Abstract
The long-term biochemical and clinical effectiveness of allogenic bone marrow transplantation (BMT) was shown in a severely affected, transfusion-dependent 18-month-old female with congenital erythropoietic porphyria (CEP), an autosomal recessive inborn error of heme biosynthesis resulting from mutations in the uroporphyrinogen III synthase (URO-synthase) gene. Three years post-BMT, the recipient had normal hemoglobin, markedly reduced urinary porphyrin excretion, and no cutaneous lesions with unlimited exposure to sunlight. The patient was homoallelic for a novel URO-synthase missense mutation, G188R, that expressed less than 5% of mean normal activity in Escherichia coli, consistent with her transfusion dependency. Because the clinical severity of CEP is highly variable, ranging from nonimmune hydrops fetalis to milder, later onset forms with only cutaneous lesions, the importance of genotyping newly diagnosed infants to select severely affected patients for BMT is emphasized. In addition, the long-term effectiveness of BMT in this patient provides the rationale for future hematopoietic stem cell gene therapy in severely affected patients with CEP.
CONGENITAL ERYTHROPOIETIC porphyria (CEP, Gunther disease) is a rare autosomal recessive disorder of heme biosynthesis that results from the markedly deficient, but not absent, activity of uroporphyrinogen III synthase (URO-synthase; EC4.2.1.75).1-4 The resultant accumulation of the nonphysiological and pathogenic porphyrins, uroporphyrin I and coproporphyrin I, in erythrocytes leads to hemolysis and the released type I isomers are deposited in tissues and bones throughout the body as well as excreted in the urine and feces. Uroporphyrin I is a photosensitizing compound, and exposure of the skin to sunlight results in blistering and vesicle formation. Secondary infection can lead to scarring, tissue loss, and deformities, including loss of digits and facial features such as eyelids, ears, and nares.5 In addition, corneal scarring can cause blindness.
The clinical severity of the anemia and cutaneous involvement in CEP is highly variable, ranging from nonimmune hydrops fetalis because of severe hemolytic anemia in utero to milder, later onset forms, which have only cutaneous lesions in adult life. Hemolysis is a feature in moderately to severely affected patients and is accompanied by anisocytosis, poikilocytosis, polychromasia, basophilic stippling, reticulocytosis, increased nucleated red cells, absence of haptoglobin, increased unconjugated bilirubin, increased fecal urobilinogen, and increased plasma iron turnover. Severely affected patients are transfusion dependent. Secondary splenomegaly enhances the anemia and also may result in leukopenia and thrombocytopenia.
The availability of the full-length cDNA and genomic clones encoding human URO-synthase6,7 permit genotype/phenotype correlations to predict the clinical severity of CEP. The single URO-synthase gene, located at chromosome 10q25.3→q26.3,8 contains 10 exons.7Mutation analysis in unrelated patients with CEP has shown a variety of lesions including gene rearrangements, splicing mutations, and single base substitutions.4,9,10 The prokaryotic expression of various missense mutations has permitted estimation of their relative residual activities for genotype-phenotype correlations.10
At present, treatment of CEP has been nonspecfic, involving avoidance of sunlight to prevent the skin lesions and splenectomy or chronic transfusions for the hemolytic anemia. Protection of the skin from sunlight and minor trauma is of the utmost importance, and aggressive antibiotic treatment of all infections is essential to minimize scaring and mutilation.3,11,12 For transfusion-dependent patients, chronic erythrocyte transfusions are lifesaving and have been used therapeutically in moderately affected patients with mild hemolytic anemia to suppress erythropoiesis and thereby decrease porphyrin production.13 This strategy has been effective until puberty when porphyrin production may increase and more aggressive treatment with hydroxyurea to reduce bone marrow porphyrin synthesis may be considered.14
To date, allogenic bone marrow transplantation (BMT) has been performed in three patients with CEP.15-17 The first patient was a moderately affected 8-year-old girl who died of cytomegalovirus (CMV) encephalitis 11 months after a successful BMT,15 whereas two other patients were engrafted, including a 2-year-old moderately affected patient transplanted in Paris who rejected the first graft, but whose second graft was successful,17 and a 4-year-old moderately affected patient transplanted in Strasbourg with cord blood.16 These patients were not transfusion dependent, and their uroporphyrinogen III synthase mutations were not reported, so their phenotypic severity could not be fully evaluated. After BMT, the two living patients had remarkably reduced levels of urinary uroporphyrin I and coproporphyrin I, normal hemoglobin values, and did not develop skin lesions on limited exposure to sunlight at 1 year post-BMT. Although encouraging, further experience must be gained with BMT to evaluate its biochemical, hematologic, and clinical effectiveness as well as its long-term ability to prevent later manifestations caused by the progressive accumulation of type I isomers. In addition, effective long-term treatment would justify the risks of BMT in newly diagnosed, severely affected CEP patients, and would provide the rationale for future hematopoietic stem cell gene therapy endeavors.18-21
In this communication, we describe a 4-year, 5-month-old transfusion-dependent girl, the product of a consanguineous union, who was homoallelic for a novel mutation (G188R) and who received a successful BMT at 18 months of age which has completely corrected her biochemical, cutaneous, and hematologic manifestations for 35 months, the longest posttransplant follow-up documented.
MATERIALS AND METHODS
Case report.
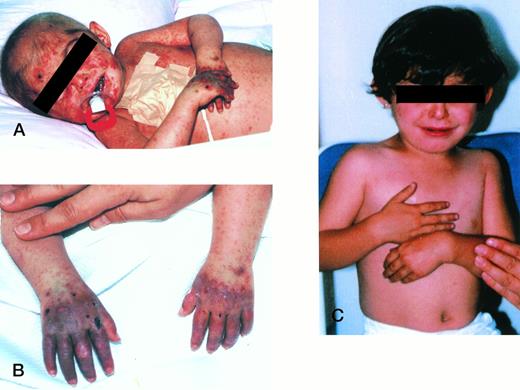
The patient was the fourth product of a first-cousin marriage of a G4P4 female who had two healthy siblings as well as a male sibling who died at 6 months of age from unknown causes. Pink-reddish urine was first noted in infancy by the parents, and splenomegaly was first detected in the first month of life. She was referred at 9 months of age to the Ihsan Dogramaci Children’s Hospital, Hacettepe University (Ankara, Turkey) for evaluation of severe anemia and hepatosplenomegaly. On physical examination, she had extreme pallor and hepatosplenomegaly. The liver and spleen were palpable 4 and 8 cm below costal margin, respectively. Pertinent laboratory results included hemoglobin, 5.6 g/dL; hematocrit, 22%; reticulocyte count, 1.8%; total leukocytes, 7,000/μL. A peripheral blood smear showed poikilocytosis, polychromasia, tear-drop cells, and normoblasts. Hemoglobin electrophoresis, osmotic fragility, pyruvate kinase, and the Ham test were normal, as was the test for unstable hemoglobin. Coomb’s tests and cold agglutinins were negative. Because of her severe hemolytic anemia, blood transfusions were required every 4 weeks. Based on the elevated level of urinary porphyrins (Table 1), the diagnosis of CEP was made. At 12 months of age, the reddish-brown discoloration of her decidous teeth was noted, and at 13 months of age, she developed crusted lesions on her face and dorsum of her hands (Fig 1A and B).
Porphyrin Levels of the Patient Before and After BMT
| Porphyrin . | Normal . | Pre-BMT . | Days Post-BMT . | ||
|---|---|---|---|---|---|
| 122 . | 241 . | 406 . | |||
| Protoporphyrins | |||||
| Serum, μg/dL | <5 | 135 | 42.3 | — | 44.4 |
| Coproporphyrins | |||||
| Serum, μg/dL | <4.5 | 208 | 37.4 | — | 24.2 |
| Urine, μg/mL | <60 | 297 | 102 | 83.4 | — |
| Uroporphyrins | |||||
| Serum, μg/dL | <3.0 | 112 | 26.2 | — | 18.3 |
| Urine, μg/mL | <10.0 | 382 | 88.7 | 52.3 | — |
| Porphyrin . | Normal . | Pre-BMT . | Days Post-BMT . | ||
|---|---|---|---|---|---|
| 122 . | 241 . | 406 . | |||
| Protoporphyrins | |||||
| Serum, μg/dL | <5 | 135 | 42.3 | — | 44.4 |
| Coproporphyrins | |||||
| Serum, μg/dL | <4.5 | 208 | 37.4 | — | 24.2 |
| Urine, μg/mL | <60 | 297 | 102 | 83.4 | — |
| Uroporphyrins | |||||
| Serum, μg/dL | <3.0 | 112 | 26.2 | — | 18.3 |
| Urine, μg/mL | <10.0 | 382 | 88.7 | 52.3 | — |
Normal levels are for children 2 to 12 years old.36Pre-BMT, the uroporphyrins and coproporphyrins were >90% type I isomers. Post-BMT, the type I isomer content was reduced to near normal type III/I ratios.
(A and B) Patient before BMT, at 18 months old; (C) patient 27 months post-BMT, at 35 months old.
(A and B) Patient before BMT, at 18 months old; (C) patient 27 months post-BMT, at 35 months old.
Patient specimens.
Peripheral blood was collected from the proband and her family members with informed consent. Lymphoid cell lines were established using cyclosporin A and Epstein-Barr virus as previously described.22 Cells were maintained by standard procedures in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum, 1% pencillin, and 1 mg/mL of streptomycin (GIBCO Laboratories, Grand Island, NY).
URO-synthase mutation analysis.
Genomic DNA was extracted from lymphoblasts using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) and each exon, including its intron/exon boundaries, was amplified by the polymerase chain reaction (PCR)23 using primer sets in which one primer was biotinylated as previously described.24 Each 50-uL amplification reaction contained 2 μg of genomic DNA; 20 pmol of each primer; 10 nmol/L of each dNTP; 50 mmol/L Tris-HCl, pH 9.0; 50 mmol/L NaCl; 10 mmol/L MgCl2; and 2 U of Taq polymerase (Promega, Madison, WI). After an initial 5-minute incubation at 94°C, amplification (30 cycles) was performed with denaturation at 94°C for 1 minute, extension at 72°C for 0.5 minutes, and annealing at the indicated conditions for each primer set.24 An aliquot (40 μL) of each amplification product was incubated with 40 μL of streptavidin-coated paramagnetic beads (Dynabeads M-280 Streptavidin; Dynal, Inc, Lake Success, NY) for 30 minutes with occasional gentle mixing. Beads with bound biotinylated PCR products were separated with a magnet as described,24the strands were denatured in 10 μL of 0.1 mol/L NaOH for 30 minutes, and the nonbiotinylated strands were eluted with two 50-μL washes of 0.1 mol/L NaOH. The biotinylated strands were washed with 50 μL of the binding and washing TE buffer (10 mmol/L tris-HC1, pH 7.5, containing 1.0 mmol/L EDTA) and 2.0 mol/L NaCl, and then with 50 μL of TE. The biotinylated strands were resuspended in 7 μL of H2O and used as templates for dideoxy chain sequencing.
Prokaryotic expression and characterization of the URO-synthase G188R mutation.
The normal and mutant G188R URO-synthase alleles were expressed inE coli strain JM 109 using the pKK223-3 vector (Pharmacia LKB Biotechnology, Inc, Piscataway, NJ) as previously described.6,24,25 Single colonies were isolated and the inserts were completely sequenced to confirm the engineered sequence and the absence of PCR errors. Bacterial growth, isopropylthiogalactoside induction, and URO-synthase assays were performed as previously described.24-26
Analysis of possible splicing abnormalities caused by G188R.
To determine if mutation G188R caused abnormal splicing of the patient’s URO-synthase RNA, reverse transcriptase (RT)-PCR was performed. mRNA was isolated from 5 × 107 lymphoid cells established from the patient’s white blood cells and from 5 × 107 cells of a control lymphoid line using the FastTrack mRNA Isolation Kit version 2.0 (Invitrogen, Carlsbad, CA). The mRNA was RT-PCR amplified using the Titan One Tube RT-PCR System (Boehringer Mannheim, Indianapolis, IN). Two different sets of PCR primers were used to detect the possible deletion of exon 9: one set amplified the URO-synthase cDNA from exon 6 to exon 10, producing a normal product of 416 bp, whereas the other set amplified a sequence from exon 8 to exon 10, producing a normal product of 223 bp. The sense primer in exon 6 was 5′-GCCTGGATACAGAAGGAGAA-3′ (cDNA nt 528-548) and the antisense primer in exon 10 was 5′-CTTGTGGCGTGGGGCTCTCT-3′ (cDNA nt 924-944). The sense primer in exon 8 was 5′-ATCCAAGGGAACCTGAACAG-3′ (cDNA nt 722-742) and the antisense primer was the above primer in exon 10. Amplification was performed according to the RT-PCR kit’s instructions. The RT-PCR products were electrophoresed in 2% agarose gels with 50-bp and 100-bp DNA ladders (Life Technologies, Gaithersburg, MD) as markers.
RESULTS
Mutation analysis and expression.
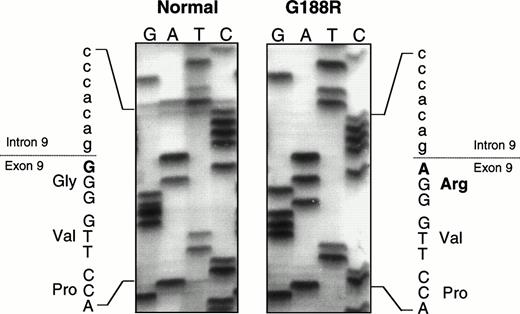
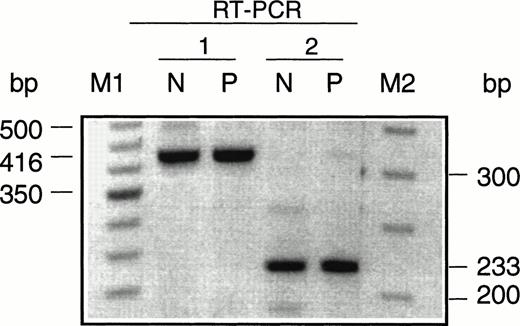
For genotype/phenotype analysis, the nature of the mutation(s) in the patient’s URO-synthase gene was determined as previously described.24,25 Sequencing of all exons and their adjacent intron/exon boundaries showed a single previously undescribed missense mutation in both alleles (Fig 2), a guanine to adenine transition at position 562 in the cDNA27 which predicted a glycine to arginine substitution at amino acid residue 188 (designated G188R). Both parents were found to be carriers of the G188R mutation. To confirm that this missense mutation altered URO-synthase function, the mutation was introduced into a prokarotyic vector and expressed in E coli. As shown in Table 2, the G188R construct expressed less than 5% of that expressed by the normal allele. To determine if this mutation in the first base of exon 9 also caused abnormal splicing, RT-PCR was performed using mRNA isolated from a lymphoid cell line established from the patient’s blood before BMT. Only PCR products that were the expected size were detected on agarose gels with both sets of primers used for RT-PCR (Fig 3), showing that the mutation did not cause abnormal splicing and skipping of exon 9.
Partial sequencing gel showing the G188R missense mutation, a G to A transition at cDNA nt 562, the first base of exon 9, which predicts the substitution of an arginine for a glycine.
Partial sequencing gel showing the G188R missense mutation, a G to A transition at cDNA nt 562, the first base of exon 9, which predicts the substitution of an arginine for a glycine.
Expression of URO-Synthase in E coli
| Construct . | URO-Synthase Activity (U/mg)* . | Percent of Mean Normal Level . | |
|---|---|---|---|
| Mean . | Range . | ||
| pKK-UROS | 73.7 | 60.4-80.0 | 100 |
| PKK-UROS-G188R | 3.20 | 2.35-4.58 | 4.3 |
| Construct . | URO-Synthase Activity (U/mg)* . | Percent of Mean Normal Level . | |
|---|---|---|---|
| Mean . | Range . | ||
| pKK-UROS | 73.7 | 60.4-80.0 | 100 |
| PKK-UROS-G188R | 3.20 | 2.35-4.58 | 4.3 |
Mean and range of activities for duplicate assays of three independent experiments induced for 3 hours with 5 mmol/L isopropylthiogalactoside. Mean background URO-synthase activity for expression of the pKK233-3 vector alone was 1.62 U/mg (range, 1.27 to 1.89 U/mg), which was subtracted from the activities of the expressed by the normal and G188R constructs.
Agarose gel electrophoresis of the URO-synthase RT-PCR products amplifed from total lymphoblast RNA from a normal individual (N) and the CEP patient (P). RT-PCR 1 used primers that amplified a normal 416-bp product from exon 6 to exon 10 and RT-PCR 2 used primers that amplified a 223-bp product from exon 8 to exon 10. Note that the RT-PCR products of the normal individual and the CEP patient were the same size.
Agarose gel electrophoresis of the URO-synthase RT-PCR products amplifed from total lymphoblast RNA from a normal individual (N) and the CEP patient (P). RT-PCR 1 used primers that amplified a normal 416-bp product from exon 6 to exon 10 and RT-PCR 2 used primers that amplified a 223-bp product from exon 8 to exon 10. Note that the RT-PCR products of the normal individual and the CEP patient were the same size.
BMT.
Allogeneic BMT was performed with marrow from the patient’s histocompatible healthy sister. For pretransplant conditioning, the patient received busulphan 16 mg/kg (total dose) for 4 days and cyclophosphamide 200 mg/kg (total dose). Cyclosporin A was administered for graft-versus-host disease (GVHD) prophylaxis. On June 21, 1995, bone marrow–nucleated cells (9 × 108/kg) were administered intravenously. The patient received granulocyte colony-stimulating factor (10 μg/kg) from day +1 to day +15 and anti-CMV hyperimmunoglobulins and acyclovir for 6 months. She developed a fever on day 3 and then purpura fulminans was noted on her hands and fingers. Staphylococcus aureus was cultured from her blood. She was treated with antibiotics, heparin, fresh frozen plasma, and high-dose methylprednisolone (30 mg/kg for 4 days). Her neutrophil count reached 500/μL on day 11. Subsequently, the purpura fulminans completely resolved without any necrosis. She continued to improve and was discharged on day 29 posttransplantation.
Beginning 1.5 months after BMT, there was a marked decrease in liver and spleen volumes and at the end of the first year posttransplant, liver and spleen were nonpalpable. There was also a dramatic improvement in her hematologic status, starting at 2 months posttransplant. Complete engraftment was documented at 8 months posttransplantation because 100% of the donor type apolipoprotein B DNA polymorphism28 was detected in peripheral leukocytes. No further blood transfusions were needed after the transplantation and her differential count has remained normal. A striking and sustained decrease in the urinary uroporphyrin and coproporphyrin levels also was observed (Table 1). At 35 months posttransplant, the patient was doing well with normal physical development, a normal hemogram, no evidence of cutaneous lesions on unlimited exposure to sunlight, or other CEP manifestations (Fig 1C).
DISCUSSION
This report documents the biochemical and clinical effectiveness of BMT in a severely affected patient with CEP and shows that successful BMT can cure the severe transfusion-dependent form of this inborn error of heme biosynthesis. Our patient, now 35 months posttransplantation, has maintained a normal hemoglobin level, markedly reduced urinary porphyrin levels, and most notably, the patient was able to resume a normal childhood, including exposure to sunlight without the development of cutaneous lesions. In addition, hepatospenomegly was no longer present. Previously, successful BMT was described in two older, less severe patients with CEP, the longest experience being only 12 months.16 17
Successful BMT in our patient resulted in remarkable biochemical and clinical improvement without GVHD. The level of plasma protoporphyrin decreased and the levels of serum and urinary uroporphyrins and coproporphyrins were markedly reduced and seemed to be decreasing with time. The post-BMT levels presumably reflected the abnormal porphyrin metabolism in extramedullary tissues such as the liver and kidneys. Because our patient and her donor were anti-CMV IgG positive, acyclovir and anti-CMV hyperimmunoglobulins were used for anti-CMV prophylaxis. No evidence of clinical CMV infection was observed during the period after transplantation. The only transplant complications were S aureus sepsis and purpura fulminans, which were successfully treated without necrosis. Purpura fulminans may have resulted from the S septicemia and hypercoagulable state after BMT (protein C, protein S, antithrombin III deficiencies, and factor V Leiden mutation were not found).29 However, the possible role of accumulated porphyrin metabolites in purpura fulminans is unknown.
Because the manifestations of CEP are highly variable, only severely affected patients should be considered for transplantation due to the morbidity and mortality associated with BMT. Most often, the diagnosis of CEP is made in the first days of life when the reddish porphyric urine is noted in the diaper. After biochemical confirmation, the major management issue is treatment, which ranges from BMT if an HLA-identical donor or cord blood is available, to observation, because nontransfusion-dependent patients may have only mild anemia and photosensitive cutaneous manifestations that can be avoided. Moderately affected patients may benefit from chronic transfusion therapy designed to maintain their hemocrit above 35 to suppress erythropoiesis.13,30 However, the benefits of this approach may decrease at puberty, perhaps because of hormonal changes that alter bone marrow and hepatic heme biosyntheses, thereby requiring more aggressive approaches like hydroxyurea treatment.14Determination of the patient’s genotype and prokaryotic expression of URO-synthase missense mutations has proven useful to estimate the function of the mutant enzyme, thereby permitting genotype/phenotype correlations.10,24 Such studies have shown that transfusion-dependent patients have more severe mutations, whereas patients with milder disease or only cutaneous lesions have at least one mutation that results in significant residual URO-synthase activity.10 In the milder cases, avoidance of sunlight and use of protective clothing will minimize the cutaneous manifestations. Thus, genotype determinations in newly diagnosed infants should be performed to predict the severity of the future clinical course, and in particular, the likelihood of transfusion dependence.
Mutation analysis showed that the patient described here was homoallelic for a previously undescribed URO-synthase missense mutation, G188R. Expression of the G188R allele in E colishowed that this mutation had markedly reduced enzymatic activity (Table 2), consistent with the patient’s transfusion-dependent phenotype. Also, because the missense mutation, a G to A transition, occurred in the first base of exon 9, it could potentially result in aberrant splicing and cause deletion of exon 9. However, RT-PCR studies did not detect aberrant splicing. This finding was consistent with the fact that either guanine or adenine can function as the first exonic base of intron-exon junctions in vertebrate genes.31 Thus, the homoallelism for the missense substitution of a positively charged hydrophilic arginine for a neutral glycine in the URO-synthase polypeptide impaired the enzyme’s function, resulting in a severe, transfusion-dependent phenotype.
The long-term effectiveness of BMT in CEP remains unknown. Whether BMT will provide sufficient Kupffer cells to offset the accumulation of type I porphyrin isomers in the liver will require further follow-up. However, the findings reported here suggest that BMT is presently the treatment of choice for CEP patients who are transfusion dependent from infancy or for newly diagnosed patients with genotypes predicting severe hemolytic disease, at least until gene therapy is developed. BMT is, of course, limited by availability of suitable bone marrow donors; however, this obstacle may be overcome with stored cord blood and bone marrow registries.32-35
ACKNOWLEDGMENT
We thank Y. Wu for mutation analysis, Prof Ayhan Gogmen for determination of porphyrin metabolites, and Raman Reddy for technical assistance.
Supported in part by grants from the National Institutes of Health, including a research grant (5 RO1 DK26824); a grant (2 MO1 RR00071) to the Mount Sinai General Clinical Research Center from the National Center for Research Resources; and a grant (5 P30 HD28822) to the Mount Sinai Child Health Research Center.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R.J. Desnick, PhD, MD, Professor and Chairman, Department of Human Genetics, Mount Sinai School of Medicine, Fifth Ave at 100th St, New York, NY 10029; e-mail:rjdesnick@vaxa.crc.mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal