Abstract
Posttransplant infection associated with host immune deficiency is the major cause of nonrelapse mortality of human bone marrow transplant recipients. In a new murine model of posttransplant infection, allogeneic bone marrow transplant recipients were infected with herpes simplex virus-1 (HSV-1) via intraperitoneal inoculation 12 weeks after transplantation. Allogeneic transplant recipients with graft-versus-host disease (GVHD) had significantly increased mortality from HSV-1 encephalitis, with deficiencies of both specific anti–HSV-1 antibody and total serum IgG2a. GVHD mice displayed a Th2 cytokine profile (increased interleukin-4 [IL-4] and decreased interferon-γ) and decreased lipopolysaccharide (LPS) responses, suggesting that both T-cell and B-cell defects contributed to the impaired production of antibody. Because passive transfer of hyperimmune serum protected mice from HSV-1 infection, we hypothesized that CD40 ligand (CD40L), which induces B-cell maturation, would protect mice from HSV-1 infection. CD40L-treated GVHD mice showed elevated IgG2a levels and increased survival compared with vehicle-treated transplant recipients.
INFECTION ASSOCIATED with posttransplant immunodeficiency is the major cause of nonrelapse death in bone marrow transplant recipients.1,2 Posttransplant patients have frequent bacterial, viral, and fungal infections, indicating that infection is the result of host immunodeficiency rather than the virulent nature of a single pathogen. Defects in both B-cell and T-cell function contribute to human posttransplant immunodeficiency.3,4 Murine posttransplant immunodeficiency is associated with multiple functional defects, including impairments in T cells, B cells, adhesion molecules, and signaling molecules.5-9
Previous studies of viral infection with concurrent murine allogeneic bone marrow transplantation showed that infection and GVHD synergized to exacerbate the infection and increase graft-versus-host disease (GVHD).10-12 Earlier work has focused on viral infection that occurred concurrently with transplantation, before engraftment. However, many severe clinical infections occur after engraftment, and murine models have not yet been developed to address the issue of late infection.
We designed a murine model consisting of allogeneic bone marrow transplantation followed by herpes simplex virus-1 (HSV-1) infection to investigate the mechanisms of posttransplant immunodeficiency and to test potential cytokine therapies. HSV-1, which produces a rapid, measurable immune response, was chosen as a model pathogen to represent the herpes viruses which often infect bone marrow transplant patients.1 The goal of this work was to evaluate the effect of the posttransplant immunodeficient state on the response to a single, representative pathogen and to test potential cytokine therapies that may decrease morbidity and mortality due to viral infection.
Because CD40L treatment accelerated B-cell maturation in a syngeneic murine bone marrow transplant system,13 we have investigated the use of this cytokine as a therapy for posttransplant immunodeficiency. The binding of CD40L to CD40 stimulates B-cell proliferation, differentiation, and antibody secretion. Other effects of CD40L binding include B7-2 upregulation and the rescue of B cells from apoptosis.14 15
MATERIALS AND METHODS
Mice.
Female CBA/J and B10.BR mice, purchased from the Jackson Laboratory (Bar Harbor, ME), were transplanted at 11 to 13 weeks of age. The CBA/J and B10.BR mouse strains, which are both H-2k, differ at multiple minor histocompatibility loci. Transplantation of bone marrow from B10.BR mice to CBA/J mice simulated bone marrow transplantation with matched unrelated donors. Female Igh-6 (B-cell–deficient mice)16 were purchased from Jackson Laboratory and used at 12 weeks of age.
Bone marrow transplantation.
Bone marrow was flushed from the femurs and tibiae of donor mice and washed one time with supplemented RPMI (RPMI1640 [Mediatech, Herndon, VA] with penicillin-streptomycin [100 IU/mL], L-glutamine [2 mmol/L], 2-ME [55 μmol/L], HEPES [10 mmol/L], and 10% heat-inactivated fetal bovine serum [Sigma, St Louis, MO]). Bone marrow cells were resuspended in Leibovitz’s L-15 medium (Life Technologies, Inc, Grand Island, NY). Recipient mice were irradiated with 1,100 cGy split dose irradiation and then injected intravenously with donor bone marrow cells (5 × 106cells). T-cell–depleted bone marrow transplant recipients were reconstituted with bone marrow that had been treated with two rounds of lysis with anti-Thy 1.2 and Complement (Accurate Chemicals & Scientific Corp, Westbury, NY). In one group, T cells (4 × 105nylon wool passed spleen cells, 75% CD3+ cells) were added to the bone marrow to produce recipient mice, which developed minimal GVHD.8 All procedures were performed in accordance with protocols approved by the Animal Care Committee of the Dana-Farber Cancer Institute.
Viral infection with HSV-1 (KOS 1.1).
The mice were infected with HSV-117 at 5 × 107 plaque forming units (pfu)/mouse via intraperitoneal injection 12 weeks after bone marrow transplantation. As in other studies of murine HSV-1 infection,18 19 examination of the central nervous system (CNS) tissues of dying GVHD mice showed the presence of HSV-1 antigen and inflammation (data not shown), suggesting HSV-1 encephalitis as the cause of death. Most deaths from HSV-1 infection occurred within 10 days of viral inoculation. Immunological studies below were performed on surviving mice.
CD40L treatment.
Trimeric murine recombinant CD40L20 was generously provided by Immunex (Seattle, WA). Mice were treated with 6 μg CD40L or vehicle (10% glycerol in saline) by intraperitoneal injection three times per week, starting the day before HSV-1 injection.
Cell culture preparation and proliferation assays.
Two weeks after HSV-1 inoculation, mice were killed by CO2asphyxiation and immunological studies were performed. Isolated splenocytes were washed and resuspended in supplemented RPMI. Viable cells were determined by Trypan blue exclusion.
Proliferation of recipient splenocytes to ultraviolet inactivated virus (4 × 105 pfu) or extract (a virus-free lysate of control Vero cells) was measured after 4 days incubation at 37°C and 5% CO2. Lipopolysaccharide (LPS; 10 μg/mL; Sigma)-stimulated proliferation was measured after 2 days. Recipient splenocytes were incubated in supplemented RPMI with LPS, inactivated virus, or extract in triplicate wells of 96-well microtiter plates with 4 × 105 cells/well. Cells were pulsed with 1 μCi3H-thymidine/well for the final 5 hours of incubation and harvested. Incorporated 3H-thymidine was determined.
Flow cytometry.
Splenocytes were stained for flow cytometry. Fc receptor binding was blocked with phosphate-buffered saline (PBS) containing 10% rat whole serum (Zymed Laboratories, San Francisco, CA). As described,17 the following monoclonal antibodies, conjugated with either fluorescein isothiocyanate, phycoerythrin, or biotin (used with Streptavidin-Red670 [Life Technologies, Inc]) were used for staining: anti-B220, anti-CD4, anti-CD8, anti-CD3.
Specific anti–HSV-1 antibody and total serum IgG1 and IgG2a measurement.
HSV-1–specific IgG1 and IgG2a antibodies in serum were determined by enzyme-linked immunosorbent assay (ELISA).21 Total serum IgG1 and IgG2a levels were determined using Radial Immunodiffusion (RID) Kits for mouse IgG1 and IgG2a (The Binding Site, Birmingham, UK). The tests were performed following the instructions of the manufacturer.
Cytokine detection.
Splenocytes from immunized recipients were cultured with inactivated virus and supernatants collected. Interferon-γ (IFN-γ) and interleukin-5 (IL-5) levels were determined by ELISA. All measurements were performed using recombinant cytokine standards and “pairs” of capture and peroxidase-conjugated secondary antibodies, all purchased from PharMingen (San Diego, CA) and used according to the protocol supplied by PharMingen. The plates were developed using avidin-peroxidase and 2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate (Sigma) and read at 405 nm using an ELISA reader. The lower detection limit of the assay was 100 pg/mL for IFN-γ. To measure IL-4, culture supernatants were tested in collaboration with Dr Abul Abbas using the cytokine-dependent cell line CT4S in the presence of IL-2 blocking antibodies.22
Passive transfer experiments.
Hyperimmune HSV-1 antiserum was generated in normal B10.BR mice by three intraperitoneal injections of HSV-1 (5 × 107pfu/mouse) given at 2-week intervals. Serum was heat inactivated for 30 minutes at 56°C, and each mouse received 200 μL via tail vein injection.
Cytotoxic T lymphocyte (CTL) assay.
Two weeks after viral infection, mice were killed and splenocyte cell suspensions (6 × 107 mononuclear cells) were prepared in 900 μL of unsupplemented RPMI. Cells were exposed to HSV-1 (ultraviolet irradiation-inactivated preparation equivalent to multiplicity of infection [MOI] of 1.5) in a total volume of 1.8 mL for 1 hour at 37°C. Supplemented RPMI 1640 (10 mL) was added and cultures were incubated for 4 days. Target L929 cells (American Type Culture Collection, Rockville, MD) were labeled overnight with 100 μCi 51Cr per 5 × 106 cells. On the day of assay, targets were incubated for 1 hour with HSV-1 (MOI = 10), washed and added at 1 × 104 cell/well to 96-well plates with effector cells at ratios of 100:1 to 3.12:1. Plates were incubated for 4 hours at 37°C and 5% CO2, centrifuged, and percent-specific lysis calculated.
RESULTS
GVHD mice have decreased survival from HSV-1.
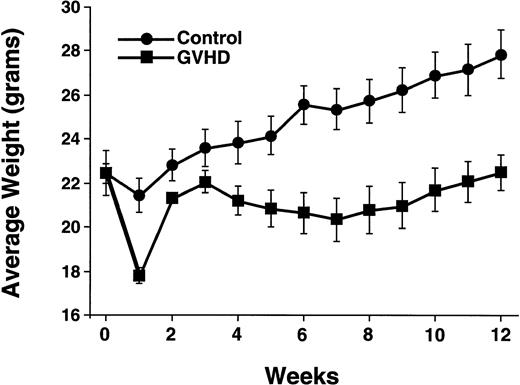
Irradiated CBA mice were reconstituted with T-cell–depleted bone marrow, or T-cell–supplemented bone marrow to generate an allogeneic control group (control) and a group with partial recovery from acute GVHD (GVHD). At 12 weeks posttransplantation, the GVHD group had a 20% weight loss due to GVHD as compared with the control group (Fig 1). In addition, the LD50of control mice was more than twofold higher than that of GVHD mice (Table 1). Normal B10.BR and CBA/J mice had LD50 values greater than both transplant groups.
GVHD transplant recipients have decreased weight gain. Posttransplant weights of the control and GVHD groups are shown. Data points represent means ± SEM of 8 to 20 mice per group. This experiment is representative of three similar experiments.
GVHD transplant recipients have decreased weight gain. Posttransplant weights of the control and GVHD groups are shown. Data points represent means ± SEM of 8 to 20 mice per group. This experiment is representative of three similar experiments.
GVHD Mice Were More Susceptible to Lethal HSV-1 Infection
Mice received intraperitoneal inoculation of HSV-1, and survival was observed daily.
LD50 values in plaque-forming units were calculated.36
Significantly different, P < .001 by logistic regression analysis.
HSV-1 immune responses of GVHD mice.
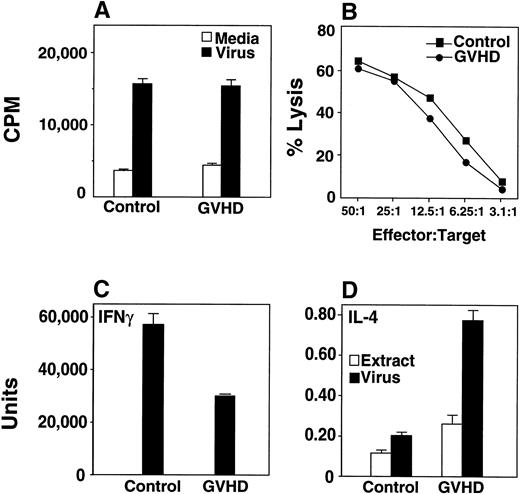
Twelve weeks after bone marrow transplantation, mice were infected with HSV-1 at a dose (5 × 107 pfu/mouse) chosen to ensure that sufficient numbers of GVHD mice survived for the analysis of the immune response. Two weeks after infection, HSV-1–specific T-cell proliferative (Fig 2A) and cytotoxic responses (Fig 2B) were equivalent in GVHD and control mice. Cells from GVHD mice produced decreased IFN-γ and increased IL-4 in response to HSV-1 (Fig 2C and D), consistent with a Th2 shift.
T cells from GVHD mice generate normal proliferative and normal CTL response to HSV-1. GVHD mice display a Th2 cytokine response to HSV-1. (A) Nylon wool–separated T cells from GVHD mice respond well to HSV-1. 3H-Thymidine incorporation by recipient splenocytes in response to inactivated virus or media alone was measured. Groups consisted of two or three mice, and the mean and SEM are shown. This experiment is representative of three similar experiments. (B) HSV-1 CTL assays showed that GVHD and control mice generated similar CTL responses after HSV-1 infection. Lysis of mock-infected L929 targets was less than 20%. The data shown were from one of three similar experiments. (C) Splenocytes were cultured in vitro with ultraviolet irradiation-inactivated HSV-1 or with an extract from uninfected Vero cells, and IFN-γ production was measured from supernatants. The mean and standard error of three replicate wells are shown; data are representative of three similar experiments with three mice per group in each experiment. The quantity of IFN-γ produced by the extract control was below the limit of detection of the assay. Significant differences were detected between the extract and virus data (P ≤ .05, Student’s t-test). (D) Splenocytes were cultured in vitro with ultraviolet irradiation-inactivated HSV-1 or with an extract from uninfected Vero cells, and IL-4 production was measured from supernatants. Data from a single experiment is shown. Significant differences were detected between the extract and virus data (P ≤ .05, t-test). Results from two additional experiments measuring IL-5 were similar to the IL-4 experiment (data not shown).
T cells from GVHD mice generate normal proliferative and normal CTL response to HSV-1. GVHD mice display a Th2 cytokine response to HSV-1. (A) Nylon wool–separated T cells from GVHD mice respond well to HSV-1. 3H-Thymidine incorporation by recipient splenocytes in response to inactivated virus or media alone was measured. Groups consisted of two or three mice, and the mean and SEM are shown. This experiment is representative of three similar experiments. (B) HSV-1 CTL assays showed that GVHD and control mice generated similar CTL responses after HSV-1 infection. Lysis of mock-infected L929 targets was less than 20%. The data shown were from one of three similar experiments. (C) Splenocytes were cultured in vitro with ultraviolet irradiation-inactivated HSV-1 or with an extract from uninfected Vero cells, and IFN-γ production was measured from supernatants. The mean and standard error of three replicate wells are shown; data are representative of three similar experiments with three mice per group in each experiment. The quantity of IFN-γ produced by the extract control was below the limit of detection of the assay. Significant differences were detected between the extract and virus data (P ≤ .05, Student’s t-test). (D) Splenocytes were cultured in vitro with ultraviolet irradiation-inactivated HSV-1 or with an extract from uninfected Vero cells, and IL-4 production was measured from supernatants. Data from a single experiment is shown. Significant differences were detected between the extract and virus data (P ≤ .05, t-test). Results from two additional experiments measuring IL-5 were similar to the IL-4 experiment (data not shown).
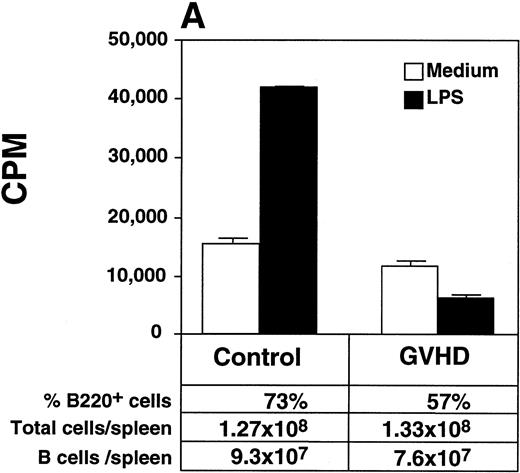
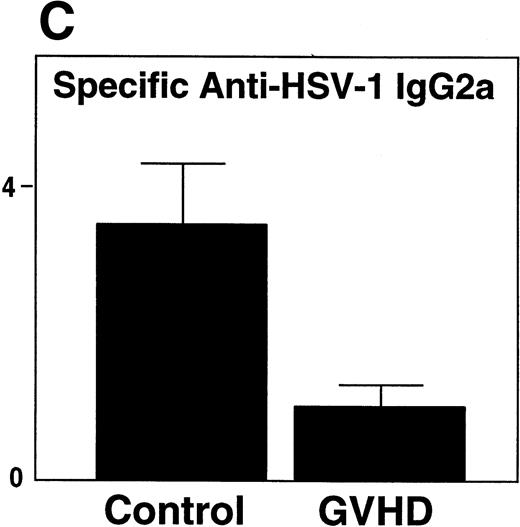
GVHD mice had impaired B-cell function, as measured by decreased proliferative response to LPS when compared with control mice (Fig 3A). Two weeks after HSV-1 infection, Control mice generated a fivefold increase in total serum IgG2a (Fig 3B), a particularly important immunoglobulin subclass for immunity to viral infection.23,24 In contrast, GVHD mice failed to generate any increase in total serum IgG2a in response to HSV-1 (Fig 3B). In response to HSV-1 inoculation, control transplant recipients generated substantial levels of specific anti–HSV-1 IgG2a. However, the anti–HSV-1 IgG2a level of GVHD mice was only 25% of the control (Fig 3C). Total serum IgG1 and specific anti–HSV-1 IgG1 levels of GVHD mice were similar to control mice (data not shown). Because Th2 cytokines were critical for the production of IgG1,25 the decreased IgG2a production of GVHD mice was consistent with the finding that the cells from GVHD mice were polarized to a Th2 phenotype, as described in chronic GVHD.26 27
GVHD splenocytes proliferated poorly in response to LPS, and GVHD mice failed to generate increased total serum IgG2a and specific anti–HSV-1 IgG2a after viral inoculation. (A) Spleen-cell proliferative response to LPS or medium alone was measured. Representative B-cell numbers show that control and GVHD mice had similar B-cell numbers. The experiment shown is representative of four similar experiments. (B) Total serum immunoglobulin levels were measured by radial immunodiffusion before and 2 weeks following HSV-1 infection. Preinfection and postinfection serum results are shown. Each mouse group consists of three mice, and the mean and SEM are shown. This experiment is representative of three similar experiments. Significant differences (P < .05, t-test) are detected between total serum IgG2a levels in control group and the GVHD group after HSV-1 infection. (C) Serum levels of specific anti–HSV-1 IgG2a were determined by ELISA. The data shown are the mean and SEM of five similar experiments that included 15 mice. The IgG2a level of the GVHD group is significantly decreased compared with the IgG2a level of control mice (P < .05, t-test).
GVHD splenocytes proliferated poorly in response to LPS, and GVHD mice failed to generate increased total serum IgG2a and specific anti–HSV-1 IgG2a after viral inoculation. (A) Spleen-cell proliferative response to LPS or medium alone was measured. Representative B-cell numbers show that control and GVHD mice had similar B-cell numbers. The experiment shown is representative of four similar experiments. (B) Total serum immunoglobulin levels were measured by radial immunodiffusion before and 2 weeks following HSV-1 infection. Preinfection and postinfection serum results are shown. Each mouse group consists of three mice, and the mean and SEM are shown. This experiment is representative of three similar experiments. Significant differences (P < .05, t-test) are detected between total serum IgG2a levels in control group and the GVHD group after HSV-1 infection. (C) Serum levels of specific anti–HSV-1 IgG2a were determined by ELISA. The data shown are the mean and SEM of five similar experiments that included 15 mice. The IgG2a level of the GVHD group is significantly decreased compared with the IgG2a level of control mice (P < .05, t-test).
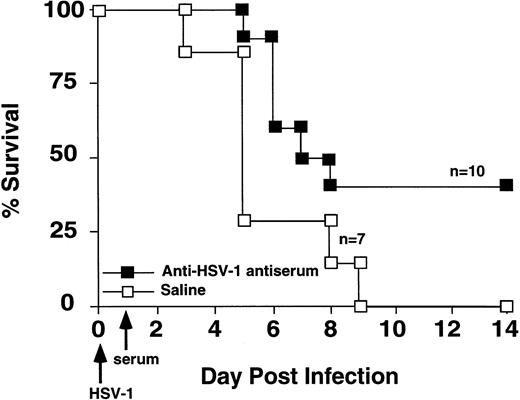
Passive transfer of HSV-1 immune serum protected GVHD mice from HSV-1 mortality.
Because B cells and antibody protected against HSV-1–induced mortality,18,28,28a we tested the hypothesis that antibody production was critical for successful immune response to HSV-1 infection. GVHD mice were given intravenous injection of either hyperimmune HSV-1 antiserum or saline on the day after inoculation with 2 × 108 pfu HSV-1. Hyperimmune HSV-1 antiserum increased the survival of HSV-1 infected GVHD mice (Fig 4).
Passive transfer of HSV-1 immune serum protected GVHD mice from HSV-1 mortality. GVHD mice were injected with hyperimmune HSV-1 serum or saline on the day following HSV-1 inoculation (2 × 108 pfu). Survival was monitored daily. Survival of the two groups was significantly different (Wilcoxon rank sum test, P= .03).
Passive transfer of HSV-1 immune serum protected GVHD mice from HSV-1 mortality. GVHD mice were injected with hyperimmune HSV-1 serum or saline on the day following HSV-1 inoculation (2 × 108 pfu). Survival was monitored daily. Survival of the two groups was significantly different (Wilcoxon rank sum test, P= .03).
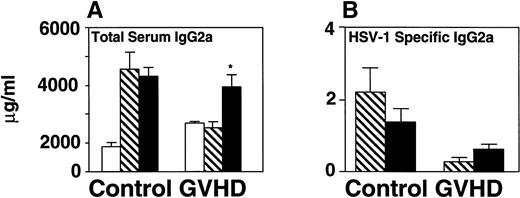
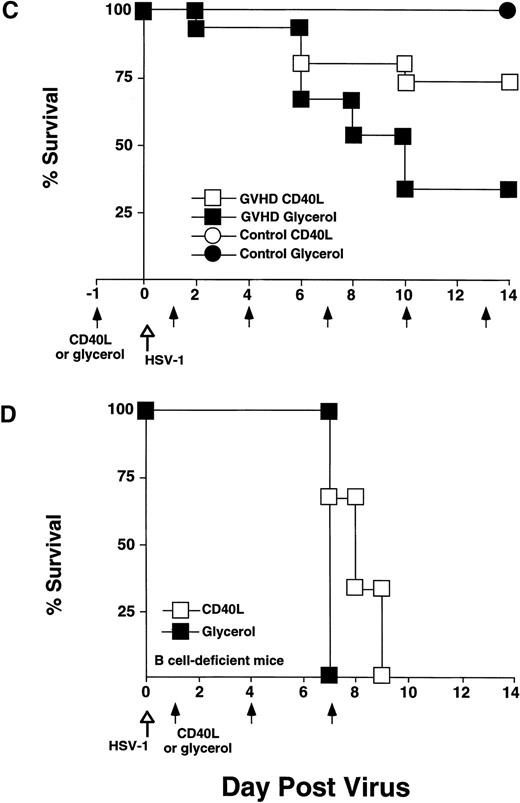
CD40L therapy prolonged survival and increased total serum IgG2a.
To stimulate B-cell differentiation, 12-week-posttransplant recipients were treated with CD40L (6 μg) three times per week for 2 weeks until they were killed. Vehicle-treated mice received a similar treatment with 10% glycerol. HSV-1 inoculation followed the first CD40L injection (schema shown on x-axis of Fig5C).
CD40L treatment stimulated IgG2a production and improved survival of GVHD mice following HSV-1 infection but did not affect survival of B-cell–deficient mice. CD40L was given intraperitoneally (6 μg/mouse, three times a week) to control and GVHD mice. Vehicle-treated control and GVHD mice received glycerol diluted with saline. (A) Mice were inoculated with HSV-1 2 weeks before sacrifice. Serum was obtained by orbital bleed on day 14 immediately before killing. Total serum IgG2a was measured by radial immunodiffusion: (□) pre–HSV-1 infection, (▧) post–HSV-1 glycerol-treated, (▪) post–HSV-1 CD40L-treated. Significant differences (P < .05,t-test) were found between the glycerol and CD40L-treated GVHD groups. (B) Serum levels of HSV-1–specific and IgG2a were determined by ELISA 2 weeks post–HSV-1 infection: (▧) post–HSV-1 glycerol-treated, (▪) post–HSV-1 CD40L-treated. The increase in HSV-1–specific IgG2a in GVHD mice does not reach statistical significance. The data shown are the mean and SEM of six similar experiments that included 50 mice. (C) Mice were monitored daily for survival after infection with HSV-1. The GVHD-CD40L and GVHD-glycerol group are significantly different (P < .05, log-rank test). Each group included 15 mice. (D) Two groups of Igh-6 (B-cell–deficient) mice were inoculated with HSV-1 (1 × 108 pfu, 4.5LD50) and treated with: (□) CD40L or (▪) glycerol. Both groups of mice have similar survival. The CD40L and glycerol groups each included three mice.
CD40L treatment stimulated IgG2a production and improved survival of GVHD mice following HSV-1 infection but did not affect survival of B-cell–deficient mice. CD40L was given intraperitoneally (6 μg/mouse, three times a week) to control and GVHD mice. Vehicle-treated control and GVHD mice received glycerol diluted with saline. (A) Mice were inoculated with HSV-1 2 weeks before sacrifice. Serum was obtained by orbital bleed on day 14 immediately before killing. Total serum IgG2a was measured by radial immunodiffusion: (□) pre–HSV-1 infection, (▧) post–HSV-1 glycerol-treated, (▪) post–HSV-1 CD40L-treated. Significant differences (P < .05,t-test) were found between the glycerol and CD40L-treated GVHD groups. (B) Serum levels of HSV-1–specific and IgG2a were determined by ELISA 2 weeks post–HSV-1 infection: (▧) post–HSV-1 glycerol-treated, (▪) post–HSV-1 CD40L-treated. The increase in HSV-1–specific IgG2a in GVHD mice does not reach statistical significance. The data shown are the mean and SEM of six similar experiments that included 50 mice. (C) Mice were monitored daily for survival after infection with HSV-1. The GVHD-CD40L and GVHD-glycerol group are significantly different (P < .05, log-rank test). Each group included 15 mice. (D) Two groups of Igh-6 (B-cell–deficient) mice were inoculated with HSV-1 (1 × 108 pfu, 4.5LD50) and treated with: (□) CD40L or (▪) glycerol. Both groups of mice have similar survival. The CD40L and glycerol groups each included three mice.
Total serum immunoglobulin and specific anti–HSV-1 antibody of GVHD mice were measured before and after treatment with CD40L or glycerol. Total serum IgG2a increased 1.5-fold in GVHD mice receiving CD40L compared with GVHD mice receiving glycerol (Fig 5A). CD40L treatment also produced a detectable but not statistically significant increase in specific anti–HSV-1 IgG2a antibody (Fig 5B). CD40L-treated GVHD mice had improved survival compared with glycerol-treated GVHD mice (Fig 5C). These data suggest that CD40L therapy, which generates increased antibody production, protected HSV-1–infected GVHD mice from death.
To determine if CD40L effects on B cells were of primary importance, we tested if CD40L treatment could protect Igh-6 (B-cell–deficient mice)16 from HSV-1 infectious death. Two groups of B-cell–deficient mice were inoculated with CD40L or glycerol (6 μg intraperitoneally 3×/wk). Both groups had similar mortality and all mice were dead by 9 days (Fig 5D). These data suggested that CD40L-mediated stimulation of B cells (rather than macrophages or CTL) was critical for HSV-1 infectious resistance.
DISCUSSION
To establish an animal model of posttransplant infection related morbidity and mortality, we inoculated murine allogeneic bone marrow transplant recipients with HSV-1 after engraftment. Allogeneic transplant recipients with GVHD had increased mortality from HSV-1 and generated decreased total serum IgG2a and decreased specific anti–HSV-1 antibody in response to HSV-1 infection. Passive transfer of immunoglobulin protected GVHD mice and B-cell–deficient mice from HSV-1 encephalitis, suggesting that B cells and immunoglobulin were of primary importance in protection from HSV-1 encephalitis.28a
Because CD40L promoted B-cell maturation in a syngeneic bone marrow transplant model,13 we tested the ability of CD40L to improve survival of allogeneic transplant recipients after HSV-1 infection. When given concurrently with HSV-1, CD40L therapy increased the level of total serum IgG2a and improved the survival of allogeneic mice with GVHD.
The concurrence of GVHD and viral infection exacerbated the pathology of both diseases. Using the Parent into F1 (P → F1) model of graft-versus-host reaction (GVHR), Shanley and colleagues found that mice with concurrent murine cytomegalovirus (MCMV) infection and GVHR developed a severe interstitial pneumonitis that was not detected in mice with either MCMV or GVHD alone.12 Cray and Levy,29 using the P → F1 model, detected increased anti-host CTL activity in MCMV-infected animals. Antihost CTL activity was a measure of increased GVHD during infection. In both of these studies viral infection and GVHR were induced simultaneously. Increased host immunodeficiency was reported by Via30 in a P → F1 mouse model in which GVHD was induced following MCMV infection. GVHD and MCMV infection reduced subsequent development of T-cytotoxic responses to trinitrophenyl-modified syngeneic cells.
In the single study involving irradiated recipients, Shanley, Korngold, and Forman31 examined cytomegalovirus (CMV) viral clearance in an irradiated allogeneic transplant model (B10.BR → CBA/J recipient). In this study, mice were infected with MCMV immediately or 14 days after transplantation. In both cases, the combined presence of GVHD and virus lead to increased viral titers in the lungs and salivary glands.
Our results confirm and extend the notion that GVHD increases susceptibility to mortality from viral infection. The novel aspect of our study was the fact that we examined a viral infection initiated after full engraftment had occurred. In addition, previous studies of viral infection and murine bone marrow transplantation were performed in a P → F1 model of GVHD that did not involve irradiation.5,12,29 30 Because patients generally receive total body irradiation, resulting in the generation of cytokines that may affect the immune response, an irradiated system of murine transplantation is a more appropriate model of human posttransplant infection. We showed that the recovery period after GVHD is associated with persistent immunodeficiency and increased morbidity and mortality due to viral infection.
In this murine GVHD model, mice develop weight loss due to GVHD in the early posttransplant period. The animals then recover and, at 12 weeks, appear well but underweight. This is an appropriate model of recipients of unrelated bone marrow transplants. These patients frequently develop mild GVHD in the immediate posttransplant period and then recover to an apparently healthy state associated with subtle immunocompromise within a few months after transplant.
Defects in B-cell function following bone marrow transplantation have also been previously documented. Kagan, Chaplin, and Saxon7reported that patients showed defective B-cell activation, proliferation, and differentiation during the first 10 months following bone marrow transplantation. Storek32 observed that human bone marrow transplant survivors continue to have low IgG production at 1 year posttransplant, perhaps due to the lack of isotype-switched B cells. From studies of murine systemic graft-versus-host reactions induced by injecting Parent lymphocytes into F1 recipients, Xenocostas reported that systemic GVHD produces a decrease in pre–B cells and early B-cell numbers in the bone marrow.5 In the P → F1 system, B-cell antibody responses are suppressed by GVHD, even after B lymphocyte numbers have recovered to normal numbers.33 GVHD due to mismatches in histocompatibility antigens resulted in a delay in the appearance and a reduction in the numbers of pre–B cells.9 We showed that minor histocompatibility mismatch induced GVHD affected B-cell function 12 weeks after transplantation and that this decreased function resulted in decreased immune response to HSV-1 infection.
CD40L treatment increased resistance to HSV-1 infection, probably as a result of an increase in total serum IgG2a. Alternative mechanisms may have contributed to CD40L-induced protection from HSV-1 mortality. CD40L may have caused B7-2 upregulation, which increased both CTL generation and/or macrophage IL-12 production. Either augmented HSV-1 CTL activity or IL-12 action could have produced HSV-1 protection. Data showing that CD40L failed to protect B-cell–deficient mice from HSV-1 infection (Fig 5D) make these explanations less likely.
Although anti-CD40L antibody treatment early after transplantation limited the development of GVHD,34 35 we did not observe any increase in GVHD in CD40L-treated mice; this may be due to the timing of our CD40L treatments. At 12 weeks after transplantation, the initial critical expansion of mature donor T cells had already occurred. Therefore, late CD40L treatment may avoid stimulation of mature donor T-cell expansion, while allowing the enhancement of newly developed immune responses.
In our new model of posttransplant immunodeficiency, HSV-1 infection of murine transplant recipients with GVHD resulted in decreased antibody production and increased mortality. CD40L therapy increased total serum IgG2a production and improved survival after HSV-1 infection, suggesting that B-cell maturation stimuli may improve posttransplant immunodeficiency.
ACKNOWLEDGMENT
The authors thank Dr Steven Burakoff for helpful conversations and Dr Paul Martin for critical reading of the manuscript.
Supported by the Mallinckrodt Foundation, a Claudia Adams Barr Investigator Award, and NIH PO1-CA39542 (I.J.R.) and a DFG grant (H.A.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ilonna J. Rimm, MD, PhD, Genetics Institute, 87 Cambridge Park Dr, Cambridge MA 02140.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal