Abstract
We investigated the oxidative state of low-density lipoprotein (LDL) in patients with β-thalassemia to determine whether there was an association with atherogenesis. Conjugated diene lipid hydroperoxides (CD) and the level of major lipid antioxidants in LDL, as well as modified LDL protein, were evaluated in 35 β-thalassemia intermedia patients, aged 10 to 60, and compared with age-matched healthy controls. Vitamin E and β-carotene levels in LDL from patients were 45% and 24% of that observed in healthy controls, respectively. In contrast, the mean amount of LDL-CD was threefold higher and lysil residues of apo B-100 were decreased by 17%. LDL-CD in thalassemia patients showed a strong inverse correlation with LDL vitamin E (r = −0.784; P < .0001), while a negative trend was observed with LDL-β–carotene (r = −0.443; P = .149). In the plasma of thalassemia patients, malondialdehyde (MDA), a byproduct of lipid peroxidation, was increased by about twofold, while vitamin E showed a 52% decrease versus healthy controls. LDL-CD were inversely correlated with plasma vitamin E (r = −0.659; P < .0001) and correlated positively with plasma MDA (r = 0.621; P < .0001). Plasma ferritin was positively correlated with LDL-CD (r = 0.583; P =.0002). No correlation was found between the age of the patients and plasma MDA or LDL-CD. The LDL from thalassemia patients was cytotoxic to cultured human fibroblasts and cytotoxicity increased with the content of lipid peroxidation products. Clinical evidence of mild to severe vascular complications in nine of the patients was then matched with levels of LDL-CD, which were 36% to 118% higher than the mean levels of the patients. Our results could account for the incidence of atherogenic vascular diseases often reported in β-thalassemia patients. We suggest that the level of plasma MDA in β-thalassemia patients may represent a sensitive index of the oxidative status of LDL in vivo and of its potential atherogenicity.
THE THALASSEMIAS ARE genetic disorders, which encompass a wide variety of clinical phenotypes, ranging in severity from clinically silent heterozygous β-thalassemia to severe transfusion-dependent thalassemia major. Among others, clinical features of thalassemias include vascular complications, such as pulmonary thromboembolism, cerebral thrombosis, and leg ulcers.1-3 Consistent with the pathogenesis of such events, the data indicate that injury of vascular endothelial cells is present in thalassemia patients.4
In recent years increasing evidence suggests that the oxidative modification of low-density lipoprotein (LDL) is the key step in the sequence of events leading to atherogenesis-related vascular alterations.5-7 Modified LDL are internalized in monocyte-derived macrophages through cell surface scavenger receptors, an event that leads to foam cell formation. Infiltration and deposition of these cells in the arterial wall are considered the initiating steps to develop atherosclerotic plaque.
Oxidation of polyunsaturated fatty acids (PUFA) is considered to be an important initiating factor in the alteration of LDL.8,9Decomposition products of PUFA gradually spread over to the protein moiety of apo B-100 and neutralize the positively charged lysil ε-amino groups.10 Little is known about the mechanism by which LDL then becomes oxidized in vivo. However, in β-thalassemia, such conditions as alteration of iron homeostasis,11interactions between ruptured erythrocytes and LDL,12,13depletion of antioxidant defenses14,15 and decrease of HDL particles16 might promote oxidative damage to circulating LDL. A recent report from our laboratory showed that LDL from β-thalassemia intermedia patients is more susceptible to the in vitro oxidation, as compared with LDL from healthy donors.15 All of the above evidence lends credence to the idea that circulating LDL from thalassemia patients may bear marked oxidative modifications.
We evaluated the extent of protein and lipid oxidation products and lipid antioxidants in LDL from patients with β-thalassemia intermedia, in which transfusion-dependent secondary iron overload is not a prominent cause of toxicity. In addition, because oxidized LDL are cytotoxic,17-19 cytotoxicity of LDL from thalassemia patients on human cultured fibroblasts was investigated as one basic parameter of its atherogenic potential. A relationship between the oxidative status of LDL and atherosclerotic vascular lesions observed in a number of patients is also reported.
MATERIALS AND METHODS
Subjects.
Twenty female and 15 male thalassemia intermedia patients, age 10 to 60 years (mean, 32 ± 14), who had been previously characterized for β-globin gene mutation were recruited for this study. Consent was obtained and individuals were under observation for 1 year. All patients were regularly interviewed and examined by a staff of physicians at intervals of 15 days to 1 month. Hemoglobin levels were 6.5 to 11.3 g/dL (mean, 8.33 ± 1.28) and patients received occasional transfusions (<3 to 6 per year). Ferritin was measured every 4 months, and cardiac, endocrinologic, and hepatologic evaluations were performed regularly. No patient was diabetic or hepatitis C virus positive or showed abnormal levels of serum alanine or aspartate aminotransferases. Some patients had experienced one or more of the following vascular complications: pulmonary hypertension (10 patients), cerebral ischemia (three patients), retinal vasculopathy (three patients), and ulcerative peripheral vasculopathy (one patient). Two of the patients were smokers. Patients were not on lipid-altering medications.
Clinical chemistry analyses.
After an overnight fast, blood from thalassemia patients was collected in EDTA (1 mg/mL-1). Blood samples from 35 apparently healthy individuals, aged 22 to 59, who were nonsmokers and who were not taking any medication, were used for the control group. Plasma was separated by centrifugation and divided in suitable aliquots to prepare LDL and perform the analytical determinations described below.
Total bilirubin, total cholesterol, and high-density lipoprotein (HDL) cholesterol were evaluated by using commercial analytical kits from Sigma (St Louis, MO). Concentration of plasma LDL cholesterol was calculated by the Friedwald formula.20 Ferritin was determined by an enzyme-immuno assay (Abbott Labs, North Chicago, IL).
Preparation of LDL.
LDL (1.019 to 1.063 g/mL) was isolated from EDTA plasma by stepwise ultracentrifugation at 4°C in a Beckman L8-70M ultracentrifuge fitted with a 50 Ti rotor using potassium bromide for density adjustments (Beckman, Palo Alto, CA), according to Kleinveld.21 The LDL fraction was shown to be free of other lipoproteins by electrophoresis on an agarose gel. EDTA and salts were removed from LDL by gel filtration on Sephadex G-25 Medium (Pharmacia Biotech, Milano, Italy).22 Proteins were determined by the Bio Rad colorimetric method.23 In typical preparations, 0.6 mg apo B-100 was obtained from 1 mL plasma. To prevent autoxidation reactions, LDL were used immediately or after an overnight storage at −70°C. Preliminary assays after overnight storage at −70°C showed that this treatment did not modify LDL composition as compared with freshly prepared LDL.
Apo B-100 analysis.
Apo B-100 lysine residues were evaluated after delipidation and acidic hydrolysis of the protein in 12 N HCl, for 18 hours at 100°C. Briefly, LDL samples (1.0 mg protein) in 1.0 mL 0.15 mol/L NaCl, mixed with 0.7 mL of a mixture of CHCl3:MeOH (2:1, vol:vol) in a Pyrex tube, were vortexed and then centrifuged at 3,000g for 10 minutes. The bottom CHCl3 layer was removed with a Pasteur pipette and discarded, and the extraction was repeated three times. The MeOH:water phase (and the residual CHCl3 were) was evaporated by placing the tubes in a boiling water bath, then the apo B precipitated on the tube walls was removed by the aid of 1.0 mL of 12 N HCl. Screw caps were tightened and hydrolysis was performed for 18 hours at 100°C in a boiling water bath. Residues in the hydrolysate were analyzed by a Beckman 6003 amino acid analyzer equipped with a Shimadzu Chromatopac C-R3A integrator (Shimadzu, Kyoto, Japan).
Biochemical analyses.
Malondialdehyde (MDA) was evaluated in 50 μL plasma samples by a colorimetric reaction with thiobarbituric acid (TBA, Sigma),24 followed by neutralization of samples with equivalent volumes of a mixture consisting of 4.5 mL 1.0 mol/L NaOH and 45.5 mL methanol. Isocratic high performance liquid chromatography (HPLC) separation of the MDA adduct was performed using a Supelco Supelcosil (Bellefonte, PA) LC-18 column (0.46 x 25 cm), eluted with 40% methanol in 50 mmol/L potassium phosphate buffer, pH 6.8, at 1.5 mL min-1. The MDA-TBA adduct was revealed at 532 nm and quantified by reference to a calibration curve of tetraethoxypropane (Sigma), submitted to the TBA colorimetric procedure. Butylated hydroxytoluene (0.03%) was added to the TBA reagent to prevent artifactual lipid peroxidation during the assay procedure. The conjugate diene lipid hydroperoxides in the lipid fraction of LDL (LDL-CD) were extracted from LDL samples (200 μg protein in 1.0 mL 0.15 mol/L NaCl) by 2.0 mL CHCl3:MeOH (2:1, vol:vol). The organic extract was dried under a nitrogen stream, resuspended in cyclohexane, and quantitated spectrophotometrically at 234 nm, using a molar absorption coefficient of 27,000.25 The results are expressed as nmol/mg LDL protein.
All-trans retinol and α-tocopherol were extracted from 200 μL of plasma samples, diluted to 1.0 mL with 0.15 mol/L NaCl, by mixing with 2 volumes of absolute ethanol, followed by two successive extractions with 6 and 2 volumes of petroleum ether. The organic extracts were gathered, dried under nitrogen, resuspended in several microliters of suitable solvent, and injected on top of an LC-18 HPLC column (see above). Analysis was performed by eluting with methanol at 1.0 mL min-1. Detection of all-trans retinol and α-tocopherol were at wavelengths of 320 nm and 290 nm, respectively. Under the conditions described, all-trans retinol eluted after 5.2 minutes and α-tocopherol after 12.8 minutes. Automatic wavelength change after 9 minutes allowed the detection of both compounds in the same sample. Alpha-tocopherol was extracted from LDL samples (50 μg protein in 1.0 mL phosphate-buffered saline [PBS]) and analyzed by HPLC as described above.
Beta-carotene was extracted from 500 μg LDL protein in a final volume of 1.0 mL PBS by mixing with 1 volume of methanol and 3 volumes of hexane:diethyl ether (1:1, vol:vol). The extracts were then dried under nitrogen, resuspended with several microliters of a mixture of acetonitrile:methanol:tetrahydrofurane (58.5:35:6.5, vol:vol:vol) and analyzed with the same solvent26 with an LC-18 Supelco column as above, at a flow rate of 2.5 mL min-1. Under these conditions, β-carotene eluted at 13.8 minutes. Revelation was at 450 nm.
Quantitation of all compounds evaluated by HPLC was performed by reference to standard curves constructed with 5 to 100 ng of each compound and by relating the amount of the compound under analysis to the peak area. All procedures were performed under dim red light to avoid artifactual photooxidation of lipids and to preserve light sensitive vitamins.
Test of the cytotoxicity of thalassemic LDL.
Human fibroblasts were obtained from small dermal specimens from the dorsal forearm of healthy donors. The epidermal layers were carefully removed and portions of the underlying dermis were cut into explants (1 mm3) and placed in flasks in complete medium (CM, GIBCO, Grand Island, NY) containing 10% heat-inactivated fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin. The flasks were incubated at 37°C with 5% CO2 and the medium changed twice weekly. When the fibroblasts were near confluence, the explanted tissue was removed, the cells trypsinized, and plated into 25 cm2 culture flasks in CM. Cytotoxicity experiments were performed with cells in passage 3 through 8, with a density of 1.7 to 2.4 × 105 cells per 35 mm culture dish. For the experiments, fibroblasts were trypsinized and plated into 35 mm culture dishes in CM with 10% heat-inactivated fetal calf serum 24 to 36 hours before the start of experimentation. After this period, all cultures were rinsed and 1 mL of either CM or CM containing 200 mg LDL protein was added. After 24 to 48 hours incubation, the cell viability was assessed on an aliquot of cell culture by Trypan blue exclusion test.
Statistical analysis.
All results are expressed as means ± standard deviation (SD). Comparison between controls and thalassemia patients was performed by the unpaired Student’s t-test. Pearson’s correlations were used to determine the relationships between covariates.
RESULTS
Hematologic data and values for major plasma lipid antioxidants of our thalassemia intermedia patients and healthy controls are summarized in Table 1. The mean concentration of serum ferritin was eight times and that of bilirubin four times higher than the control values, thus indicating the rather large hemolysis and increased iron absorption in the thalassemic patients (Table 1). Total cholesterol, as well as HDL and LDL cholesterol, appeared markedly lower than relevant controls, which is peculiar of the disease,27 28 whereas triglycerides were not significantly varied (Table 1). A marked decrease of lipid antioxidants such as vitamin A and vitamin E was observed. However, because of the strong fall in the cholesterol level, when normalized to plasma lipids (cholesterol + triglycerides), the lipid-corrected vitamin E and vitamin A did not appear significantly different with respect to control (Table 1).
Hematologic Data and Major Lipid Antioxidants in Plasma From β-Thalassemia Intermedia Patients and Controls
| . | Controls n = 35 . | β-Thalassemia Patients n = 35 . | P . |
|---|---|---|---|
| Ferritin (ng/mL) | 80 ± 10 | 687 ± 343 | <.001 |
| Bilirubin (mg/dL) | 0.68 ± 0.16 | 2.7 ± 1.36 | <.001 |
| Total cholesterol (mmol/L) | 5.53 ± 0.18 | 2.78 ± 0.51 | <.001 |
| HDL cholesterol (mmol/L) | 1.42 ± 0.15 | 0.85 ± 0.16 | <.001 |
| LDL cholesterol (mmol/L) | 3.62 ± 0.41 | 1.42 ± 0.20 | <.001 |
| Triglycerides (mmol/L) | 1.17 ± 0.27 | 1.22 ± 0.30 | .466 |
| Vitamin E (μmol/L) | 18.9 ± 4.0 | 9.26 ± 2.94 | <.001 |
| Vitamin A (μmol/L) | 1.79 ± 0.13 | 1.43 ± 0.7 | .004 |
| Lipid-corrected vitamin E* | 2.82 ± 0.55 | 2.31 ± 0.99 | .010 |
| Lipid-corrected vitamin A* | 0.26 ± 0.07 | 0.30 ± 0.1 | .057 |
| . | Controls n = 35 . | β-Thalassemia Patients n = 35 . | P . |
|---|---|---|---|
| Ferritin (ng/mL) | 80 ± 10 | 687 ± 343 | <.001 |
| Bilirubin (mg/dL) | 0.68 ± 0.16 | 2.7 ± 1.36 | <.001 |
| Total cholesterol (mmol/L) | 5.53 ± 0.18 | 2.78 ± 0.51 | <.001 |
| HDL cholesterol (mmol/L) | 1.42 ± 0.15 | 0.85 ± 0.16 | <.001 |
| LDL cholesterol (mmol/L) | 3.62 ± 0.41 | 1.42 ± 0.20 | <.001 |
| Triglycerides (mmol/L) | 1.17 ± 0.27 | 1.22 ± 0.30 | .466 |
| Vitamin E (μmol/L) | 18.9 ± 4.0 | 9.26 ± 2.94 | <.001 |
| Vitamin A (μmol/L) | 1.79 ± 0.13 | 1.43 ± 0.7 | .004 |
| Lipid-corrected vitamin E* | 2.82 ± 0.55 | 2.31 ± 0.99 | .010 |
| Lipid-corrected vitamin A* | 0.26 ± 0.07 | 0.30 ± 0.1 | .057 |
Results are expressed as mean ± SD. P values were determined by the Student’s t-test. Hematologic data from thalassemia patients are the mean of the values from each patient during 1 year of observation. LDL cholesterol was calculated by the Friedwald formula: LDL cholesterol (mmol/L) = Total cholesterol (mmol/L) − [HDL cholesterol (mmol/L) + (0.42 × triglycerides (mmol/L)]).
Lipid-corrected -vitamin E or -vitamin A refers to the ratio vitamin E or vitamin A (μmol/L)/[total cholesterol + triglycerides (mmol/L)]. Values are obtained from determinations performed in duplicate on blood samples from different subjects. Each thalassemia patient contributed the mean of two values measured during 1 year of observation.
Oxidation parameters in plasma and LDL and the major lipid LDL-antioxidants of thalassemia patients are shown in Table 2. Plasma lipid peroxides measured as MDA were about twofold that of healthy controls. CD lipid hydroperoxides in LDL from β-thalassemia patients ranged from 4.63 to 49.34 nmol/mg LDL protein (mean amount, 22.60 ± 12.84) and were significantly higher than the CD found in control LDL (6.25 ± 3.04 nmol/mg LDL protein, range, 4.03 to 10.5, Table 2).
Plasma MDA and Oxidation Indices and Lipid Antioxidants in LDL From β-Thalassemia Patients and Control Subjects
| . | Controls n = 35 . | β-Thalassemia n = 35 . |
|---|---|---|
| Plasma MDA (μmol/L) | 0.90 ± 0.28 | 1.80 ± 0.59* |
| LDL-conjugated dienes (nmol/mg LDL prot) | 6.25 ± 3.04 | 22.60 ± 12.84* |
| Apo B lysine residues (nmol/mg LDL prot) | 500 ± 12 | 416 ± 24* |
| LDL-Vitamin E (nmol/mg LDL prot) | 13.83 ± 3.63 | 6.31 ± 3.21* |
| LDL-β-carotene (nmol/mg LDL prot) | 0.66 ± 0.2 | 0.16 ± 0.12* |
| . | Controls n = 35 . | β-Thalassemia n = 35 . |
|---|---|---|
| Plasma MDA (μmol/L) | 0.90 ± 0.28 | 1.80 ± 0.59* |
| LDL-conjugated dienes (nmol/mg LDL prot) | 6.25 ± 3.04 | 22.60 ± 12.84* |
| Apo B lysine residues (nmol/mg LDL prot) | 500 ± 12 | 416 ± 24* |
| LDL-Vitamin E (nmol/mg LDL prot) | 13.83 ± 3.63 | 6.31 ± 3.21* |
| LDL-β-carotene (nmol/mg LDL prot) | 0.66 ± 0.2 | 0.16 ± 0.12* |
Values are the mean ± SD of determinations performed in duplicate on blood samples and homologous LDL from different subjects. Each thalassemia patient contributed the mean of two values measured during 1 year of observation.
With respect to the relevant control, values were significant withP < .001 (Student’s t-test).
LDL oxidation involves the loss of lysine ε-amino groups from apo B-100. Quantitative analysis of lysine residues in LDL samples showed a mean loss of 17% (Table 2) in LDL in the thalassemia patients. Table 2also shows the concentration of the major LDL-associated antioxidants. The mean amount of vitamin E and β-carotene in LDL from patients was 48% and 24% of controls, respectively.
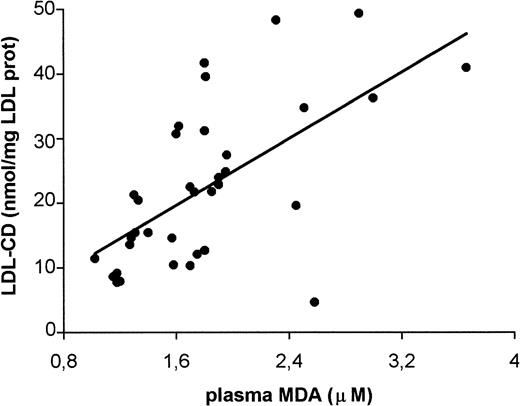
The amount of CD hydroperoxides in LDL from thalassemia patients showed a strong inverse correlation with both plasma vitamin E (r = −0.659; P < .0001) and vitamin E in LDL (r = −0.784; P < .0001) (Fig 1). A negative trend was observed with β-carotene in LDL (r = −0.443; P = .149, not shown). A positive correlation was found between LDL-CD and plasma MDA (r = 0.621; P < .0001, Fig 2). Plasma ferritin positively correlated with CD hydroperoxides in LDL (r = 0.583; P = .0002, Fig 3). No correlation existed between either LDL-CD or plasma MDA and the age of patients.
Correlation between conjugated diene lipid hydroperoxides in LDL (LDL-CD) and the amounts of vitamin E in plasma (A) and in LDL (B) from β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of plasma vitamin E (n = 35; A: r = −.659; P < .0001; B: r = −.784; P < .0001).
Correlation between conjugated diene lipid hydroperoxides in LDL (LDL-CD) and the amounts of vitamin E in plasma (A) and in LDL (B) from β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of plasma vitamin E (n = 35; A: r = −.659; P < .0001; B: r = −.784; P < .0001).
Correlation between conjugated diene lipid hydroperoxides in LDL (LDL-CD) and plasma malondialdehyde (MDA) from β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of plasma MDA (n = 35; r = .621; P < .0001).
Correlation between conjugated diene lipid hydroperoxides in LDL (LDL-CD) and plasma malondialdehyde (MDA) from β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of plasma MDA (n = 35; r = .621; P < .0001).
Correlation between ferritin and LDL-CD in β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of ferritin (n = 35; r = .583; P = .0002).
Correlation between ferritin and LDL-CD in β-thalassemia intermedia patients. Each blood sample was simultaneously processed for isolating and analyzing LDL and for the analysis of ferritin (n = 35; r = .583; P = .0002).
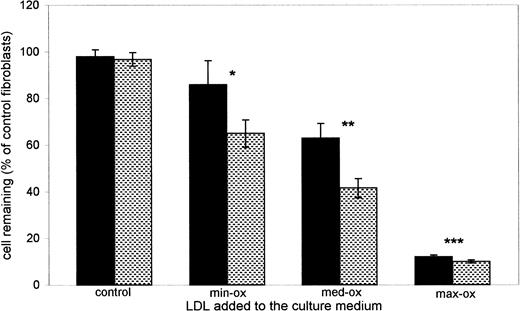
Content of lipid peroxidation products in LDL has been linked to the cytotoxic potency of LDL.29 30 The cytotoxicity of LDL from thalassemia patients was therefore assayed by incubating LDL with cultured fibroblasts for 24 to 48 hours. When compared with the fibroblasts incubated with the culture medium alone, the viability of fibroblasts did not appear affected by incubation with 200 μg protein of control LDL (Fig 4). No significant toxicity was demonstrated by control LDL even at a protein amount of 300 μg (not shown). On the contrary, incubation with LDL from thalassemia patients caused a decrease in cell viability, which increased with the extent of the oxidative modification of LDL. Minimum oxidized LDL (min-ox LDL, average LDL-CD 10.41 ± 1.58 nmol/mg LDL protein; range, 4.63 to 13.16) caused a decrease of cell viability of about 14% after a 24-hour incubation and of 35% after 48 hours. Exposure of cultured cells to medium-oxidized LDL (med-ox LDL, average LDL-CD 20 ± 3.41 nmol/mg LDL protein; range, 14.58 to 27.45) determined a loss of viable cells of 37% and 60% after 24 and 48 hours, respectively. After incubation with maximally modified LDL (max-ox LDL average LDL-CD 32.91 ± 4.7 nmol/mg LDL protein; range, 30.7 to 49.37), the survival of fibroblasts was about 10% after 24 hours and did not differ significantly after 48 hours (Fig 4).
Cytotoxicity of LDL to cultured human fibroblasts. Treatment of fibroblasts was as in Materials and Methods. Bars refer to the percent of viable fibroblasts remaining after incubation with 200 μg protein of either control LDL, or LDL from β-thalassemia intermedia patients, for 24 hours (full bars) or 48 hours (shaded bars). Each value is the mean ± SD of values obtained with n LDL samples from different healthy controls or patients, each examined in duplicate. Control LDL, n = 12; minimum oxidized LDL (min-ox LDL), n = 11; medium oxidized LDL (med-ox LDL), n = 14; maximum oxidized LDL (max-ox LDL), n = 10. With respect to fibroblasts incubated for the relevant time * with control LDL, P < .001; ** with min-ox LDL, P < .001; *** with med-ox LDL, P < .001; Student’s t-test.
Cytotoxicity of LDL to cultured human fibroblasts. Treatment of fibroblasts was as in Materials and Methods. Bars refer to the percent of viable fibroblasts remaining after incubation with 200 μg protein of either control LDL, or LDL from β-thalassemia intermedia patients, for 24 hours (full bars) or 48 hours (shaded bars). Each value is the mean ± SD of values obtained with n LDL samples from different healthy controls or patients, each examined in duplicate. Control LDL, n = 12; minimum oxidized LDL (min-ox LDL), n = 11; medium oxidized LDL (med-ox LDL), n = 14; maximum oxidized LDL (max-ox LDL), n = 10. With respect to fibroblasts incubated for the relevant time * with control LDL, P < .001; ** with min-ox LDL, P < .001; *** with med-ox LDL, P < .001; Student’s t-test.
Atherosclerotic vascular lesions are frequent in β-thalassemia intermedia.1-4 Nine of our patients showed evidence of atherogenesis-related vascular complications. A description of the patients including plasma MDA and LDL-CD values are reported in Table 3. It is noteworthy that the lipid peroxidation products in LDL are 36% to 118% higher than the mean level of the thalassemia patients as a group.
Characterization of Patients Showing Atherogenesis-Related Vascular Complications
| Patients3-150 . | Sex/Age . | Blood Transfusions Per Year . | Ferritin3-151 (ng/mL) . | Vascular Complication . | LDL-CD3-151 (nmol/mg LDL prot) . | Plasma MDA3-151 (nmol/mL) . |
|---|---|---|---|---|---|---|
| 1 | M/33 | 6 | 1,100 | Ulcerative peripheral vasculopathy | 36.26 | 3.00 |
| 2 | F/29 | 6 | 950 | Severe cerebral ischemia | 48.33 | 2.31 |
| 3 | M/41 | <3 | 1,350 | Pulmonary hypertension | 34.7 | 2.51 |
| Retinal vasculopathy | ||||||
| 4 | M/31 | 6 | 1,050 | Pulmonary hypertension | 31.94 | 1.65 |
| 5 | F/42 | <3 | 750 | Severe cerebral ischemia | 39.58 | 1.81 |
| 6 | M/27 | <3 | 450 | Pulmonary hypertension | 31.17 | 1.80 |
| 7 | F/12 | <3 | 450 | Pulmonary hypertension | 30.7 | 1.70 |
| 8 | M/34 | <3 | 1,900 | Pulmonary hypertension | 41.6 | 1.80 |
| Retinal vasculopathy | ||||||
| 9 | M/40 | <3 | 900 | Pulmonary hypertension | 45.38 | 2.90 |
| Patients3-150 . | Sex/Age . | Blood Transfusions Per Year . | Ferritin3-151 (ng/mL) . | Vascular Complication . | LDL-CD3-151 (nmol/mg LDL prot) . | Plasma MDA3-151 (nmol/mL) . |
|---|---|---|---|---|---|---|
| 1 | M/33 | 6 | 1,100 | Ulcerative peripheral vasculopathy | 36.26 | 3.00 |
| 2 | F/29 | 6 | 950 | Severe cerebral ischemia | 48.33 | 2.31 |
| 3 | M/41 | <3 | 1,350 | Pulmonary hypertension | 34.7 | 2.51 |
| Retinal vasculopathy | ||||||
| 4 | M/31 | 6 | 1,050 | Pulmonary hypertension | 31.94 | 1.65 |
| 5 | F/42 | <3 | 750 | Severe cerebral ischemia | 39.58 | 1.81 |
| 6 | M/27 | <3 | 450 | Pulmonary hypertension | 31.17 | 1.80 |
| 7 | F/12 | <3 | 450 | Pulmonary hypertension | 30.7 | 1.70 |
| 8 | M/34 | <3 | 1,900 | Pulmonary hypertension | 41.6 | 1.80 |
| Retinal vasculopathy | ||||||
| 9 | M/40 | <3 | 900 | Pulmonary hypertension | 45.38 | 2.90 |
No patient was a smoker.
Values are the mean of two determinations performed in duplicate on blood samples and homologous LDL during 1 year of observation.
DISCUSSION
Oxidative stress is a consequence of the disease process in β-thalassemia.31-34 This is evident as an increase of plasma lipid peroxidation products such as MDA and a marked decrease of plasma lipid antioxidants such as vitamin E and vitamin A, as compared with healthy controls.14 The presence of MDA in plasma suggests that circulating lipoprotein particles are enriched in oxidatively modified components.35 In accordance with this hypothesis, our quantitative evaluation of LDL oxidation shows high amounts of CD lipid hydroperoxides in LDL from patients. At the same time, oxidation of the apo B is indicated by the loss of specific lysil residues.
If LDL is exposed to prooxidative conditions, it becomes depleted of its antioxidants, with α-tocopherol being the first to be lost and β-carotene the last.36 We found a strong depletion of these antioxidants in LDL from thalassemia patients, which was inversely correlated with the level of conjugated diene lipid hydroperoxides in LDL. Hence, due to the ongoing oxidative stress in β-thalassemia, plasma antioxidant defenses are overwhelmed and LDL is no longer adequately protected and undergoes oxidation. On the basis of the conjugated dienes and lysil residues measured in LDL from thalassemia intermedia patients and from healthy controls, the mean amount of oxidized LDL in the patients may be calculated in the range 17% to 27%.
The characteristics of oxidized LDL are under extensive investigation, as oxidation of lipoproteins and damage to vascular wall constituents have been identified as early events in the pathogenesis of atherosclerosis.29,30 A number of studies have focused on the capacity of oxidized LDL to injure cultured vascular cells, fibroblasts and macrophages, and rat endothelial cells in vivo,17-19 37 pointing to its role in the atherogenetic disease. In accordance, we found that LDL from thalassemia patients is cytotoxic to cultured human fibroblasts, with the level of cytotoxicity well correlated to the content of CD lipid hydroperoxides.
Risk factors for high levels of oxidized LDL are not well established and may be important in identifying individuals who may benefit from antioxidant supplementation. The suggestion that plasma MDA may be taken into account as a biomarker of oxidative stress in exposed populations has been recently put forward.38 Because plasma MDA correlates positively with LDL-CD in thalassemia patients, an interesting suggestion from our analysis is that this plasma lipid peroxidation marker can be useful for predicting the potential cytotoxicity and possibly the atherogenicity of thalassemic LDL. This may be recommended in thalassemia patients, in that traditional lipid and lipoprotein risk factors could be biased because of the altered lipid pattern. According to our prediction, nine of our β-thalassemia intermedia patients, with clinical evidence of severe atherogenesis-related vascular complications exhibit very high levels of LDL-CD and have plasma MDA levels twofold to threefold higher than control.
Intervention to impair oxidative modifications of LDL may be proven of benefit in the attenuation of atherosclerotic processes. Vitamin E administration to selected thalassemia intermedia patients has recently started at our center.
Such features of thalassemia as hemolysis, iron loading, and the increased iron absorption due to ineffective erythropoiesis could have a role in the observed LDL oxidation. Although the correlation between plasma ferritin and LDL-CD suggests an involvement of high iron levels, it is difficult to decide whether this is the only factor or the most prominent factor that promotes oxidized LDL production in thalassemia patients.
Iron accumulation is involved in cardiac injury,39 but its role in the oxidation of LDL and development of atherogenesis-related pathologies is doubtful.40 Serum iron and iron stores, expressed as elevated ferritin levels, have been implicated in coronary artery disease.41-44 The interaction between iron, oxygen free radicals and LDL, leading to oxidized LDL particles, progression of atherosclerosis, and finally to acute myocardial infarction has been hypothesized to account for this evidence. However, recent epidemiologic studies showed that moderately elevated serum ferritin concentrations (200 to 500 μg/mL) are a strong risk factor for acute myocardial infarction,45 a finding that was not associated with atherogenic LDL. In addition, premature atherosclerosis is not a prominent feature in hemochromatosis,46 a common genetic disease causing severe iron accumulation in plasma and liver, although congestive heart failure is characteristic of these patients. This suggests that the elevation of iron alone may not bring about the free radical reactions causing oxidative stress to LDL and would indicate that additional factors are required. Unpaired hemoglobin chains and red blood cell hemolysis products may have more importance.12 This is supported by very recent in vitro studies47 in which oxidative interactions of hemoglobin α-chains with LDL apo B serve as triggers of oxidative modification of LDL. It is also supported by consideration of the increased in vivo oxidation of LDL in uremic patients undergoing hemodialysis,48,49 a practice in which chronic hemolysis has been demonstrated in vitro and in vivo.50,51 As further evidence, although the oxidative status of LDL has not been investigated in subjects affected by sickle cell anemia, another hemolytic disorder in which reactive iron is produced, clinical parameters establish these patients to be at risk for atherogenesis.52 53 It may be worthwhile to investigate to what extent the transfusion-dependent secondary iron overload would affect the oxidative status of circulating LDL in thalassemia major patients. Oxidized LDL could further contribute to the pathogenesis of the heart disease related to the myocardial iron storage.
ACKNOWLEDGMENT
The cooperation of the staff of the “Servizio Talassemia,” Ospedale V. Cervello di Palermo is gratefully acknowledged.
Supported by Assessorato Sanità Regione Sicilia and Grant No. CNR 95.04669.ST75.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M.A. Livrea, PhD, Istituto di Farmacologia e Farmacognosia, Via C. Forlanini, 1, 90134 Palermo, Italy; e-mail mal96@mbox.unipa.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal