on the activity of the endoglin promoter.
The presence of consensus TGF-β–responsive elements within the endoglin promoter (Table 2) suggested transcriptional regulation by members of the TGF-β family of proteins and prompted us to assay the endoglin promoter activity in the presence of TGF-β1. As shown in Fig9A, the luciferase activity of the −400/+341 fragment was found to be induced by TGF-β in a dose-dependent fashion. The stimulation by TGF-β could be detected in three different endoglin promoter constructs (Fig 9B). An average of threefold increased activity using the −400/+341, −224/+341, or −141/+341 fragments was observed upon the addition of TGF-β1. This fold-induction was of the same order of magnitude as the TGF-β–inducible reporter construct p3TP-lux, which yielded a 5.5-increased activity upon TGF-β treatment. By contrast, no stimulation was produced in the cadherin promoter construct pxp-200, included as a negative control. The TGF-β inducibility of the endoglin promoter could be explained by the presence of putative TGF-β–responsive elements present in the three constructs assayed, including TGF-β activation (TAE), control (TCE), and inhibitory (TIE) elements and Mad binding sites (Fig 9C). These results are consistent with a previous report showing that transcript and protein levels of endoglin can be upregulated by TGF-β113 and suggest that the mechanism of the TGF-β regulation resides, at least in part, at the level of transcription initiation.
Effect of TGF-β treatment on the endoglin promoter activity. BAEC were transiently transfected with the indicated promoter constructs and TGF-β1 was added 24 hours posttransfection to half of the transfected cells. Luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors, used as positive and negative controls, respectively. The promoter activity of the pGL2-SV40 vector was arbitrarily considered as 100. (A) Effect of increasing concentrations of TGF-β. BAEC were transiently transfected with the pCD105 (−400/+341) plasmid and stimulated with TGF-β1 at the concentrations indicated. This is a representative experiment of five separate experiments. (B) TGF-β inducibility in different endoglin promoter constructs. BAEC were transiently transfected with the indicated plasmids and incubated either in the absence (▪) or in the presence (▨) of 10 ng/mL of TGF-β1. The TGF-β–inducible reporter construct p3TP-lux was included as a positive control. The P-cadherin promoter construct pxp-200 failed to respond to TGF-β. A representative experiment of five different experiments is shown. (C) A representative scheme of the different constructs used with the relative location of TGF-β–responsive elements is shown. TCE, TGF-β control element; TIE, TGF-β inhibitory element; TAE, TGF-β activation element; Mad, Mothers against decapentaplegic complex.
Effect of TGF-β treatment on the endoglin promoter activity. BAEC were transiently transfected with the indicated promoter constructs and TGF-β1 was added 24 hours posttransfection to half of the transfected cells. Luciferase activity was determined 48 hours after transfection. Correction for transfection efficiency was made by cotransfection with β-galactosidase expression vectors and parallel transfections with the pGL2-SV40 and pXP2 vectors, used as positive and negative controls, respectively. The promoter activity of the pGL2-SV40 vector was arbitrarily considered as 100. (A) Effect of increasing concentrations of TGF-β. BAEC were transiently transfected with the pCD105 (−400/+341) plasmid and stimulated with TGF-β1 at the concentrations indicated. This is a representative experiment of five separate experiments. (B) TGF-β inducibility in different endoglin promoter constructs. BAEC were transiently transfected with the indicated plasmids and incubated either in the absence (▪) or in the presence (▨) of 10 ng/mL of TGF-β1. The TGF-β–inducible reporter construct p3TP-lux was included as a positive control. The P-cadherin promoter construct pxp-200 failed to respond to TGF-β. A representative experiment of five different experiments is shown. (C) A representative scheme of the different constructs used with the relative location of TGF-β–responsive elements is shown. TCE, TGF-β control element; TIE, TGF-β inhibitory element; TAE, TGF-β activation element; Mad, Mothers against decapentaplegic complex.
DISCUSSION
In the present study, we describe the characterization of the promoter for human endoglin gene. To this end, a genomic clone of 3.3 kb encompassing the 5′ flanking region of the endoglin gene was isolated and sequenced from a human genomic library. A cluster of transcriptional start sites spanning 78 nucleotides has been identified within the 741-bp BamHI/BbrPI fragment, being the site numbered as +1 located 350 nucleotides upstream from the translation initiation site. No consensus TATA or CAAT boxes were found within the 741-bp BamHI/BbrPI fragment, but GC-rich boxes (−5 to +16 and −47 to −29) and a consensus Sp1 site (−37) were localized near the initiation site. The presence of GC-rich sequences within the 5′-flanking region is a feature of TATA-less genes, which usually contain Sp1 binding sites37and display multiple transcription start sites. The lack of TATA and CAAT boxes is a common feature in other components of the mammalian TGF-β system, including human TGF-β1,48 mouse TGF-β1,49 mouse inhibin/activin βc gene,50or human TGF-β receptor type II.51 52
An Alu sequence was found along the 5′ region of the endoglin promoter. The polymorphic short interspersed elements of the Alu family are ubiquitous in the genome of primates and are often found within the 5′ flanking regions of regular transcription units. This supports the notion that the minimal promoter region should be contained between the Alu sequence and the transcription initiation site. Similar arrangements of Alu sequences located 5′ to the promoter have been described for the von Willebrand factor and PECAM-1 genes expressed by endothelial cells.30,53 54
The location of transcription initiation is in agreement with the high promoter activity of the 741-bp BamHI/BbrPI fragment, which displayed between twofold and sixfold higher activity than an SV40 promoter-enhancer construct in bovine endothelial cells. The specificity of this fragment for endothelial cells was demonstrated by the fact that its transcriptional activity in bovine and human endothelial cells was between 12- and 40-fold higher than in other cell lineages lacking endoglin expression. The specificity of this promoter is in agreement with the fact that the endothelium constitutively express endoglin at high levels.1,55 These results suggest that this region contains most of the signals necessary to direct endothelial cell expression and thus is a potentially valuable tool to be used in those gene therapy protocols in which endothelial gene delivery is required. Supporting this view is the fact that transgene expression driven by the 741-bp BamHI/BbrPI fragment has been detected in murine vascular endothelium of all organs examined.56 The existence of several potential transcription factor binding sites, also present in other endothelial gene promoters, might account for the restricted cell expression of endoglin. These elements include NFκB, GATA, AP-2, Sp1, and ets (Table 1). Functionally active GATA elements have been identified within promoters of a large number of genes constitutively expressed by vascular cells. Similarly to endoglin, a subset of these promoters are TATA-less, including human PECAM-1, rat platelet factor 4 (PF4), human GPIIb, and human GPIX.30,54 It has been shown that GATA motifs of these promoters can serve not only as a binding site for the GATA family of transcription factors, but also as a recognition element for basal transcription factors.57 Two NFκB consensus sequences were also identified in the endoglin promoter. NFκB motifs are responsive to a large number of agents, such as bacterial and viral pathogens, inflammatory cytokines, or mechanical forces.36,58,59 Interestingly, endothelial cells are subjected to the hemodynamic forces of the bloodstream, suggesting that the NFκB consensus sequences found in the endoglin promoter might regulate endoglin transcription, as in the case of other endothelial genes.59 The presence of the transcription factors Sp1 and AP-2 in the endoglin promoter is a feature shared by promoters regulating the expression of other components of the TGF-β system, including those of TGF-β1, TGF-β2, and TGF-β3; biglycan; and TGF-β receptor type I.60-62
The endoglin promoter also contains five ets consensus sequences. The ets family of transcription factors is a large set of winged helix-loop-helix DNA binding proteins.34 Many of these factors are expressed in endothelial cells and are involved in the transcription of several endothelial gene promoters,46,47and data presented in this report suggest an important role of ets in endoglin transcription. Because endoglin is a component of the TGF-β receptor complex, it is interesting to note that an ets member has also been reported to regulate expression of the TGF-β receptor type II.63
The endoglin promoter activity was found to be clearly stimulated by the presence of TGF-β1 (Fig 9). This result agrees with our previous findings showing that TGF-β1 is able to increase the levels of transcripts and cell surface expression of endoglin.13Thus, TGF-β1 seems to regulate the expression of a component of its membrane receptor system. The transcriptional modulatory effect of TGF-β is likely mediated by several elements responsive to TGF-β found in the endoglin promoter. These include TGF-β activation elements previously described in α1 (I) collagen gene,41TGF-β control elements identified in the c-mycpromoter,42 TGF-β inhibitory elements characterized in the transin/stromelysin gene,43 and a TGF-β–related signalling protein Mad,9 whose DNA binding properties have been recently described inDrosophila.40 Further experiments are required to unveil the specific proteins involved in the TGF-β inducibility of the endoglin promoter.
The presence of consensus TGF-β and steroid responsive elements within the endoglin promoter suggests a role on the in vivo transcriptional regulation that might have important implications in HHT1. Recently, a haploinsufficiency model has been postulated as the pathogenic mechanism of this disorder.5,7,8 According to this model, reduced expression of endoglin from the mutant allele creates a situation in which endoglin function falls below a critical threshold, predisposing toward the development of the vascular lesions observed in HHT. In this sense, the stimulation of the endoglin promoter activity by TGF-β1 and the putative regulatory role of steroids might serve to overcome the threshold levels. In favor of this hypothesis is the fact that estradiol has been found to be beneficial in the treatment of HHT gastrointestinal bleedings.64 The involvement of TGF-β1 in endoglin gene transcription is also supported by the fact that TGF-β1 knockout mice display vascular defects that share histological similarities to lesions seen in HHT patients.65 Thus, it will be of interest to investigate the therapeutic use of TGF-β1 in HHT.
ACKNOWLEDGMENT
The authors thank Drs Michelle Letarte and Peter Cowan for sharing their unpublished data, Dr Arun Seth for antibodies to ets-2, Dr Fernando Larcher for porcine keratinocytes, and Drs Angel Corbı́, Santiago Rodrı́guez de Cordoba, Santiago Lamas, Joan Massagué, and Amparo Cano for reagents and discussions.
Supported by grants from Comisión Interministerial de Ciencia y Tecnologı́a (CICYT-SAF97-0034), Comunidad Autónoma de Madrid (CAM), and Biomed Program of the European Community (BMH4-CT95-0995) to C.B.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Carmelo Bernabéu, PhD, Centro de Investigaciones Biológicas, CSIC, Velázquez 144, 28006 Madrid, Spain.





![Fig. 8. Identification of an ets motif within the −88/−59 fragment. Nuclear extracts from TGF-β–treated or untreated U937 cells and BAEC were incubated with the [32P]-labeled −88/−59WT (A and B) or −88/−59Mut fragments (A), in the absence or in the presence of competitor oligonucleotides or specific antibodies as indicated. Excess of unlabeled −88/−59 probe was used as a control for specificity (A and B). The oligonucleotide CD11c-PU.1, containing a consensus binding site for the PU.1 member of the ets family of transcription factors, and fragment BR1, a negative control, were used in competition experiments (B). Specific antibodies to ets-2 or fos/jun were incubated with the nuclear extracts, using a preimmune serum as a negative control (B). Samples were electrophoresed on a 5% polyacrylamide gel and autoradiographed. The presence of specific complexes is indicated by an arrow at the margins. The open arrowheads indicate complexes formed by proteolytic products of ets as previously described.66](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4677/5/m_blod42433008w.jpeg?Expires=1769250320&Signature=kOb5UNsGbrxB5AG9A0w~dCNn8NJnoLxc-2MfeqKo7dIRSVlhR8i3B6VXRVQQHMSzHdRcWBiUnBMONnHYuTIBNvaMvnEdpoHDaDY6E-po6jNmadkdLWPqPi6sy7GjgQIZ8vRDmJhJytsA7j13QLPY9ycSm8aE5VYmTtCiYWDWENDIZvHAeVQm~8ovc2Klgu2HzndgAiAA3IypVFLXtgkQfw3tvtdS0olB7k8RH7Yo6uYSwByeDz94-Axv-bu-cm-vSZh5XKoQ6CK8acNnnzfT3cDnLJoABjjE0-Ukjb9ohqNH0nHx4Ut6WH191Dh1WeaQKhHJfbjZ35LPdcIPiOEmmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal