To the Editor:
As antiproliferative agents, glucocorticoids (GCs) exert their effects by several mechanisms, the most significant of which being inhibition of cytokine production and action.1 In addition, GCs upregulate the expression of select proinflammatory cytokine receptors directly or indirectly in association with growth factors or other factors,2,3 thereby augmenting cytokine effects on target cells. This enhancement of cytokine receptor was specific for the GCs, required de novo receptor synthesis, and resulted in increased receptor density but not affinity.4,5 However, the effect of GCs on interleukin-2 receptor (IL-2R) remain controversial, because they were shown to either inhibit IL-2Rα6 or enhance high-affinity IL-2R directly or indirectly in association with IL-2,3 thus raising the possibility that the reduction in IL-2Rα expression may be due to earlier blockade of IL-2 synthesis by GCs. Furthermore, it was reported that GCs may decrease or increase IL-2R expression and hence the response to IL-2 stimulation depending on the extent of T-cell activation.7 The effect of GCs on IL-2R must then be viewed in the context of direct effect of GCs on IL-2R and the contribution of IL-2 blockade by GCs on subsequent IL-2R expression.
In light of the reported worsening of the outcome of diseases treated by GCs, including allograft rejection and autoimmunity, upon short-term/abrupt GCs withdrawal, which often necessitated brief pulses of high-dose GCs,8 we assessed the effect of GCs withdrawal on IL-2R expression and the subsequent response to recombinant IL-2 (rIL-2) stimulation. Our working hypothesis was that pre-exposure of activated T cells to GCs enhances cytokine receptor expression, thereby conferring a higher stimulation index upon restimulation with the respective ligand after GCs are withdrawn. To mimic GCs withdrawal, human peripheral blood T cells were treated with the GCs dexamethasone (DEX) or prednisolone (PRED); preactivated with mitogens (phytohemagglutinin [PHA] + phorbol myristate acetate [PMA]), receptor cross-linking antibodies (anti-CD28 Ab + PMA), or the CD3-bypass stimulation regimen PMA + ionomycin; and cultured for 4 to 48 hours at 37°C. The cells were then washed (at least 3× with phosphate-buffered saline [PBS] ) and reactivated with rIL-2. Cytokine receptor expression was determined by FACS analysis, and T-cell proliferation was quantitated by measuring the cellular uptake of tritiated thymidine.
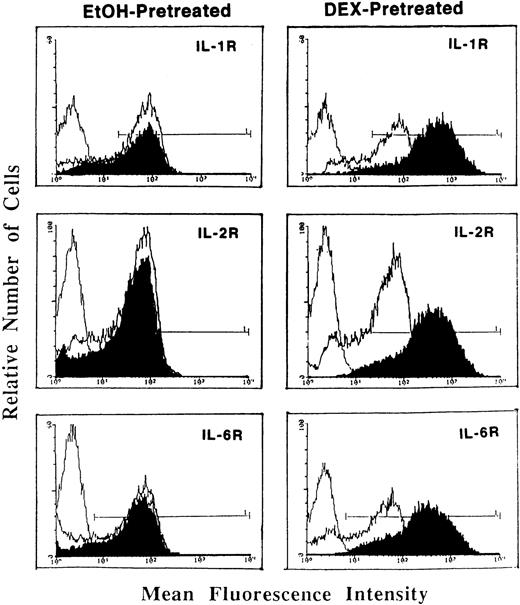
Whereas DEX and PRED directly suppressed IL-1, IL-2, IL-6, and other cytokine mRNA expression at the transcriptional and posttranscriptional levels,1 pretreatment of activated T cells with DEX (Fig1) or PRED (data not shown) resulted in a significant enhancement in (type I) IL-1R, IL-2Rα (CD25), and IL-6R (Fig 1). This GCs-induced increase in cytokine receptor expression was the result of enhanced mRNA expression for the three receptors (determined by reverse transcription-polymerase chain reaction [RT-PCR]) and was blocked by the antimetabolites actinomycin D and cycloheximide, indicating the requirement for de novo receptor synthesis.
Flow cytometric analysis of (type I) IL-1R, IL-2R (CD25), and IL-6R expression on T cells pretreated with DEX (0.1 μmol/L; right panels) or a corresponding volume of ethanol (left panels) and stimulated with PHA (5 μg/mL) + PMA (5 ng/mL; dark tracings). Light tracings represent cytokine receptor expression by PHA + PMA-stimulated cells; the first (light) tracing represents background fluorescence. Shown is a representative of three individually performed experiments.
Flow cytometric analysis of (type I) IL-1R, IL-2R (CD25), and IL-6R expression on T cells pretreated with DEX (0.1 μmol/L; right panels) or a corresponding volume of ethanol (left panels) and stimulated with PHA (5 μg/mL) + PMA (5 ng/mL; dark tracings). Light tracings represent cytokine receptor expression by PHA + PMA-stimulated cells; the first (light) tracing represents background fluorescence. Shown is a representative of three individually performed experiments.
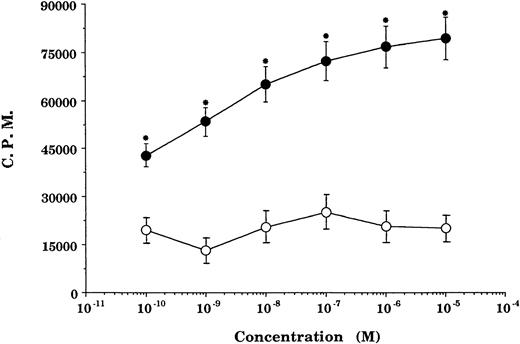
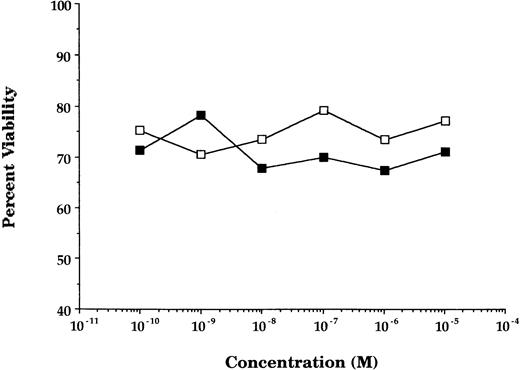
GCs enhancement of cytokine receptor expression resulted in augmented cytokine effect on GCs-pretreated cells. In GCs-pretreated and mitogen-preactivated T-cell cultures, stimulation with rIL-2 resulted in a significant concentration-dependent enhancement in proliferation (P < .05 v ethanol- or medium-pretreated cultures) at all concentrations of GCs (DEX) tested (Fig2). This enhancement in rIL-2–driven responses was not specific for the stimulation regimen (PHA + PMA) used in the preactivation phase, because comparable responses were obtained using anti-CD28 Ab + PMA or PMA + ionomycin as an alternative stimulation regimen. Furthermore, the enhancement in IL-2Rα (CD25) was not due to possible GCs-induced alteration in cell viability/apoptosis,9 because the cell viability of GCs-pretreated and PHA + PMA-preactivated cultures was essentially similar to that of ethanol-pretreated and preactivated cultures (Fig3).
Pretreatment with GCs enhances rIL-2–stimulated proliferation. Proliferation of T cells preactivated and pretreated with DEX (•) or a corresponding volume of ethanol (○) and reactivated with rIL-2 + PMA. Data points represent the mean ± SEM of four individually performed experiments. Proliferation of unstimulated cells (negative control), 1,023 ± 403; rIL-2 ± PMA-stimulated cells (positive control), 27,076 ± 2,944. *P< .05 versus ethanol-pretreated cells.
Pretreatment with GCs enhances rIL-2–stimulated proliferation. Proliferation of T cells preactivated and pretreated with DEX (•) or a corresponding volume of ethanol (○) and reactivated with rIL-2 + PMA. Data points represent the mean ± SEM of four individually performed experiments. Proliferation of unstimulated cells (negative control), 1,023 ± 403; rIL-2 ± PMA-stimulated cells (positive control), 27,076 ± 2,944. *P< .05 versus ethanol-pretreated cells.
Pretreatment with GCs does not alter cell viability. The percentage of viability (determined by trypan blue exclusion principle) of T cells activated and treated with DEX (▪) or a corresponding volume of ethanol (□). Data points represent the mean of 10 individually performed experiments.
Pretreatment with GCs does not alter cell viability. The percentage of viability (determined by trypan blue exclusion principle) of T cells activated and treated with DEX (▪) or a corresponding volume of ethanol (□). Data points represent the mean of 10 individually performed experiments.
In essence, GCs upregulation of cytokine receptor expression and the accompanying enhancement in cytokine-driven response (proliferation) are not transient events and are distinct from GCs effects on cytokine gene expression or on cytokine-driven responses, both of which are profoundly inhibited by GCs treatment. In contrast to suppression of cytokine expression and cytokine-stimulated responses that require continued GCs presence, this GCs-induced rebound phenomenon does not require continued GCs presence. Whereas washing off GCs from GCs-treated and stimulated cultures followed by resumption of activation restored cytokine expression at the transcriptional and posttranscriptional levels, it did not affect enhanced cytokine receptor expression; in fact, a further increase in receptor densities were detected in GCs-pretreated cultures. It is tempting to suggest that this GCs-induced rebound is due to receptor supersensitivity, where the expression of a specific receptor is upregulated in the face of lost ligand (cytokine) expression.
Although the conclusion reached here was based on in vitro observation using well-defined culture conditions; nevertheless, it provides a plausible explanation for the reported increased morbidity that frequently accompanies short-term GCs withdrawal. Although the cause of this GCs-induced rebound phenomenon is not yet completely understood, our study suggests that enhanced cytokine receptor expression on activated T cells (and other cell types, most notably macrophages, fibroblasts, and epithelial cells) by GCs,1 coupled with sustained cytokine secretion reinstated after removal of GCs, induces an exaggerated T-cell effector function that ultimately results in increased morbidity. This also explains the reported efficacy of high-dose GCs pulse or rapamycin (sirolimus) and other agents that act by suppressing cytokine-mediated signaling events, but not cyclosporin A, in controlling this GCs-induced rebound. Collectively, this underscores the dual nature of GCs in regulating cytokine and cytokine receptor expression; in essence, GCs suppress cytokine expression while enhancing the expression of their high-affinity receptors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal