Abstract
In vitro infection of human B lymphocytes by Epstein-Barr virus (EBV) results in their growth transformation and establishment of immortalized lymphoblastoid cell lines. The virus was found to encode a homologue of the pleiotropic cytokine interleukin-10 (IL-10), which has wide-ranging effects on the immune system. We investigated the effect of human IL-10 (hIL-10) and viral IL-10 (vIL-10) on EBV-specific immunological memory, as assessed by the inhibition of EBV-induced B-cell transformation by the autologous T cells. We found that IL-10 abrogates the inhibitory capacity of T cells. This IL-10 effect is mediated through suppression of T-cell activation-induced IL-2 and interferon-γ production and through a direct enhancement of EBV-infected B-cell growth.
INTERLEUKIN-10 (IL-10) is an 18-kD glycoprotein produced by activated T cells, monocytes, B cells, and thymocytes. Analysis of the IL-10 coding sequence showed a high degree of homology to the Epstein-Barr virus (EBV) open reading frame (ORF) BCRF1.1,2 The mature product of this ORF was shown to share many of the functional properties to its cellular counterpart and hence it was termed viral IL-10 (vIL-10). IL-10 affects the functions of many cell types and has wide-ranging effects on human B cells. It acts as a costimulatory factor for activated B cells and also as a B-cell differentiation factor that promotes secretion of IgA, IgM, and IgG.3 A pleiotropic effect of IL-10 on apoptosis has also been described. The cytokine induces apoptosis of chronic lymphocytic leukemia cells and B cells stimulated with Staphylococcus aureus Cowan strain (SAC),4 whereas it rescues germinal center B cells,5 suggesting that the effect of IL-10 depends on the state of B-cellular activation.
IL-10 regulates the growth and differentiation of T cells. It inhibits antigen-specific activation and proliferation of human T cells and T-cell clones,6,7 as well as the proliferation, generation of cytotoxic activity, and cytokine production in mixed lymphocyte reactions.8 These effects are partially mediated through inhibition of the function of antigen-presenting cells,9,10partly through interference of T-cell activation that results in inhibition of IL-2 production.11,12 Moreover, recent studies indicate that IL-10 induces a long-lasting antigen unresponsiveness against alloantigens13 and drives the generation of T cells with suppressor activity.14
EBV establishes a latent infection in human B cells both in vivo and in vitro. The virus is associated with several B-cell tumors including Burkitt’s lymphoma (BL), immunoblastic lymphomas in immunosuppressed individuals, and about 50% of the cases of Hodgkin’s disease (HD).15 Some, but not all, BL lines, acquired immunodeficiency syndrome (AIDS)-associated lymphomas and HD specimens have been shown to produce IL-10.16-19 In addition, the development of EBV-carrying lymphomas in severe combined immunodeficiency (SCID)-hu mice involves abundant production of hIL-10,20 suggesting that IL-10 may play a role in lymphomagenesis.
In EBV seropositive individuals, the EBV-specific memory response is manifested in vitro as the ability of T cells to inhibit the growth of EBV-infected B lymphocytes, a phenomenon designed as outgrowth inhibition (OI).21 Given that IL-10 has profound effects on cells involved in the immune response, the aim of this work was to determine the influence of IL-10 on the immunity relevant to EBV infection. Our results show that IL-10 abrogates the growth inhibitory capacity of the T cells and also affects the growth of EBV-infected B cells.
MATERIALS AND METHODS
Medium and reagents.
Cultures were maintained in RPMI 1640 (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin and 2 mmol/L L-glutamine, referred to as complete medium. Recombinant hIL-10 (specific activity 107 U/mg) and vIL-10 (specific activity 5 × 106 U/mg) were kindly provided by DNAX Research Institute, Palo Alto, CA. Unless otherwise indicated, the concentration of IL-10 used was 100 U/mL. Recombinant human IL-2 and interferon-γ (IFN-γ) were generously provided by Hoffman LaRoche (Nutley, NJ) and were used at a final concentration of 20 U/mL and 100 U/mL, respectively.
Outgrowth inhibition assay.
OI assays were performed as described earlier.22 Briefly, Ficoll isopaque-separated peripheral blood lymphocytes (PBLs) from EBV seropositive donors (EBV S+) were depleted of macrophages by plastic adherence and EBV infected by exposure to supernatants of the virus producer B958 cell line for 2 hours at 37°C. The infected PBLs were seeded by doubling dilutions ranging from 8 × 105 to 1.5 × 104 cells/well in flat-bottomed microtiter plates in a final volume of 200 μL. Five replicates of each dilution were performed in the absence and in the presence of 100 U/mL of IL-10. In some experiments IL-10 was added at day 4 after infection. The cultures were incubated at 37°C/5% CO2 and fed once per week by replacing half of the medium with fresh complete media with or without IL-10. Four to 5 weeks later, the growth was evaluated visually22 and by3H-thymidine (TdR) incorporation. The strength of the OI is expressed as OI index that reflects the minimum number of cells/well required for 50% reduction of B-cell growth. This value was calculated by plotting the mean % inhibition versus the log of the cell concentration/well. A regression line was obtained from which the minimum number of cells/well required for 50% inhibition was calculated. Wells were pulsed with 1 μCi of 3H-TdR (Amersham, Amersham, UK) 12 hours before harvesting onto a fiberglass filter and the 3H-TdR incorporation was determined by liquid scintillation counting.
Isolation of CD4+ and CD8+enriched populations.
CD4+ and CD8+ populations were obtained by negative selection. Aliquots of PBLs depleted of macrophages by plastic adherence were incubated with saturating concentrations of anti-CD4 or anti-CD8 monoclonal antibody (MoAb) for 30 minutes at 4°C. Cells were washed twice with Hanks’ Balanced Salt Solution (HBSS) and subsequently rosetted with magnetic beads coated with sheep antimouse IgG (Dynabeads M-450 sheep antimouse IgG, Dynal AS, Oslo, Norway) at a bead to cell ratio of 20:1. The mixture was incubated for 30 minutes at 4°C with gentle shaking before removal of rosetted cells with a magnetic particle concentrator according to the manufacturer’s recommendations. The resulting cell preparations contained less than 5% of the depleted population as assessed by staining with fluorescein isothiocyanate (FITC) goat anti-mouse Ig.
Analysis of IL-10 effect on EBV-induced B-cell proliferation.
Purified B cells were obtained from PBL depleted of adherent cells by plastic adherence and iron phagocytosis and further fractionated into T- and B-cell enriched populations by nylon wool passage.23B cells were collected from the nylon adherent fraction and further purified by negative selection with anti-CD3 MoAb-coated magnetic beads (Dynabeads M-450 anti CD3; Dynal) according to manufacturer’s recommendations. The recovered cells were exposed to B958 cell supernatants for 2 hours at 37°C, washed, and resuspended in complete media. The infected cells were seeded in triplicates at a concentration of 5 × 104/well in 200 μL vol in 96-flat bottomed microplates in the presence of various concentrations of IL-10. Seven days later, B-cell proliferation was assessed by3H-TdR incorporation during the last 8 hours of the incubation period.
Lymphokine determination.
Supernatants collected at days 1, 2, and 3 after EBV infection were assayed as duplicates for their content of IL-2, IL-4, IL-5, IL-6, IL-10, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-α (TNF-α) by lymphokine-specific enzyme-linked immunosorbent assays (ELISA) as described previously.8 The sensitivity of the various ELISA were: 10 pg/mL for IL-2; 40 pg/mL for IL-4, IL-5, and IL-10; 100 pg/mL for GM-CSF, IL-6, and IFN-γ; and 20 pg/mL for TNF-α.
RESULTS
IL-10 prevents the inhibition of EBV-induced B-cell transformation by autologous T cells.
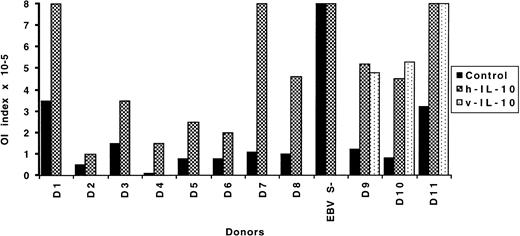
The effects of exogenous IL-10 (human and viral) on the T-cell–mediated inhibition of EBV-induced transformation of B cells was analyzed in OI assays that are known to reflect an EBV-specific memory response. The strength of the OI is expressed in terms of the initial number of cells/well required for 50% reduction of B-lymphocyte growth. The reported mean value for healthy seropositive individuals is 1.9 × 105 cells/well with a range between 0.3 to 6.3 × 105.24 One seronegative and 11 seropositive donors were tested. When IL-10 was added to the cultures immediately after EBV infection, it reduced the growth inhibitory capacity in all instances, independent of the initial strength of the inhibition. The same effect was observed with both hIL-10 and vIL-10 (donors 1 to 8 and 9 to 11, respectively). When tested in parallel, human- and viral-IL–10 displayed comparable inhibitory capacity (donors 9 to 11). The outcome of the assay was not affected in one case where the donor was EBV seronegative and thus did not exhibit OI response (Fig 1).
Effect of IL-10 on the OI. EBV-infected PBL from 11 seropositive and one seronegative (EBV S−) donors were seeded at various cell concentrations in flat-bottomed microtiter plates in medium control or medium containing 100 U/mL of IL-10. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
Effect of IL-10 on the OI. EBV-infected PBL from 11 seropositive and one seronegative (EBV S−) donors were seeded at various cell concentrations in flat-bottomed microtiter plates in medium control or medium containing 100 U/mL of IL-10. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
IL-10 potentiates the growth of EBV-transformed cells.
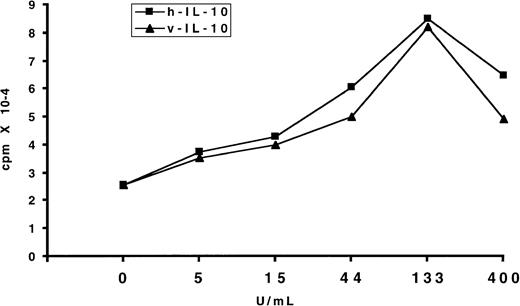
IL-10 is known to be involved in the proliferation and autonomous growth of EBV-transformed B cells.25-27 Therefore, we tested the effect of exogenous IL-10 on the EBV-induced B-cell transformation. To this end, purified B cells were exposed to the virus and cultured in the presence of various concentrations of hIL-10 and vIL-10. Seven days later, the proliferation was assessed by3H-TdR incorporation. IL-10 increased the proliferation of B cells in a dose-dependent fashion. At high doses, ie, 400 U/mL, the enhancing effect declined (Fig 2). A potentiating effect of IL-10 on B-cell growth/transformation was also observed in the OI assays when the number of PBL seeded per well was equal or below 2.5 × 104. Under these conditions, the number of EBV-specific T-cell precursors is too low to prevent EBV-induced transformation; in addition, the proportion of B cells that undergo transformation is also low, and/or a feeder effect is required to sustain growth. In the presence of IL-10, there was a higher frequency of wells with transformation compared with control cultures. This effect was reflected in the 3H-TdR incorporation values (Table 1). In accordance with previous reports,25 27 B-cell transformation was strongly inhibited by the addition of anti–IL-10 antibodies (data not shown).
Effect of IL-10 on the proliferation of EBV-infected B lymphocytes. Purified B cells were infected with EBV and cultured in flat-bottomed microtiter plates in the presence of different concentrations of IL-10. Seven days later the proliferation was evaluated by 3H-TdR incorporation. The results expressed mean cpm of triplicate samples. The standard deviation (SD) was lower than 10%. One representative experiment of three is shown.
Effect of IL-10 on the proliferation of EBV-infected B lymphocytes. Purified B cells were infected with EBV and cultured in flat-bottomed microtiter plates in the presence of different concentrations of IL-10. Seven days later the proliferation was evaluated by 3H-TdR incorporation. The results expressed mean cpm of triplicate samples. The standard deviation (SD) was lower than 10%. One representative experiment of three is shown.
IL-10 Enhances the Transformation of B Cells
| Experiment . | Cells/ Well . | Control . | IL-10 . | ||
|---|---|---|---|---|---|
| Incidence of Growth . | Mean cpm . | Incidence of Growth . | Mean cpm . | ||
| 1 | 1.5 × 104 | 1/4 | 606 | 4/4 | 11,737 |
| 2 | 1.5 × 104 | 1/4 | 597 | 4/4 | 8,116 |
| 3 | 2.5 × 104 | 0/4 | 147 | 4/4 | 22,200 |
| Experiment . | Cells/ Well . | Control . | IL-10 . | ||
|---|---|---|---|---|---|
| Incidence of Growth . | Mean cpm . | Incidence of Growth . | Mean cpm . | ||
| 1 | 1.5 × 104 | 1/4 | 606 | 4/4 | 11,737 |
| 2 | 1.5 × 104 | 1/4 | 597 | 4/4 | 8,116 |
| 3 | 2.5 × 104 | 0/4 | 147 | 4/4 | 22,200 |
PBL were infected with EBV and cultured with or without 100 U/mL of hIL-10 in flat-bottomed microtiter plates at the indicated cell concentration/well. Four to 5 weeks later the cultures were visually scored for incidence of transformation. Proliferation was evaluated by3H-TdR incorporation. The SD of the replicates was ≤12%.
Kinetics of the IL-10 effect.
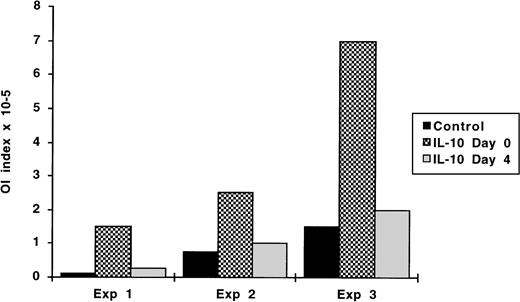
We have shown previously that the first 3 days after viral infection are crucial for the outcome of the OI. When T cells or cytokines, such as IFN-γ are added after this period, their inhibitory effects are no longer observed or are substantially reduced.24 28Therefore, we tested whether IL-10 can exert its effect if added 72 hours after infection. Experiments were performed in which hIL-10 was added immediately after infection or at day 4. As shown in Fig 3, IL-10 was effective in abrogating the OI response only if present from the outset of the culture period, when added at day 4, the OI values were as those of the control cultures. The kinetics of the IL-10 effect was the same independently of the initial strength of the OI response.
Kinetics of the IL-10 effect on the OI. OI assays were performed as described in Materials and Methods. IL-10 was added either from the outset of the culture period or on day 4. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
Kinetics of the IL-10 effect on the OI. OI assays were performed as described in Materials and Methods. IL-10 was added either from the outset of the culture period or on day 4. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
IL-10 inhibits cytokine production.
IL-10 appears to act predominantly as an immunosuppressive agent, inhibiting the proliferation of T cells and the production of cytokines in response to allo- and soluble antigens.8,9 11 Thus, we set out to test the effect of IL-10 on the production of IL-2, IL-3, IL-4, IL-5, IL-6, IL-13, GM-CSF, IFN-γ, and TNF-α in the EBV-infected cultures. A single determination was performed at day 3 in most experiments. However, given the fact that various cytokines have different kinetics of production, supernatants from EBV-infected PBLs cultured with or without IL-10 were harvested at days 1, 2, and 3 in two experiments. Five different experiments are shown in Table 2 where results from the experiments 1, 2, and 3 correspond to the OI assays of donors 1, 3, and 6 depicted in Fig 1. We could not detect production of IL-4, IL-5, and GM-CSF (not shown), while significant levels of IL-2, IL-6, IL-10, TNF-α, and IFN-γ were found in the supernatants. Addition of IL-10 resulted in substantial reduction of IL-2 and TNF-α production. The production of IFN-γ and IL-6 was affected to a lesser extent, although the levels were significantly decreased compared with the control cultures (P values < .05) (Table 2).
Effect of Exogenous IL-10 on Cytokine Production by EBV-Infected PBL
| Experiment . | Day . | IL-2 pg/mL . | IL-6 pg/mL . | IL-10 pg/mL . | IFN-γ pg/mL . | TNF-α pg/mL . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control . | IL-10 . | Control . | IL-10 . | Control . | Control . | IL-10 . | Control . | IL-10 . | ||
| 1 | 3 | 80 | 0 | 983 | 0 | 218 | 1,603 | 1,113 | 61 | 27 |
| 2 | 3 | 40 | 12 | 329 | 0 | 829 | 2,156 | 629 | NT | NT |
| 3 | 3 | 60 | 0 | 818 | 0 | 631 | 2,838 | 1,176 | NT | NT |
| 4 | 1 | 45 | 26 | 2,000 | 537 | 147 | NT | NT | 740 | 237 |
| 2 | 295 | 66 | 1,077 | 188 | 277 | NT | NT | 214 | 35 | |
| 3 | 260 | 65 | 1,394 | 182 | 255 | 1,897 | 365 | 221 | 41 | |
| 5 | 1 | 44 | 16 | 1,005 | 995 | 165 | NT | NT | 772 | 130 |
| 2 | 71 | 13 | 1,390 | 565 | 290 | NT | NT | 91 | 37 | |
| 3 | 164 | 52 | 2,002 | 482 | 427 | 2,636 | 547 | 52 | 22 | |
| Experiment . | Day . | IL-2 pg/mL . | IL-6 pg/mL . | IL-10 pg/mL . | IFN-γ pg/mL . | TNF-α pg/mL . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control . | IL-10 . | Control . | IL-10 . | Control . | Control . | IL-10 . | Control . | IL-10 . | ||
| 1 | 3 | 80 | 0 | 983 | 0 | 218 | 1,603 | 1,113 | 61 | 27 |
| 2 | 3 | 40 | 12 | 329 | 0 | 829 | 2,156 | 629 | NT | NT |
| 3 | 3 | 60 | 0 | 818 | 0 | 631 | 2,838 | 1,176 | NT | NT |
| 4 | 1 | 45 | 26 | 2,000 | 537 | 147 | NT | NT | 740 | 237 |
| 2 | 295 | 66 | 1,077 | 188 | 277 | NT | NT | 214 | 35 | |
| 3 | 260 | 65 | 1,394 | 182 | 255 | 1,897 | 365 | 221 | 41 | |
| 5 | 1 | 44 | 16 | 1,005 | 995 | 165 | NT | NT | 772 | 130 |
| 2 | 71 | 13 | 1,390 | 565 | 290 | NT | NT | 91 | 37 | |
| 3 | 164 | 52 | 2,002 | 482 | 427 | 2,636 | 547 | 52 | 22 | |
PBL from EBV-seropositive individuals were exposed to the virus and cultured in medium control or medium containing 100 U/mL of hIL-10. The cell-free supernatants were collected and analyzed for cytokine content with cytokine specific ELISA.
Abbreviation: NT, not tested.
The inhibitory effect of IL-10 can be neutralized by exogenous IL-2.
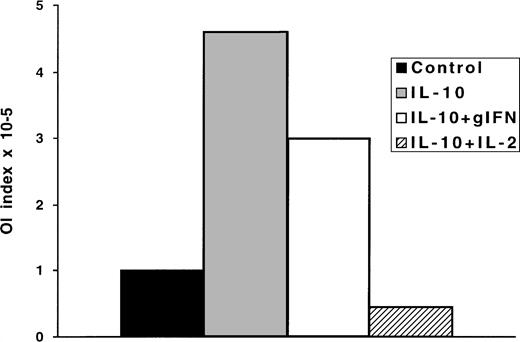
The reduction of IL-2 and IFN-γ production are well-documented effects of IL-10, which are responsible for the inhibition of T-cell responses.8,11,12 Originally, IL-10 was described as a cytokine that inhibits IFN-γ production by peripheral blood mononuclear cell (PBMC) after activation by phytohemagglutinin (PHA) or anti-CD3 MoAb.29 IL-10 was shown to act directly on human T cells triggering a signalling pathway that specifically inhibits IL-2 synthesis.11 12 Hence, we investigated whether exogenously added IL-2 or IFN-γ could overcome the inhibitory effects of IL-10 in the OI. To this end, IL-2 or IFN-γ were added to the IL-10 containing cultures from day 0. Addition of IL-2 neutralized the effect of IL-10; the inhibition of transformation in the IL-2 supplemented cultures was even stronger than in the control cultures. In contrast, IFN-γ could only partially overcome the inhibitory effect (Fig 4).
The OI is reconstituted by exogenous IL-2, but not by IFN-γ. Four replicate plates were performed for OI as follows: (1) medium control, (2) medium containing 100 U/mL of IL-10, (3) medium containing 100 U/mL of IL-10 and 10 U/mL of IL-2, (4) medium containing 100 U/mL of IL-10 and IFN-γ. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation. One representative experiment of three is shown.
The OI is reconstituted by exogenous IL-2, but not by IFN-γ. Four replicate plates were performed for OI as follows: (1) medium control, (2) medium containing 100 U/mL of IL-10, (3) medium containing 100 U/mL of IL-10 and 10 U/mL of IL-2, (4) medium containing 100 U/mL of IL-10 and IFN-γ. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation. One representative experiment of three is shown.
The inhibitory effect of IL-10 is potentiated in the presence of CD4+ T cells.
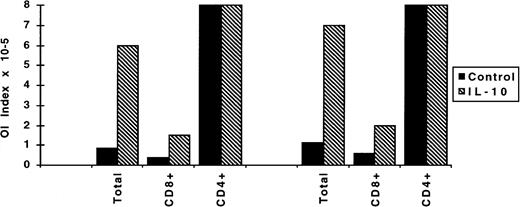
We have demonstrated previously that the OI is the end result of a combination of inhibitory activities mediated by soluble factors as well as cytotoxic cell responses exerted by T cells and natural killer (NK) cells.22,24,28 Studies involving the separation of T cells into CD4+ or CD8+ populations showed that the CD8+ cell compartment is the main effector in the OI.30 To dissect the effect of IL-10 on CD4+ or CD8+ T cells, OI assays were performed with PBLs enriched in CD4+ or CD8+ cells by negative selection. Using these fractionated populations, we confirmed that the CD8+, but not the CD4+ cells, play a major role in the OI. In the absence of CD8+ cells, 50% inhibition was not observed even at the highest cell number seeded, ie, 8 × 105/well. Conversely, the OI capacity of the CD8+-enriched population was stronger than that of the total population (twofold reduction in the number of cells/well required for 50% inhibition). The IL-10 inhibitory effect was more evident in the total population. The inhibitory effect of the CD8+-enriched population was reduced by threefold compared with sevenfold reduction in the total population (Fig 5).
Effect of IL-10 on the outgrowth inhibitory capacity of CD4+ and CD8+ populations. CD4+- and CD8+-enriched PBL populations were obtained by negative depletion of CD8+ and CD4+ cells, respectively, and thereafter infected with EBV. OI assays were performed as described in medium alone or medium containing 100 U/mL of IL-10. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
Effect of IL-10 on the outgrowth inhibitory capacity of CD4+ and CD8+ populations. CD4+- and CD8+-enriched PBL populations were obtained by negative depletion of CD8+ and CD4+ cells, respectively, and thereafter infected with EBV. OI assays were performed as described in medium alone or medium containing 100 U/mL of IL-10. Four to 5 weeks later the transformation was visually scored. The results are expressed as OI index representing the minimum number of cells/well required to achieve 50% inhibition of EBV-induced transformation.
DISCUSSION
In this study, we show that IL-10 abrogates the inhibitory activity that T cells from EBV seropositive individuals have on the EBV-induced transformation of autologous B cells. This reflects a combination of enhancing effects on EBV-induced B-cell proliferation on one hand and inhibitory effects on T-cell reactivation on the other.
EBV appears to have acquired a number of unique functions that enhance its ability to colonize the B-cell system and to persist as a latent infection within the B-cell pool despite the continuous presence of cytotoxic T lymphocyte (CTL) surveillance. IL-10 contributes to these strategies in several ways. IL-10 promotes the efficiency of virus-induced transformation through its capacity to enhance the proliferation and survival of the infected B cells. Viral IL-10 is expressed during the late phase of the lytic cycle, but has also been detected by reverse transcriptase-polymerase chain reaction (RT-PCR) within 6 hours after viral infection.31 This early expression of the viral product is followed, 20 to 30 hours later, by the virally induced expression of hIL-10.25,31 Expression of the EBV latent membrane antigen (LMP-1) upregulates hIL-10 production in transfected sublines of EBV- Burkitt’s lymphomas.32 Studies involving vIL-10 showed an enhancing effect on EBV-induced transformation that was due to an increase in the viability of the infected B cells.26 Addition of exogenous hIL-10 potentiates the proliferation of infected B cells,25and hIL-10 was shown to act as an autocrine factor promoting the growth of spontaneously derived lymphoblastoid cell lines from patients with EBV-associated lymphoproliferative disorders.27 Therefore, vIL-10 may exert these growth enhancing effects in the earlier phases after EBV infection, before the induction of the cellular IL-10, which is known to peak 3 to 4 days after infection.31 In our hands, this promoting effect was reflected in the levels of proliferation in the transformation assays as measured by3H-TdR incorporation and also in the OI assays. In the latter, when the number of cells/well seeded was low (below or equal to 2.5 × 104 total PBL with a proportion of B cells between 10% to 15%), the incidence of transformation was enhanced in the presence of IL-10. These findings are in line with previous reports25 31 and our own observation that B-cell transformation is affected in the presence of anti–IL-10 antibodies.
Relevant to the B-cell growth promoting effect of IL-10 is also its capacity to inhibit the production of IFN-γ, which is important in the early control of EBV infection/transformation. IFN-γ inhibits EBV-induced B-cell proliferation only if added during the first 72 hours after viral infection, before the transformed phenotype is established.33 Notably, while the transforming ability of viruses in which the vIL-10 gene was deleted was not affected, the lymphoblastoid cell lines (LCLs) obtained by transformation with the mutant viruses induced higher levels of IFN-γ production by allogeneic PBL than the LCLs established with the wild-type virus.34
A direct effect of IL-10 on the T-cell–mediated control of B-cell transformation may involve modulation of CTL recognition. Recently, it was shown that vIL-10 downregulates the expression of the transporter associated with antigen presentation-1 (TAP1) in primary B lymphocytes after EBV infection. As a consequence, vIL-10 causes a general reduction of surface major histocompatibility complex (MHC) class I molecules that could affect the presentation of viral antigens to T cells.35 Similar mechanisms are used by other herpes viruses.36 An immediate early gene product of Herpes simplex virus (HSV) inhibits the TAP-dependent peptide translocation.37,38 Various early gene products of human cytomegalovirus (HCMV) prevent antigen presentation in a sequential multistep process by modifying the intracellular transport of MHC class I molecules and also by preventing peptide loading within the endoplasmic reticulum.39,40 It is noteworthy that EBV has evolved a specialized strategy for preventing presentation of a latency-associated antigen, EBNA-1. Recognition of EBNA-1 by MHC class I restricted CTLs is inhibited by the presence of a Gly-Ala repeat domain, which appears to prevent ubiquitin/proteasome-dependent processing.41 The product of the BZLF2 ORF encodes a type II membrane glycoprotein expressed during lytic infection that binds the β chains of MHC class II and thus blocks class II antigen presentation.42
The present report suggests an additional advantage for the virus in capturing this cytokine homologue, namely the suppression of specific T-cell responses manifested as the capacity of T cells to inhibit EBV-induced B-cell transformation. We have previously shown that activation of T cells, as well as IFN-γ production during the early phases of EBV infection, are crucial in determining the outcome of the OI. Cyclosporin-A abolished the OI capacity of T cells if present during the first 3 days after viral infection and exogenously added IL-2 could neutralize this effect.28 The kinetics of the IL-10 effect showed the same pattern. In the OI system, the IL-10 effect appears to be explained mainly by abrogation of the IL-2 production. This assumption is substantiated by the fact that addition of exogenous IL-2 could restore the OI, while IFN-γ had only a partial effect. Therefore, the critical consequence of the IL-10 effect is the inhibition of expansion of the reactivated T cells. We have described a similar IL-10 effect on the proliferative and cytotoxic responses to alloantigens.8 In that study, we showed that the effect of IL-10 is at the level of IL-2 production rather than IL-2 consumption because the inhibitory effect of IL-10 was also evident in the presence of anti–IL-2R antibodies.
Our results differ from those reported by Stewart and Rooney43 who showed that transfection of vIL-10 into an EBV+ BL line, or addition of vIL-10 to PBL cultures stimulated with autologous or allogeneic LCLs, results in enhanced generation of allo-specific CTL, EBV-specific CTL, and HLA unrestricted killing. The discrepancy may have several explanations. In the experiments of Stewart and Rooney, EBV-specific CTLs were reactivated by exposure to already transformed B cells. Thus, the enhancing effects of IL-10 could be partially explained by an activating effect on B-cell lines. It is noteworthy that in our previous studies on modulation of alloreactivity, the inhibitory effect of IL-10 on T-cell proliferation and generation of cytotoxicity was not evident when EBV-transformed LCLs were used as stimulators.8 In the present study, IL-10 was added from the beginning of the cultures when T cells are exposed to infected B cells at a very early stage of transformation. It is likely that the early steps of T-cell activation are influenced by parameters such as the number and phenotype of the stimulator cells where expression of costimulatory molecules and/or different patterns of viral antigens may be critical. Furthermore, it was recently shown that IL-10 induces antigen-specific unresponsiveness13; whether this is an additional mechanism in the IL-10–mediated inhibition of EBV-specific responses remains to be determined.
In accordance with a previous study,30 the CD8+population seems to play a major role in the OI. We have observed that although the strength of the CD8+-mediated inhibition was reduced twofold to threefold in the presence of IL-10, the inhibitory effect of IL-10 was more evident on the total population where the reduction was sixfold to sevenfold. This indicates that the IL-10 effect may require and/or is potentiated in the presence of CD4+ cells. This finding is interesting in light of a recent report where IL-10 was shown to drive the generation of CD4+ clones that suppress antigen-specific responses.14
Our results support the idea that IL-10 plays a pivotal role in the establishment and maintenance of EBV carrier state through its ability to enhance transformation of B cells during an initial infection and to suppress the immune responses triggered by primary infection or during subsequent reactivation of the virus. In the strategy of persistence of herpes viruses, a reactivation step is required for spread to a new host. This reactivation occurs in immunocompetent individuals. The inhibitory mechanisms evolved by the viruses are partial and allow delay of rejection until the productive virus cycle is completed. During primary infection, the EBV replication is rapidly controlled by a vigorous T-cell response, which includes CTLs specific for various antigens of the lytic cycle.44, 45 The presence of vIL-10 and the virally induced hIL-10 may delay or partially reduce such response so that the number of virally infected cells may reach a sufficiently high level, before the onset of the rejection response, to secure the access of the virus to an immunologically protected latent reservoir. IL-10 was reported to prevent the apoptotic death of T cells from infectious mononucleosis patients in vitro.46 This promoting activity on the survival of T cells may be instrumental in allowing the establishment of immunological memory after resolution of the illness.
The inhibitory effect of IL-10 on EBV-specific memory response reported in this study could also play an important role in viral pathogenesis. The IL-10 effect on preventing T-cell reactivation could be one of the mechanisms underlying the local suppression of EBV-specific T-cell responses that we have shown in HD biopsies.47 In addition, the combined effects of IL-10 described above may be responsible for the association of IL-10 production and conditions of lymphoproliferation such as lymphomagenesis in SCID-hu mice, AIDS-associated lymphomas, and HD.6-9,20 27
ACKNOWLEDGMENT
We thank Dr Rene de Waal Malefyt (DNAX Research Institute, Palo Alto, CA) for the generous gift of IL-10.
Supported by the Swedish Cancer Society, the Lars Hiertas Minne Foundation, the Gunnar Arvid and Elisabeth Nilsson Foundation, and the Karolinska Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M.T. Bejarano, MD, MTC, Karolinska Institutet, Box 280, S-171 77 Stockholm, Sweden; e-mail:maria.teresa.bejarano@mtc.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal