Abstract
P-selectin is an adhesion receptor for leukocytes expressed by activated platelets and endothelial cells. To assess a possible role of P-selectin in platelet clearance, we adapted an in vivo biotinylation technique in mice. Wild-type and P-selectin–deficient mice were infused with N-hydroxysuccinimido biotin. The survival of biotinylated platelets was followed by flow cytometry after labeling with fluorescent streptavidin. Both wild-type and P-selectin–deficient platelets presented identical life spans of about 4.7 days, suggesting that P-selectin does not play a role in platelet turnover. When biotinylated platelets were isolated, activated with thrombin, and reinjected into mice, the rate of platelet clearance was unchanged. In contrast, storage of platelets at 4°C caused a significant reduction in their life span in vivo but again no significant differences were observed between the two genotypes. The infused thrombin-activated platelets rapidly lost their surface P-selectin in circulation, and this loss was accompanied by the simultaneous appearance of a 100-kD P-selectin fragment in the plasma. This observation suggests that the platelet membrane P-selectin was shed by cleavage. In conclusion, this study shows that P-selectin, despite its binding to leukocytes, does not mediate platelet clearance. However, the generation of a soluble form of P-selectin on platelet activation may have biological implications in modulating leukocyte recruitment or thrombus growth.
RELEASED FROM precursor megakaryocytes, human platelets will circulate for 10 to 12 days1 until they are recognized as senescent, or can be consumed by hemostatic processes. This duration of life seems to be precisely controlled by an as yet unknown mechanism.
Platelets are responsible in vivo for the maintenance of vessel integrity by rapidly reacting to any injured surface leading to platelet adhesion, platelet-platelet interaction, and plug formation. On the other hand, platelets undergoing uncontrolled activation have been implicated in vascular occlusion because of thrombus formation. Thus, it has been reasonably postulated that, in the regulation of homeostasis, activated platelets have to be cleared. In apparent contradiction, two groups have reported that thrombin-activated platelets were not cleared more rapidly from the circulation than resting platelets.2-5
Although platelet clearance has already been studied in physiological and pathological conditions and shown to occur mainly in the liver and the spleen,6 it is still not clear what mechanisms are responsible for recognition and removal of either senescent or damaged platelets by the scavenging system. However, modifications of platelet membrane receptor distribution and the loss of sialic acid from the platelet surface have been proposed as potential markers.7-9
P-selectin is a cell adhesion molecule of platelets and endothelial cells stored in secretory granules and rapidly expressed on the plasma membrane on activation.10-13 After expression on the cell surface both endothelial and platelet P-selectin mediate leukocyte adhesion.14 Whereas endothelial P-selectin has clearly been shown to be involved in leukocyte and platelet rolling,15,16 the role of platelet P-selectin is less clear. However, in view of P-selectin implication in leukocyte binding, and because of the role of the leukocytes as cofactors in coagulation,17,18 a role for platelet P-selectin in the regulation of hemostasis has been postulated18,19 as well as a direct function in activated platelet scavenging.20 21
In this report, using both wild-type and P-selectin–deficient mice and a technique of platelet biotinylation,22 we studied the implications of P-selectin in platelet clearance after physiological aging, after platelet activation, and in a model of platelet storage at 4°C, a process which induces irreversible damage to platelets, leading to their rapid clearance from the blood stream.
MATERIALS AND METHODS
Materials.
N-hydroxysuccinimido biotin (NHS-biotin) and phycoerythrin-streptavidin (PE-SA) were purchased from Calbiochem (La Jolla, CA). Mouse and human thrombin, prostaglandin E1 (PGE1), and dimethyl sulfoxide (DMSO) were from Sigma (St Louis, MO). Hirulog BG8967 was kindly provided by J. Maraganore (Biogen, Cambridge, MA). Rat anti-mouse GPIIb-IIIa monoclonal antibody (MoAb) was a generous gift from A. K. Ng (University of Southern Maine, Scarborough, ME). Goat anti-rat fluorescein isothiocyanate (FITC)-conjugated antibody was purchased from Cappel Laboratories (Downington, PA). Rabbit anti-human P-selectin was kindly provided by Dr Michael C. Berndt (Baker Medical Research Institute, Prahran, Victoria, Australia).
Animals.
Male and female C57Bl/6/129SV mice, wild-type and P-selectin–deficient,15 were used with approval from the Animal Care and Use Committee of the Center for Blood Research (Boston, MA). Blood for platelet preparation was obtained from mice of any age. Recipient mice were matched by age.
In vivo biotinylation.
Mouse platelets were in vivo biotinylated by infusion of NHS-biotin, similar to the procedures described in dogs.22 23Specifically, 10 mg NHS-biotin/kg body weight was dissolved in DMSO and diluted into 500 μL of sterile saline. The solution was slowly injected via the lateral tail vein of the mouse with a 27-gauge needle.
Blood collection and preparation of washed platelets.
Mice treated with biotin were anesthetized with 2.5% tribromoethanol (0.15 mL/10 g) and blood was collected by retro-orbital venous plexus sampling into polypropylene tubes (Eppendorf; Marsh Biochemical Products, Rochester, NY) containing 10% final volume of ACD buffer (38 mmol/L citric acid, 75 mmol/L trisodium citrate, 100 mmol/L dextrose). Platelet-rich plasma (PRP) was prepared by centrifugation at 200g for 7 minutes. The plasma and the buffy coat were gently transferred to a fresh tube, and the centrifugation was repeated at 200g for 2 minutes. The PRP was incubated for 15 minutes with PGE1 at 0.1 μg/mL, platelets were isolated by centrifugation at 850g for 7 minutes, and the pellet was resuspended in a washing buffer (129 mmol/L NaCl; 13.6 mmol/L trisodium citrate; 11.1 mmol/L dextrose, 1.6 mmol/L KH2PO4, pH 6.8). Platelets were then washed twice in the washing buffer and finally resuspended in resuspension buffer (137 mmol/L NaCl; 4 mmol/L KCl; 0.5 mmol/L MgCl2; 0.5 mmol/L sodium phosphate; 11.1 mmol/L dextrose; 0.1% bovine serum albumin [BSA]; 10 mmol/L HEPES, pH 7.4). When activation was needed, platelets were prepared without PGE1 and were treated with mouse or human thrombin (1 U/mL) for 15 minutes at 37°C and the reaction was stopped by adding hirulog at 1 μg/mL.
Platelet cooling.
To study the effects of cold temperature storage on platelet survival, samples of isolated platelets in the resuspension buffer were either kept at room temperature (controls) or 4°C for 2 hours and rewarmed at 37°C for 15 minutes before reinjection.
Flow cytometry.
The percentage of biotinylated platelets in an individual blood sample was determined by using two-color flow cytometry. One or two drops of blood were collected from anesthetized mice by retro-orbital bleeding into 500 μL of Hanks’ Balanced Salt Solution (HBSS) without Ca++ and Mg++ plus 50 μL of ACD. Platelets were isolated and resuspended in HBSS. Samples were incubated with rat anti-mouse MoAb directed against GPIIb-IIIa (0.86 ng/μL) and goat anti-rat FITC-conjugated antibodies (1/500 dilution) whereas biotinylated platelets were identified by labeling with PE-SA (1/500 dilution). When needed, P-selectin expression on platelet membrane was monitored by fluorescence-activated cell sorting (FACS) analysis using a polyclonal rabbit anti-human P-selectin which cross-reacts with mouse P-selectin and a donkey anti-rabbit Cyanine-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA). Flow cytometric analyses were performed on a Becton-Dickinson FACSCalibur (Franklin Lakes, NJ). The light scatter and the fluorescent channels were set at logarithmic gain (forward scatter was E00 with a threshold of 52 and side scatter was 273). Ten thousand events were collected for each sample.
Enzyme-linked immunosorbent assay (ELISA) for soluble P-selectin.
Blood samples from retro-orbital plexus bleeding were collected in polypropylene tubes containing 10% volume of ACD and centrifuged at 14,000g for 10 minutes to remove the cells and collect the plasma. Microtiter plates were coated overnight at 4°C with an anti-mouse P-selectin MoAb (Pharmingen, San Diego, CA) at 2 μg/mL in phosphate-buffered saline (PBS). The plates were washed three times with PBS containing 0.1% Tween 20 (PBS-T) and 0.5% BSA and blocked with PBS-T containing 1% BSA for 30 minutes at room temperature. Plasma samples were diluted in PBS-T containing 0.5% BSA and 0.3% gelatin and were incubated in coated wells for 2 hours at 37°C. After washing, a biotinylated rabbit anti–P-selectin antibody (Pharmingen) was added to the plates and incubated for 2 hours. Extravidin-conjugated alkaline phosphatase was added after three washes and the activity was revealed with its substrate pNPP (Sigma). The plates were read at 405 nm in an ELISA reader.
Western blot.
Platelets were washed to remove serum proteins and lysed by addition of 1% NP40 and 1 mmol/L EDTA. Plasma or platelet lysate samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto nitrocellulose, and probed with a specific polyclonal anti–P-selectin antibody. An electrochemiluminescence-kit (Sigma) was used to visualize the antigens using X-ray films (Eastman Kodak, Rochester, NY).
Data analysis.
All values are reported as mean ± SEM. Statistical significance was assessed by Student’s t-test. Data in Fig 1 are presented as linear fitted curves.
Survival of in vivo biotinylated wild-type and P-selectin–deficient platelets. Blood from mice of both genotypes was in vivo biotinylated as described in Materials and Methods. The percentage of biotinylated cells present at various time intervals after biotinylation was determined by flow cytometry. Because the absolute levels of biotinylated platelets varied among mice, the least square-fitted curve for each mouse was normalized with respect to their value at time zero (% of biotinylated platelets and red blood cells at time zero = 100). Each curve represents a composite curve and linear fit (r = 0.99) for the data of eight mice in each genotype. Superimposed curves were obtained with wild-type (P-sel+/+) (△) and P-selectin–deficient (P-sel-/-) (◊) platelets with a mean life span of 4.72 ± 0.32 and 4.71 ± 0.3 days, respectively. Red blood cells from wild-type animals (◍) were used as a control for the stability of the biotinylation.
Survival of in vivo biotinylated wild-type and P-selectin–deficient platelets. Blood from mice of both genotypes was in vivo biotinylated as described in Materials and Methods. The percentage of biotinylated cells present at various time intervals after biotinylation was determined by flow cytometry. Because the absolute levels of biotinylated platelets varied among mice, the least square-fitted curve for each mouse was normalized with respect to their value at time zero (% of biotinylated platelets and red blood cells at time zero = 100). Each curve represents a composite curve and linear fit (r = 0.99) for the data of eight mice in each genotype. Superimposed curves were obtained with wild-type (P-sel+/+) (△) and P-selectin–deficient (P-sel-/-) (◊) platelets with a mean life span of 4.72 ± 0.32 and 4.71 ± 0.3 days, respectively. Red blood cells from wild-type animals (◍) were used as a control for the stability of the biotinylation.
RESULTS
In vivo determination of resting platelet clearance in wild-type and P-selectin–deficient mice.
Previous studies have shown that biotinylation of platelets did not interfere either with their function or their life span in vivo22-24 and therefore it constitutes a reliable nonradioactive technique for platelet life span determinations. In this study, labeling of platelets was performed with minimum platelet manipulation. Mice were intravenously infused with NHS-biotin, leading to biotinylation of 93% to 95% of the platelets. These biotinylated platelets could be easily identified by flow cytometry using PE-SA. Blood collection was performed daily up to day 5. The stability of the biotinylation was assessed by examining the in vivo-biotinylated red blood cells which have a life span of about 60 days.25 As shown in Fig 1, only a slight reduction in the biotinylated red blood cell population occurred during the 5-day period. The survival of biotinylated platelets was followed as the ratio between total platelet population labeled for GPIIb-IIIa and the biotinylated platelets labeled with PE-SA. The life span of biotinylated wild-type platelets was found to be 4.7 ± 0.3 days, the same as described previously by others using 51Cr or 111In labeling.26 No differences in platelet life span were observed between wild-type and P-selectin–deficient mice (Fig 1), suggesting that P-selectin does not play a role in normal clearance of aged platelets in vivo.
Clearance of thrombin-activated platelets.
To ascertain a possible role for P-selectin in platelet clearance, we induced its expression on the platelet plasma membrane by thrombin activation. In vivo biotinylated wild-type platelets were isolated and activated with 1 U/mL of mouse thrombin, and the enzyme was then inhibited by hirulog. Under these conditions, 60% to 90% of platelets were P-selectin positive as determined by anti–P-selectin polyclonal antibody binding. When these activated platelets were injected into mice, their clearance was identical to that of resting platelets (Fig 2). This observation reinforced the idea that P-selectin was not involved in platelet clearance and confirmed that platelet activation was not necessarily the ultimate stage of platelet life.
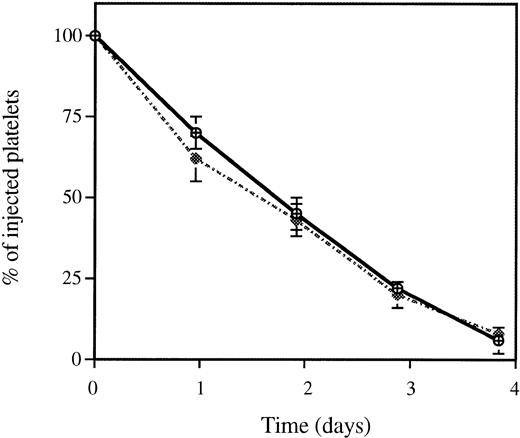
Clearance of thrombin-activated wild-type platelets. After treatment of biotinylated platelets with thrombin at 1 U/mL, activated (dotted line) or resting (solid line) platelets were injected into a mouse. The fraction of biotinylated platelets was quantified from time zero (<2 minutes after infusion) to 72 hours. The fraction of biotinylated platelets at time zero was called 100%. Platelet activation did not affect the rate of platelet clearance; n = 6. P-selectin–deficient platelets behaved in the same manner (not shown).
Clearance of thrombin-activated wild-type platelets. After treatment of biotinylated platelets with thrombin at 1 U/mL, activated (dotted line) or resting (solid line) platelets were injected into a mouse. The fraction of biotinylated platelets was quantified from time zero (<2 minutes after infusion) to 72 hours. The fraction of biotinylated platelets at time zero was called 100%. Platelet activation did not affect the rate of platelet clearance; n = 6. P-selectin–deficient platelets behaved in the same manner (not shown).
P-selectin shedding.
In a baboon model, the infusion of activated platelets has been associated with an increase of plasma P-selectin lost from the activated platelets.5 To examine in our model whether platelet P-selectin was indeed shed from the plasma membrane and not recycled to the granules, as happens in endothelial cells on in vitro activation,27 the presence of soluble P-selectin in plasma was evaluated. Activated wild-type platelets were injected into P-selectin–deficient recipients and 15 minutes later blood was collected. The plasma samples were analyzed by ELISA and by Western blotting using anti–P-selectin antibody. The ELISA showed the presence of a soluble form of P-selectin in the recipients (Fig 3A), which was identified as a 100-kD fragment of the protein by Western blotting (Fig 3B). This P-selectin fragment was observed after injection of thrombin-activated platelets, whereas no signal was seen after the injection of resting platelets (Fig 4A and B). The size of the soluble fragment indicates that platelet surface P-selectin was removed by a proteolytic cleavage rather than shed through vesiculation. Because the MoAb used in the ELISA is directed to the extracellular domain of P-selectin,28 we believed that the fragment corresponds to the N-terminal extracellular portion of the protein. To ascertain that most of the P-selectin was indeed shed rather than internalized, thrombin-activated biotinylated platelets were reinfused into a mouse and allowed to circulate for 2 hours. Their surface P-selectin was lost and no re-expression was observed on thrombin or calcium ionophore A23187 reactivation (not shown). We then examined whether P-selectin was cleaved by proteases present in plasma, on platelets themselves, or other blood cells. Wild-type platelets were activated with thrombin, incubated under different conditions, and their P-selectin expression followed over time. In vitro incubation of activated platelets in resuspension buffer or platelet-poor plasma for up to 4 hours did not modify the level of platelet P-selectin expression, whereas incubation with whole blood at room temperature or reinjection of thrombin-activated platelets into a mouse induced a significant decrease in P-selectin expression (Fig 4). These results suggest that signals in addition to thrombin activation are required for P-selectin shedding from platelets.
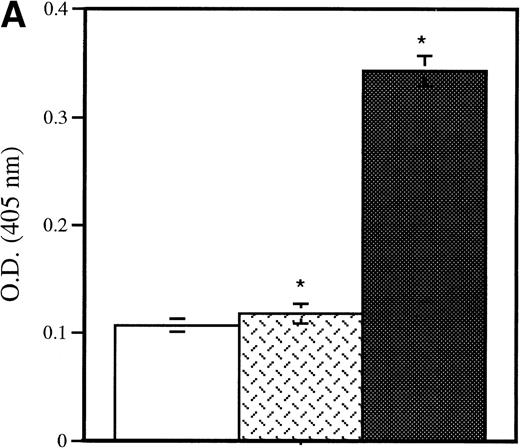
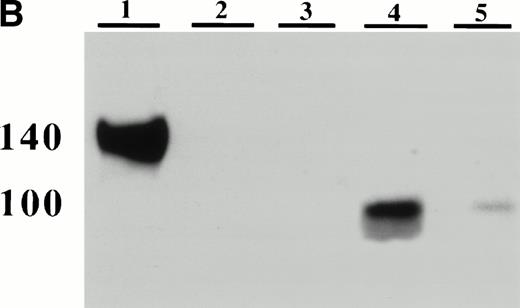
Detection of platelet-derived soluble P-selectin. To detect the shedding of platelet P-selectin into plasma, resting or thrombin-activated wild-type platelets were injected into P-selectin–deficient recipients. After 15 minutes mice were bled and plasma was isolated by centrifugation. (A) As revealed by ELISA, injection of resting platelets () did not induce significant changes in the level of soluble P-selectin compared with untreated P-selectin–deficient mice (□). Meanwhile, injection of thrombin-activated platelets (▪) generated a significant increase in soluble P-selectin in the plasma of the recipient mice (P < .0001). (B) A Western blot analysis with anti–P-selectin antibody showed the presence of an approximately 100-kD P-selectin fragment in mice injected with activated platelets (lane 4) but not with resting platelets (lane 3). Wild-type and P-selectin–deficient platelet lysates were used as reference (lane 1 and 2). In wild-type plasma from untreated animals, a small amount of P-selectin fragment was also detected (lane 5).
Detection of platelet-derived soluble P-selectin. To detect the shedding of platelet P-selectin into plasma, resting or thrombin-activated wild-type platelets were injected into P-selectin–deficient recipients. After 15 minutes mice were bled and plasma was isolated by centrifugation. (A) As revealed by ELISA, injection of resting platelets () did not induce significant changes in the level of soluble P-selectin compared with untreated P-selectin–deficient mice (□). Meanwhile, injection of thrombin-activated platelets (▪) generated a significant increase in soluble P-selectin in the plasma of the recipient mice (P < .0001). (B) A Western blot analysis with anti–P-selectin antibody showed the presence of an approximately 100-kD P-selectin fragment in mice injected with activated platelets (lane 4) but not with resting platelets (lane 3). Wild-type and P-selectin–deficient platelet lysates were used as reference (lane 1 and 2). In wild-type plasma from untreated animals, a small amount of P-selectin fragment was also detected (lane 5).
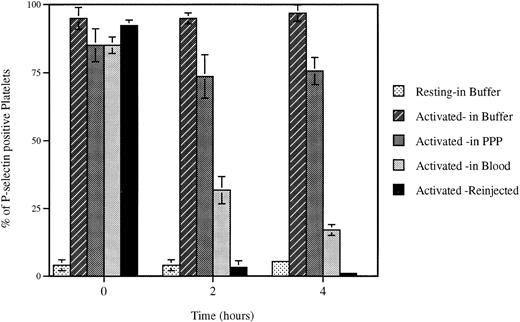
Loss of surface P-selectin from platelets. Platelets were activated (Activated) with mouse thrombin (1 U/mL) and incubated for indicated times under defined conditions. The percent of P-selectin–positive platelets was then evaluated by FACS analysis. The incubation of platelets up to 4 hours in the resuspension buffer (pH 7.3) (Buffer) or platelet-poor plasma (PPP) did not significantly alter the expression of P-selectin on the activated platelets, whereas reinjection (Reinjection) after biotinylation into an animal or incubation at room temperature with whole blood (Blood) induced a dramatic decrease in plasma membrane P-selectin. Incubation of resting platelets (Resting) in the resuspension buffer did not upregulate P-selectin expression.
Loss of surface P-selectin from platelets. Platelets were activated (Activated) with mouse thrombin (1 U/mL) and incubated for indicated times under defined conditions. The percent of P-selectin–positive platelets was then evaluated by FACS analysis. The incubation of platelets up to 4 hours in the resuspension buffer (pH 7.3) (Buffer) or platelet-poor plasma (PPP) did not significantly alter the expression of P-selectin on the activated platelets, whereas reinjection (Reinjection) after biotinylation into an animal or incubation at room temperature with whole blood (Blood) induced a dramatic decrease in plasma membrane P-selectin. Incubation of resting platelets (Resting) in the resuspension buffer did not upregulate P-selectin expression.
Clearance of platelets stored at 4°C for 2 hours.
From the experiments described above, P-selectin did not seem to be involved in the clearance of either aged or thrombin-activated platelets. To extend our analysis to damaged platelets, we developed a cooling protocol to evaluate a possible role for P-selectin in platelet clearance after storage at low temperature. In human blood transfusion protocols, it has been shown that cooled platelets have a much decreased life span in comparison with platelets stored at room temperature.29 30 Similarly, in our model, 2 hours of storage at 4°C reduced the survival of platelets on reinfusion from 65% to 25% at 24 hours (Fig 5). The same effect of cold storage was observed with both wild-type and P-selectin–deficient platelets infused into mice of the same genotype, thus excluding a role for P-selectin in the clearance of cooled platelet.
Survival of cooled platelets. In vivo biotinylated platelets were isolated and stored at 4°C (dotted line) or room temperature (solid line) for 2 hours without stirring and reinjected into a mouse. Blood samples were collected from time zero (<2 minutes, percentage of biotinylated platelets at time zero = 100) to 48 hours. The survival of platelets stored at room temperature and cooled platelets was compared for both wild-type (A) and P-selectin–deficient (B) mice. In both genotypes, cooling the platelets dramatically reduced their life span. No statistically significant differences between genotypes were detected; n = 6.
Survival of cooled platelets. In vivo biotinylated platelets were isolated and stored at 4°C (dotted line) or room temperature (solid line) for 2 hours without stirring and reinjected into a mouse. Blood samples were collected from time zero (<2 minutes, percentage of biotinylated platelets at time zero = 100) to 48 hours. The survival of platelets stored at room temperature and cooled platelets was compared for both wild-type (A) and P-selectin–deficient (B) mice. In both genotypes, cooling the platelets dramatically reduced their life span. No statistically significant differences between genotypes were detected; n = 6.
DISCUSSION
The platelet adhesion receptor for leukocytes, P-selectin, was proposed as the mediator of platelet clearance from circulation.20,21,31-35 However, a recent study has shown that infusion of activated platelets into baboons causes them to lose P-selectin into the blood without significantly affecting the life span of these platelets, as compared with infused resting platelets.5 Thus, neither platelet activation nor P-selectin expression affected platelet life span. We confirmed and extended the study by Michelson et al5 by showing that P-selectin does not play a role in the removal of naturally senescent platelets nor of platelets that were damaged by cold storage ex vivo. In addition, we found that the expression of P-selectin on platelets is followed by its rapid cleavage in vivo, producing a soluble 100-kD P-selectin fragment. This 100-kD fragment is generated only in the presence of other blood cells and may have substantial biological significance.
To unequivocally examine the role of P-selectin in platelet clearance, we have taken advantage of P-selectin–deficient mice generated a few years ago15 and adapted the techniques of in vivo platelet labeling and platelet transfusion to the mouse. In vivo platelet biotinylation has an advantage over platelet labeling ex vivo in that platelets can be studied with minimum manipulation. The platelet transfusion protocol, on the other hand, allows for specific platelet treatment or genotype switching before reinfusion. These mouse protocols could be exploited for other blood cell types, and with the availability of mice deficient in various adhesion molecules, cytoskeletal, or signaling elements, the protocols could be used to study the role of these molecules in blood cell clearance or in testing new blood banking conditions.
Our study and several others dating back to the 1970s2-5,7clearly show that activated platelets are not preferentially cleared from the circulation, but rather these thrombogenic sticky platelets seem to undergo changes which return them to a more resting, nonadhesive state. This transformation has been best documented in vitro for the integrin receptor GPIIb-IIIa. When activated platelets bind fibrinogen through this receptor and are not involved in a platelet aggregate, the integrin with its bound fibrinogen will be rapidly internalized, thus downregulating platelet adhesiveness. These platelets lose their ability to aggregate, but they can be brought to re-express fibrinogen and to aggregate by a secondary stimulation.36 We now present another mechanism of downregulation in the adhesiveness of activated platelets through proteolytic cleavage of P-selectin. Although shedding of P-selectin from activated platelets was also observed in the baboon model,5 the nature of the shed P-selectin was not examined.
To our knowledge, this is the first description of a prominent naturally occurring cleavage product of P-selectin. We also find this fragment in the plasma of normal mice but in much smaller amounts than on infusion of activated platelets (Fig 3). In mouse blood, we see only a trace of P-selectin of molecular size similar to the intact molecule. This could be either the alternatively spliced soluble isoform of P-selectin,37 whose message has not yet been reported in the mouse, or uncleaved P-selectin in the membranes of micro particles. The protease(s) responsible for the cleavage generating the 100-kD fragment remains to be identified. It will be interesting to see whether the metalloprotease inhibitors that inhibit L-selectin shedding38 also affect the shedding of P-selectin from platelets.
In human plasma, soluble P-selectin is found at concentrations of about 0.1 to 0.2 μg/mL.39-42 This soluble species of P-selectin is assumed to be mainly the alternatively spliced form of P-selectin lacking the transmembrane domain43 which was also found in platelet releasate.37,39 Increased levels of soluble P-selectin have been described in diseases such as ischemic stroke, atherosclerosis, hypertension, thrombotic thrombopenic purpura, eclampsia, thromboembolic diseases, malaria, and diabetes as well as in different stages during the menstrual cycle of healthy women.42,44-52 Comparisons of levels of circulating protein markers of endothelial cell or platelet activation with that of soluble P-selectin have suggested that activated platelets were the major source of the excess of soluble P-selectin,47,53 which could therefore be used as a marker of platelet activation.54 55 In the above investigations the levels of P-selectin in plasma were measured by ELISA and the size or molecular nature of soluble P-selectin was not examined. Assuming it indeed is generated from activated platelets, we suspect that the increased levels of P-selectin may include the cleavage product identified in our study.
Aside from rendering the circulating activated platelets less adhesive for leukocytes, what could be the biological significance of P-selectin shedding? During a severe vascular injury massive degranulation of platelets will occur and large amounts of soluble P-selectin will be locally generated. The role of this P-selectin could be twofold: first, it might inhibit excessive leukocyte recruitment by inhibiting leukocyte adhesion to the endothelium as was shown with purified platelet P-selectin56 and downregulate neutrophil activation57; second, it could inhibit resting platelet rolling on P-selectin16 and thus prevent excessive platelet recruitment to the growing platelet thrombus. A soluble form of P-selectin containing the N-terminal portion of the protein has been shown to functionally bind leukocytes through its ligand PSGL-1.40 58
Our study ruled out any role for endothelial or platelet P-selectin in mediating platelet clearance. The mechanism(s) involved in recognition of senescent or damaged platelets is still not known. Storage of human platelets at a temperature below 20°C has been shown to induce platelet lesions including cytoskeletal reorganization,30,59 receptor redistribution,32metabolism shut down,29 and membrane phospholipids disturbance,60 resulting in a shortening of platelet survival on reinfusion. In our model, even a short cooling period significantly increased the rate of platelet clearance, thus replicating in the mouse the long-standing observations with human cooled platelet transfusion. Although the clearance of cooled platelets was also P-selectin independent, we hope that this new mouse transfusion model will help to define the essential factors necessary for platelet survival and eventually lead to better platelet blood banking.
In conclusion, our results, while ruling out a direct function of P-selectin in platelet clearance after in vivo aging, thrombin activation, or cold temperature storage, lead us to hypothesize and to further study a possible function for platelet P-selectin through its shedding as a soluble regulator of cell adhesion.
ACKNOWLEDGMENT
We acknowledge John Hartwig, Richard Hynes, and Joan Brugge for their advice; Paul Frenette for helpful discussions; and Lesley Cowan for help with preparation of the manuscript.
Supported by National Institutes of Health Grant No. PO1 HL56949.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Denisa D. Wagner, PhD, The Center for Blood Research, Harvard Medical School, 800 Huntington Ave, Boston, MA 02115.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal