Abstract
B-cell chronic lymphocytic leukemia (B-CLL) and autoimmune disease are a related event, and genetic factors are linked to both diseases. As B-CLL is mainly of B-1 cell type that participates in autoantibody production, genetically-determined regulatory abnormalities in proliferation and/or differentiation of B-1 cells may determine their fate. We earlier found that, in H-2–congenic (NZB × NZW) F1 mice, while H-2d/z heterozygosity predisposes to autoimmune disease, H-2z/z homozygosity predisposes to B-CLL. Studies also suggested the involvement of non–H-2-linked NZW allele(s) in leukemogenesis. Using H-2–congenic NZW and B10 mouse strains, their F1 and backcross progeny, we have now identified three major NZW susceptibility loci for abnormal proliferation of B-1 cells, which form the basis of leukemogenesis; one H-2–linked locus on chromosome 17 and the other two non–H-2-linked loci, each on chromosome 13 and chromosome 17. Each susceptibility allele functioned independently, in an incomplete dominant fashion, the sum of effects determining the extent of aberrant B-1 cell frequencies. The development of leukemia was associated with age-related increase in B-1 cell frequencies in the blood. Thus, these alleles probably predispose B-1 cells to accumulate genetic alterations, giving rise to B-CLL. Potentially important candidate genes and correlation of the findings with autoimmune disease are discussed.
CHRONIC LYMPHOCYTIC leukemia (CLL), mostly the B-cell type, has two intriguing characteristics; one, the significance of hereditary factors in the pathogenesis, and the other, frequent association of immunologic abnormalities, including autoimmune diseases among patients or their family members. First-degree relatives of patients develop B-CLL or other lymphoid neoplasms at more than three times as high a risk as found in the general population.1 While B-CLL is the most common adult leukemia in Caucasians, it is rare in Asians, including those who immigrated to the United States.2-5 The frequent association of autoimmunity with B-CLL has been well described. Patients with B-CLL or their family members frequently have immunologic abnormalities, including autoimmune hemolytic anemia, thrombocytopenic purpura, systemic lupus erythematosus (SLE), and Sjögren’s syndrome.6-11 Patients with B-CLL often share common HLA haplotypes with relatives who have autoimmune diseases.12
Evidence suggesting that most B-CLL cells originate from the CD5+ B (B-1) cell lineage has implicated B-1 cells as a crossroad between B-CLL and autoimmune diseases.13,14B-CLL cells and intact B-1 cells share several characteristics, including production of autoantibodies,15-20 usage of rather restricted repertoires of nonmutated Ig V genes,21-24 cross-reactive idiotypes,13,20,25 and surface markers.26,27Murine B-1 cells, which belong to a developmental lineage distinct from that of conventional B (B-2) cells, have unique surface phenotypes and are localized in distinctly different patterns from conventional B cells; these cells are maintained by a self-renewal capacity.28,29 B-1 cells, in mice and in humans, participate in immune systems mainly by providing natural immunity and even autoimmunity, as opposed to conventional B-2 cells in acquired immunity. B-1 cells produce most IgM natural antibodies, the majority of which are polyreactive and cross-react with a variety of self-antigens.29,30 At least part of the B-1 cell repertoire appears to be selected and maintained by self-antigens and can protect themselves from elimination by bystander activated T cells through downregulation of Fas levels.31 Further selection, class-switch, and affinity maturation of such a long-lived B-1 cell repertoire are implicated in the development of highly pathogenic autoantibodies in genetically susceptible autoimmune disease-prone strains of mice.32,33 Hence, it is highly likely that certain regulatory abnormalities in proliferation and/or differentiation of B-1 cells are involved in both B-CLL and autoimmune disease.14
In earlier studies on newly established H-2-congenic New Zealand mouse strains, NZB, NZW, and (NZB x NZW) F1, carrying either homozygous or heterozygous haplotypes of H-2dderived from NZB and H-2z from NZW, we provided evidence that different, but related major histocompatibility complex (MHC) haplotypes predispose either to autoimmune disease resembling SLE or to B-CLL, in which while H-2d/z heterozygosity acts as one genetic predisposing element for SLE,34,35H-2z/z homozygosity acts as one element for B-CLL.36,37 For example, while H-2d/zheterozygous (NZB x NZW) F1 mice spontaneously developed SLE in association with pathogenic, high affinity IgG anti-DNA antibodies,H-2z/z homozygous mice, which differ from the former at only one locus or a cluster of loci, did not, but instead developed abnormal proliferation of B-1 cells both in the peripheral blood and in lymphoid tissues. Such B-1 cells showed an age-dependent oligoclonal to monoclonal expansion, giving rise to B-CLL.37 B-CLL also developed in H-2z/zhomozygous NZB and NZW, hence a gene or a cluster of genes located in the vicinity of NZW H-2z was suggested to play a critical role in the process of leukemogenesis.
In these studies, we also noted that the H-2-congenic B10.NZW strain carrying homozygous H-2zhaplotype38 did not manifest as much increase in peripheral B-1 cell frequencies as that seen inH-2z-homozygous NZW and NZB, and B-CLL did not occur. Thus, it is clear that, in addition to theH-2z-linked gene, non–H-2z-linked gene(s) also controls abnormal proliferation and subsequent leukemogenesis of B-1 cells in New Zealand mouse strains. Taking advantage ofH-2–congenic strains and their crosses and of analyses using microsatellite markers as tools for genome-wide linkage studies,39 we did chromosomal mapping of susceptibility alleles and analyzed their patterns of inheritance.
MATERIALS AND METHODS
Mice.
NZW and B10.D2 mice were originally obtained from the Shizuoka Laboratory Animal Center (Shizuoka, Japan). NZW.H-2d strain was established by selective backcrossing of the (NZB x NZW) F1 hybrid to NZW for 12 generations.35 B10.NZW mice were kindly donated by Dr J. Klein, Max-Planck-Institute for Biology, Tubingen, Germany.38 The F1 hybrid mice between strains NZW, B10.D2, and B10.NZW and the (NZW x B10.NZW) x B10.NZW backcross progeny were bred and maintained in our animal facility. Genotyping of backcross progeny was performed as described below. Only female mice were used in the present studies.
Cell counts and cytologic examination.
Peripheral blood was taken from the periorbital sinus. White blood cell counts were performed using a MEK-6158 Automatic Blood Cell Counter (Nihon Koden, Tokyo, Japan) according to the manufacturer’s instructions. White blood cell-rich populations were separated from 40 μL of heparinized blood using a density gradient lymphocyte separator M-SMF (Japan Immuno Research Laboratories Co, Ltd, Takasaki, Japan). Leukocyte film for cytologic examination was prepared using the Cytospin 3 Cell Preparation System (Shandon Scientific Ltd, Cheshire, UK) and stained with Giemsa.
Flow cytometry.
Peripheral blood was taken from the periorbital sinus, followed by lysis of red blood cells with ammonium chloride. For flow cytometric analysis, aliquots of 5 to 10 × 105 cells in 20 μL of phosphate-buffered saline (pH7.4) supplemented with 0.2% bovine serum albumin and 0.05% NaN3 were incubated with fluorescein isothiocyanate (FITC)-labeled rat antimouse CD5 (clone 53-7.3) monoclonal antibodies and biotinylated rat antimouse CD45R (B220) (clone RA3-6B2) antibodies, followed by phycoerythrin (PE)-avidin (Becton-Dickinson, Mountain View, CA). All incubations were run for 30 minutes at 4°C. The stained cells were examined using FACStar (Becton Dickinson), equipped with the FITC/PE filter system.
Genotyping of mice.
Genomic DNA was extracted from murine tail skins using standard techniques. Chromosomal markers consisting of simple-sequence length polymorphisms were identified by polymerase chain reaction (PCR).39 The primers were purchased from Research Genetics (Huntsville, AL). PCRs were performed in the presence of radioactively-labeled primers with [γ-32P] adenosine triphosphate (ATP), using T4 kinase (Takara Shuzo, Kyoto, Japan) according to the manufacturer’s instruction. A 40-ng aliquot of genomic DNA was amplified in 10 μL of PCR solution containing Taq polymerase (Takara Shuzo). A three temperature PCR protocol (94°C, 55°C, and 72°C, 25 to 30 cycles or 94°C, 50°C, and 72°C, 35 cycles) was conducted in a Geneamp 9600 Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT). PCR products were diluted twofold with loading buffer consisting of xylene cyanol and bromophenol blue dyes in 100% formamide and were run on 7% polyacrylamide gels. After electrophoreses, gels were dried and examined using a Bio-imaging analyzer BAS 2000 (Fuji Film, Tokyo, Japan).
Statistics.
The linkage of a particular locus with increased peripheral frequency of B-1 cells was estimated using χ2 analysis with a standard (2 × 2) contingency matrix. Interval mapping was done using MAPMAKER/EXP and MAPMAKER/QTL40 to identify chromosomal locations of quantitative trait loci (QTL). The likelihood ratio statistic (base-10 lod score) of ≥1.9 and ≥3.3 was used as thresholds for statistically suggestive and significant linkage, respectively, and support intervals were determined according to Lander et al.41 42 Analysis of variance (ANOVA) was used to determine the difference in B-1 cell frequencies among each group with different combinations of susceptibility alleles.
RESULTS
Involvement of both H-2z–linked and nonlinked genes in aberrant B-1 cell proliferation in peripheral blood of New Zealand mice.
Table 1 compares age-associated changes in the proportion of peripheral blood CD5+ B (B-1) cells per total B cells in mouse strains with different genetic backgrounds, NZW (H-2z/z), NZW.H-2d(H-2d/d), B10.NZW (H-2z/z), B10.D2 (H-2d/d), (NZW x B10.NZW) F1 (H-2z/z), (NZW x B10.D2) F1 (H-2z/d), and (NZW x B10.NZW) F1 x B10.NZW backcross mice at 8, 12, and 16 months of age. H-2z-homozygous NZW mice at any given age showed much higher B-1 cell frequencies compared with NZW.H-2d mice, a finding consistent with our earlier observation that the H-2z-linked gene(s), provisionally designated Bpal-1 (B1 cell proliferation-associated locus), acts as one major predisposing genetic element for abnormal proliferation of B-1 cells.36,37Because, the H-2-congenic NZW.H-2d strain was established by selective backcrossing of (NZW x NZB) F1 to NZW for 12 generations,35Bpal-1 is estimated to be located within or in close proximity of the H-2 complex. Compared with findings in H-2z/z-homozygous NZW, however, B-1 cell frequencies in H-2z/z-homozygous B10.NZW mice at any given age were much less, indicating that an additional non–H-2-linked NZW gene or genes are also involved.
Frequencies of Peripheral Blood B-1 Cells in Control and Backcross Mice
| Strains . | H-2 Haplotypes . | Age (months) . | ||
|---|---|---|---|---|
| 8 . | 12 . | 16 . | ||
| NZW | z/z | |||
| No. of mice | 20 | 20 | 19 | |
| Frequency* | 24.4 ± 2.4 | 46.5 ± 3.7 | 65.8 ± 5.6 | |
| NZW.H-2d | d/d | |||
| No. of mice | 7 | 7 | 19 | |
| Frequency | 5.4 ± 0.6 | 12.7 ± 1.4 | 25.2 ± 6.0 | |
| B10.NZW | z/z | |||
| No. of mice | 13 | 10 | 8 | |
| Frequency | 2.1 ± 0.2 | 5.2 ± 1.1 | 7.3 ± 1.0 | |
| B10.D2 | d/d | |||
| No. of mice | 9 | 9 | 9 | |
| Frequency | 1.7 ± 0.2 | 1.9 ± 0.3 | 1.3 ± 0.2 | |
| (NZW × B10.NZW)F1 | z/z | |||
| No. of mice | 10 | 10 | 9 | |
| Frequency | 14.6 ± 3.9 | 27.1 ± 7.8 | 32.5 ± 8.8 | |
| (NZW × B10.D2)F1 | z/d | |||
| No. of mice | 15 | 15 | 14 | |
| Frequency | 7.3 ± 0.4 | 8.1 ± 0.4 | 23.3 ± 5.5 | |
| (NZW × B10.NZW) F1 × B10.NZW | z/z | |||
| No. of mice | 140 | 134 | 127 | |
| Frequency | 4.7 ± 0.2 | 7.3 ± 0.6 | 14.0 ± 1.1 | |
| Strains . | H-2 Haplotypes . | Age (months) . | ||
|---|---|---|---|---|
| 8 . | 12 . | 16 . | ||
| NZW | z/z | |||
| No. of mice | 20 | 20 | 19 | |
| Frequency* | 24.4 ± 2.4 | 46.5 ± 3.7 | 65.8 ± 5.6 | |
| NZW.H-2d | d/d | |||
| No. of mice | 7 | 7 | 19 | |
| Frequency | 5.4 ± 0.6 | 12.7 ± 1.4 | 25.2 ± 6.0 | |
| B10.NZW | z/z | |||
| No. of mice | 13 | 10 | 8 | |
| Frequency | 2.1 ± 0.2 | 5.2 ± 1.1 | 7.3 ± 1.0 | |
| B10.D2 | d/d | |||
| No. of mice | 9 | 9 | 9 | |
| Frequency | 1.7 ± 0.2 | 1.9 ± 0.3 | 1.3 ± 0.2 | |
| (NZW × B10.NZW)F1 | z/z | |||
| No. of mice | 10 | 10 | 9 | |
| Frequency | 14.6 ± 3.9 | 27.1 ± 7.8 | 32.5 ± 8.8 | |
| (NZW × B10.D2)F1 | z/d | |||
| No. of mice | 15 | 15 | 14 | |
| Frequency | 7.3 ± 0.4 | 8.1 ± 0.4 | 23.3 ± 5.5 | |
| (NZW × B10.NZW) F1 × B10.NZW | z/z | |||
| No. of mice | 140 | 134 | 127 | |
| Frequency | 4.7 ± 0.2 | 7.3 ± 0.6 | 14.0 ± 1.1 | |
Frequencies of peripheral blood B-1 cells are expressed as mean percentage ± standard error of CD5+ B cells in total 6B2+ B cells.
Comparison of data shown in Table 1 indicate several inheritance patterns of the H-2z–linked and nonlinked susceptibility alleles: (1) because B-1 cell frequencies were higher in strains bearing either one of the H-2–linked or nonlinked genes (B10.NZW and NZW.H-2d/d, respectively) than those found in the B10.D2 strain, which lacks both genes, each gene can act to propagate B1 cells in an independent manner; (2) becauseH-2z/d–heterozygous (NZW x B10.D2) F1 at any given age had lower B-1 cell frequencies than did H-2z/zhomozygous (NZW x B10.NZW) F1 mice and becauseH-2z/z homozygous (NZW x B10.NZW) F1 had higher B-1 cell frequencies than did H-2z/z homozygous B10.NZW, the H-2z–linked and the non–H-2z-linked genes both appeared to be inherited in an incomplete dominant fashion; and (3) because the NZW strain, which carries all susceptibility alleles, has the highest frequencies of B-1 cells compared with others bearing fewer or none such as (NZW x B10.NZW) F1, (NZW x B10.D2) F1 and B10.D2, the extent of B-1 cell frequencies in the progeny appeared to depend on additive effects of the sum of each H-2z-linked and non–H-2z-linked susceptibility allele.
Mapping of non–H-2z-linked susceptibility alleles for aberrant B-1 cell proliferation.
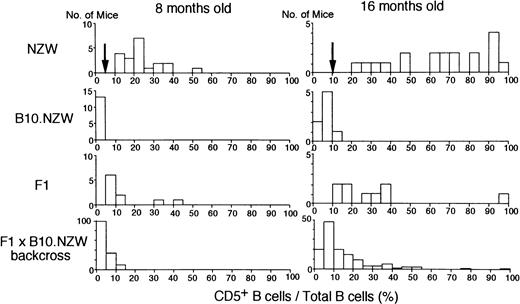
To map the non–H-2z-linked NZW locus or loci for abnormal proliferation of B-1 cells, (NZW x B10.NZW) F1 x B10.NZW female backcross mice, all bearing homozygousH-2z/z, were generated and genotyped using microsatellite markers. Of a total of 504 markers screened, 192 markers were polymorphic between the two parental strains and 103 markers were selected for further studies (Fig 1). Figure 2 illustrates histograms of the distribution of blood B-1 cell frequencies in NZW, B10.NZW, the F1 hybrid and the F1 x B10.NZW backcross mice at 8 and 16 months of age. When cut-off points were determined based on criteria that B-1 cell frequencies of over 5% and 10% were regarded as abnormal levels at age 8 and 16 months, respectively, χ2 analyses using 127 to 140 backcross mice showed that NZW/B10.NZW (NB) genotype at loci on each chromosome 13 and 17 was significantly associated with abnormal proliferation of B-1 cells (Table 2).
Polymorphic microsatellite markers used in this study. Linkage relationships for the 103 polymorphic markers were determined by analysis of 140 (NZW x B10.NZW) F1 x B10.NZW backcross mice. Genetic maps for each chromosome were prepared using MAPMAKER/EXP.
Polymorphic microsatellite markers used in this study. Linkage relationships for the 103 polymorphic markers were determined by analysis of 140 (NZW x B10.NZW) F1 x B10.NZW backcross mice. Genetic maps for each chromosome were prepared using MAPMAKER/EXP.
Histograms of proportions of CD5+B (B-1) cells in total peripheral B cells in NZW, B10.NZW, (NZW x B10.NZW) F1 and (NZW x B10.NZW) F1 x B10.NZW backcross mice at 8 and 16 months of age. Cut-off point used in χ2 test is indicated by an arrow.
Histograms of proportions of CD5+B (B-1) cells in total peripheral B cells in NZW, B10.NZW, (NZW x B10.NZW) F1 and (NZW x B10.NZW) F1 x B10.NZW backcross mice at 8 and 16 months of age. Cut-off point used in χ2 test is indicated by an arrow.
Linkage of Particular Loci With B-1 Cell Proliferation in the Backcross Progeny
| Chr* . | Locus . | Distance† . | Direction‡ . | 8 Months2-153 . | 16 Months2-153 . | Lod2-155 . | |||
|---|---|---|---|---|---|---|---|---|---|
| χ2 . | P . | χ2 . | P . | 8 Months . | 16 Months . | ||||
| 13 | D13MIT80 | 15.8 | NB > BB | 1.8 | 1.1 | ||||
| D13MIT136 | 4.9 | NB > BB | 9.48 | .0021 | 6.04 | .014 | 4.0 | 2.0 | |
| D13MIT61 | 11.3 | NB > BB | 8.38 | .0038 | 7.44 | .0064 | 3.5 | 1.7 | |
| D13MIT13 | 19.0 | NB > BB | 8.38 | .0038 | 14.42 | .0001 | 3.2 | 3.0 | |
| D13MIT126 | 15.1 | NB > BB | 8.08 | .0045 | 1.5 | 1.6 | |||
| D13MIT47 | 11.4 | NB > BB | 1.3 | ||||||
| D13MIT31 | — | ||||||||
| 17 | D17MIT222 | 10.6 | NB > BB | 6.02 | .014 | 8.44 | .0037 | 2.2 | 1.3 |
| D17MIT167 | 8.1 | NB > BB | 3.41 | .065 | 1.5 | ||||
| D17MIT137 | 6.6 | NB > BB | 2.78 | .096 | 1.4 | ||||
| D17MIT67 | 3.5 | NB > BB | 6.79 | .0092 | 1.2 | ||||
| D17MIT9 | 6.4 | NB > BB | 6.79 | .0092 | |||||
| D17MIT87 | 11.8 | ||||||||
| D17MIT159 | 4.2 | ||||||||
| D17MIT218 | 9.8 | ||||||||
| D17MIT2 | — | ||||||||
| Chr* . | Locus . | Distance† . | Direction‡ . | 8 Months2-153 . | 16 Months2-153 . | Lod2-155 . | |||
|---|---|---|---|---|---|---|---|---|---|
| χ2 . | P . | χ2 . | P . | 8 Months . | 16 Months . | ||||
| 13 | D13MIT80 | 15.8 | NB > BB | 1.8 | 1.1 | ||||
| D13MIT136 | 4.9 | NB > BB | 9.48 | .0021 | 6.04 | .014 | 4.0 | 2.0 | |
| D13MIT61 | 11.3 | NB > BB | 8.38 | .0038 | 7.44 | .0064 | 3.5 | 1.7 | |
| D13MIT13 | 19.0 | NB > BB | 8.38 | .0038 | 14.42 | .0001 | 3.2 | 3.0 | |
| D13MIT126 | 15.1 | NB > BB | 8.08 | .0045 | 1.5 | 1.6 | |||
| D13MIT47 | 11.4 | NB > BB | 1.3 | ||||||
| D13MIT31 | — | ||||||||
| 17 | D17MIT222 | 10.6 | NB > BB | 6.02 | .014 | 8.44 | .0037 | 2.2 | 1.3 |
| D17MIT167 | 8.1 | NB > BB | 3.41 | .065 | 1.5 | ||||
| D17MIT137 | 6.6 | NB > BB | 2.78 | .096 | 1.4 | ||||
| D17MIT67 | 3.5 | NB > BB | 6.79 | .0092 | 1.2 | ||||
| D17MIT9 | 6.4 | NB > BB | 6.79 | .0092 | |||||
| D17MIT87 | 11.8 | ||||||||
| D17MIT159 | 4.2 | ||||||||
| D17MIT218 | 9.8 | ||||||||
| D17MIT2 | — | ||||||||
Chromosome number.
Distance in centiMorgans (Kosambi function) between two consecutive markers as calculated by MAPMAKER/EXP. Markers are arranged from centromeric to teromeric.
NB, heterozygous loci of NZW and B10.NZW; BB, homozygous loci of B10.NZW. An inequality NB > BB indicates that the phenotype is biased to progeny genotyped as NB.
Peripheral B-1 cell frequencies are considered as increased if exceeding 5% in mice 8 months of age or 10% in mice 16 months of age.
Log of the likelihood ratio (lod) scores are analyzed by MAPMAKER/QTL. Lod scores only greater than 1 are indicated.
Interval mapping of data from backcross mice at 8 and 16 months of age using MAPMAKER/QTL showed that the locus on chromosome 13, provisionally designated Bpal-2, is located on the centromeric portion and is either significantly or suggestively linked toD13MIT136, D13MIT61, and D13MIT13. However, second peaks were always identified within each MAPMAKER/QTL analysis. In mice aged 8 months, the locus was most closely linked toD13MIT136 and, in mice aged 16 months, it was closely linked to D13MIT13 (Table 2 and Fig 3). The existence of these two peaks and the large size of the one-log confidence support interval (29 centiMorgans) obtained by analysis in mice aged 8 months suggest the possible existence of two susceptibility loci in this region (Fig 3). As numbers of recombinants between D13MIT136 and D13MIT13 were few, we could not confirm this possibility.
MAPMAKER/QTL scans on chromosomes 13 and 17 for aberrant B-1 cell proliferation in blood of 127 to 140 (NZW x B10.NZW) F1 x B10.NZW backcross mice. Lod score curves (bold lines, 8 months of age; dotted line, 16 months of age) are shown on the right with scale on the top. Map positions of markers are arranged from centromere to telomere on the left side of the chromosome line. An “error” bar represents the one-lod support interval of Bpal-2. Candidate genes within the support interval are also listed.
MAPMAKER/QTL scans on chromosomes 13 and 17 for aberrant B-1 cell proliferation in blood of 127 to 140 (NZW x B10.NZW) F1 x B10.NZW backcross mice. Lod score curves (bold lines, 8 months of age; dotted line, 16 months of age) are shown on the right with scale on the top. Map positions of markers are arranged from centromere to telomere on the left side of the chromosome line. An “error” bar represents the one-lod support interval of Bpal-2. Candidate genes within the support interval are also listed.
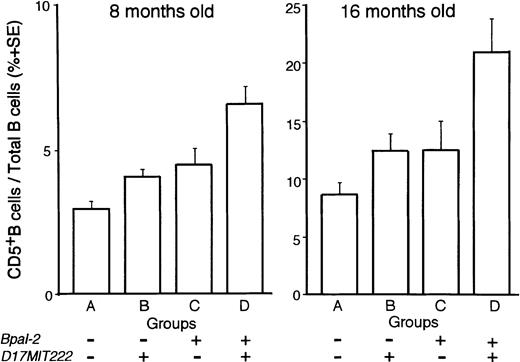
On the other hand, the non–H-2-linked locus on chromosome 17 showed a suggestive association in lod scores with D17MIT222 in MAPMAKER/QTL analysis of 8 months old mice. Although the lod scores were not in the range of significant values, the existence of QTL close to this locus was suggested in ANOVA, as based on four groups of backcross progeny, classified according to combinations of genotypes for D13MIT136 (Bpal-2) and D17MIT222, ie, group A, B10.NZW/B10.NZW (BB) genotype for both D13MIT136 andD17MIT222; groups B and C, either one of the two loci is NZW/B10.NZW (NB) and the other is BB; and group D, NB for both loci (Fig 4). Among these four groups, the extent of B-1 cell frequencies was in the order of group D, groups B and C, and group A, indicating that the frequencies are increased in a manner depending on the number of the corresponding NZW susceptibility alleles. The differences were statistically significant in mice at 8 and at 16 months of age (P < .0001 at 8 months, and P< .01 at 16 months) by ANOVA. Thus, in addition to the effects ofBpal-1 (H-2–linked) and Bpal-2, the third non–H-2-linked locus on NZW chromosome 17, tentatively designated Bpal-3, is also likely to play a role in the abnormal proliferation of B-1 cells.
ANOVA among groups classified by combined genotyping. (NZW x B10.NZW) F1 x B10.NZW backcross mice were subdivided into four groups (A to D) according to combined genotyping of loci,D13MIT136 (Bpal-2) and D17MIT222. Symbol ‘+’, genotyped as NB (heterozygous for NZW and B10.NZW) and ‘−’, genotyped as BB (homozygous for B10.NZW). P values of post hoc test (Fisher’s Protected Least Significant Difference test) were as follows: at 8 months old; A versus C, P < .05; A versus D, P < .0001; B versus D, P < .0001; C versus D, P < .005; at 16 months old; A versus D, P< .0005; B versus D, P < .005; C versus D, P < .05.
ANOVA among groups classified by combined genotyping. (NZW x B10.NZW) F1 x B10.NZW backcross mice were subdivided into four groups (A to D) according to combined genotyping of loci,D13MIT136 (Bpal-2) and D17MIT222. Symbol ‘+’, genotyped as NB (heterozygous for NZW and B10.NZW) and ‘−’, genotyped as BB (homozygous for B10.NZW). P values of post hoc test (Fisher’s Protected Least Significant Difference test) were as follows: at 8 months old; A versus C, P < .05; A versus D, P < .0001; B versus D, P < .0001; C versus D, P < .005; at 16 months old; A versus D, P< .0005; B versus D, P < .005; C versus D, P < .05.
Development of B-CLL in backcross progeny.
At the age of 23 months, a time when 70% of NZW mice had developed B-CLL, as determined by blood smear samples, 12% of the (NZW x B10.NZW) x B10.NZW backcross mice developed leukemia, and this was associated with age-related abnormal increase in the frequency of B-1 cells in the blood. Figure 5 shows a representative result of age-associated changes in frequencies of B-1 cells in the blood, as determined by fluorescence-activated cell sorting (FACS) analysis and in cytospinned leukocyte films in a (NZW x B10.NZW) x B10.NZW backcross mouse at ages 8, 16, and 23 months. FACS profiles showed that proportions of B-1 cells per total B cells were progressively increased in this mouse with aging (11%, 44%, and 97% at 8, 16, and 23 months of age, respectively). In the leukemic stage in the mouse 23 months of age, total leukocyte counts were markedly high (35,500/mm3). Morphologically, most lymphocytes in the peripheral blood in the 8-month-old mouse were small and had condensed nuclear chromatin and a scant cytoplasm. At 16 months of age, there occasionally appeared relatively larger lymphocytes with a basophilic cytoplasm, some of which had irregular nuclei with irregular networks of chromatin. The majority of blood lymphocytes at the leukemic stage were composed of lymphoid cells with a basophilic cytoplasm and nuclei with a coarse granular chromatin. Smudged cells and broken lymphocytes were frequent (data not shown). Surface markers of leukemic cells were positive for surface IgM, CD2, CD5, CD19, and MHC class II and negative for CD25 and CD38, with patterns similar to those seen in human B-CLL cells.43
Representative flow cytometry profiles and blood films of peripheral blood lymphocytes from a single (NZW x B10.NZW) F1 x B10.NZW backcross mouse carrying all Bpal-1, -2 and -3 alleles, at 8, 16, and 23 months of age. CD5+ B-1 cells are boxed. Cytospinned lymphocyte films were stained with Giemsa. Proportions of B-1 cells in total peripheral B cells and total white blood cell counts were 11% and 7,200/mm3 in mice at 8 months, 44% and 7,000/mm3 at 16 months, and 97% and 35,500/mm3at 23 months of age, respectively.
Representative flow cytometry profiles and blood films of peripheral blood lymphocytes from a single (NZW x B10.NZW) F1 x B10.NZW backcross mouse carrying all Bpal-1, -2 and -3 alleles, at 8, 16, and 23 months of age. CD5+ B-1 cells are boxed. Cytospinned lymphocyte films were stained with Giemsa. Proportions of B-1 cells in total peripheral B cells and total white blood cell counts were 11% and 7,200/mm3 in mice at 8 months, 44% and 7,000/mm3 at 16 months, and 97% and 35,500/mm3at 23 months of age, respectively.
DISCUSSION
We identified three susceptibility loci responsible for abnormal proliferation of B-1 cells, an event that forms the basis of leukemogenesis in a B-CLL model of NZW strain. All three susceptibility alleles function independently and are inherited in an incomplete dominant fashion. The extent of B-1 cell frequencies in the peripheral blood depends on the sum of each susceptibility allele, indicating additive effects of these alleles. These features are consistent with the polygenic inheritance of the abnormality as a threshold liability. It is highly plausible that these susceptible alleles predispose B-1 cells to accumulate genetic alterations, thus giving rise to B-CLL.
As usual in studies of interval mapping of genes with incomplete penetrance, support intervals associated with B-CLL susceptibility alleles were long. Thus, further approaches for identification of the causative gene in each interval include the establishment of a congenic strain for support intervals, followed by exon-trapping. Another approach would be characterization of relevant candidate genes potentially related to the dysregulation of B-1 cell proliferation. In this context, because Bpal-1, anH-2z–linked susceptibility allele, retains the effect in H-2z-congenic B10.NZW mice that have been established by selective backcrossing of mice for 12 generations.38 the candidate gene appears to be located within or in close proximity of the H-2 complex. A potentially important polymorphic candidate gene for Bpal-1 may be a structural gene of MHC class II antigens. Evidence is accumulating that B-CLL cells use restricted repertoires of nonmutated Ig V genes.21,22 VH genes of B-CLL are preferentially selected from relatively small VH subgroups and the structure of the complementarity-determining region (CDR) 3 is biased to be longer than that of the normal counterpart,20suggesting that an antigen-driven, strong selective force is operative on B-1 cells during leukemogenesis. Considering that the majority of B-1 cells can cross-react with a variety of self-antigens, which can be processed and presented as an MHC class II-peptide complex, chronic stimulation via a certain ubiquitous self-peptide plus class II may serve as a selective force for the restricted repertoires, and the risk for neoplastic transformation into B-CLL would increase. In this regard, the H-2z haplotype of NZW strain is unique. Our earlier studies using H-2–congenic New Zealand mouse strains showed that the major NZW contribution to severe SLE in (NZB x NZW) F1 mice is the H-2z–linked locus that forms H-2d/z heterozygosity in the F1 hybrid mice.34,35 Importance of the H-2 heterozygosity for SLE has been confirmed in F244 and backcross45analyses, except for one that showed a minor influence.46The most plausible hypothesis for the difference between homozygousH-2z/z for B-CLL and heterozygousH-2d/z for autoimmune disease in (NZW x NZB) F1 mice is that, in the latter, the formation of mixed haplotype-class II molecules, ie, Aαdβu, Aαuβd, Eαdβu, and Eαuβd allows selected B-1 cells to undergo class switch and affinity selection, giving rise to plasma cells producing pathogenic autoantibodies.34,35 Because Aαdβu molecule-specific autoreactive T-cell clones derived from aged (NZW x NZB) F1 mice were capable of inducing IgG anti-DNA antibody production on transfer to young mice, the Aαdβu is thought to be the most plausible candidate for restriction element for autoreactive T cells.47,48 In contrast, in the formerH-2z/z homozygotes, because of the lack of genetic element (mixed haplotype class II molecules) required for such B-1 cell maturation, only signals for proliferation would be functioning.14
Another candidate for Bpal-1 may be the tumor necrosis factor (TNF) gene. Both structural and regulatory genes of TNF-α and -β are mapped to the D subregion of the H-2complex.49 It was reported that although TNF-α weakly triggers the growth of B-CLL cells,50 it does exert a synergistic proliferative effect in combination with interleukin (IL)-2.51 Serum levels of TNF-α are increased in B-CLL patients compared with findings in healthy age-matched individuals.52 Thus, it is possible that the polymorphic TNF-α gene in the H-2 complex controls the proliferation of B-1 cells in NZW mice. However, this is less likely because production of TNF-α in the NZW strain is downregulated, rather than upregulated, by the unique polymorphic NZW TNF-α allele,53 and because this NZW TNF-α allele upregulates SLE in (NZB x NZW) F1 mice.53 Our recent genetic studies suggested that both class II and NZW TNF-α polymorphisms appear to be functioning asH-2–linked prediposing genetic elements for SLE, and that the TNF-α polymorphism functions to modulate an initial process of the autoimmune disease in these mice.54
One-log confidence interval containing Bpal-2 on chromosome 13 covers potent candidate loci, ie, encoding T-cell receptor γchain (Tcrg), inhibin βA (Inhba), prolactin (Prl), and Friend murine leukemia virus (MuLV) integration site-1 (Fim-1). Among these, Inhba deserves attention. A homodimer of inhibin βA is activin A produced in stromal cells in the bone marrow55 and regulates differentiation and proliferation of cells, including hematopoietic cells and leukemic cells.56,57 We are now determining if Inhba is polymorphic and has the potential to proliferate B-1 cells. This segment of murine chromosome 13 is homologous to portions of human chromosomal regions 7p15 (as for Tcrg and Inhba) and 6p23 (as for Prl).58
Among genes on the centromeric portion of chromosome 17, Igf2r(insulin-like growth factor 2 receptor [IGF2R]) is a plausible candidate for Bpal-3.59 Evidence is accumulating that Igf2r acts as a tumor suppressor gene in both humans and mice. Loss of IGF2R function leads to an increased extracellular concentration of IGF2 and a decreased level of activated TGF-β, a condition under which human hepatocytes are susceptible to malignant transformation.60 In human breast cancers, loss of heterozygosity was found at the locus of Igf2r.61Overexpression of IGF2 can increase frequencies of diverse malignancies, including lymphoma in mice.62 Thus, a potential genetic polymorphism of the NZW Igf2r gene may be involved in leukemogenesis. To examine all of these possibilities, we are now generating interval-specific congenic strains forBpal-2 and Bpal-3. Such strains will also be useful for the analysis of epistatic effects between susceptibility alleles.
Raveché et al63,64 reported that aged NZB mice, a spontaneous model of autoimmune hemolytic anemia, exhibit a clonal expansion of hyperdiploid B-1 cells that resemble B-CLL. In our earlier studies, the frequencies of B-1 cells in the NZB strain (H-2d) were much lower than found in the homozygousH-2z/z–congenic NZB strain.36,37However, considering the disparity in B-1 cell frequencies between the H-2z/z-congenic NZB36,37 and the H-2z/z–congenic B10.NZW (present study), it is highly plausible that the NZB strain also carries certain susceptibility alleles such as Bpal-2 and/or Bpal-3 for abnormal proliferation of B-1 cells. In concert with effects of the susceptibility alleles for aberrant B-1 cell differentiation,65 66 such Bpal-2 and/orBpal-3 may possibly relate to the autoimmune disease seen in NZB and (NZB × NZW) F1.
In conclusion, New Zealand mouse models provide a valuable tool for determination of the genetic basis of not only B-CLL ontogeny, but also autoimmune disease, in which dysregulated proliferation of B-1 cells forms the basis of B-CLL and the associated aberrant maturational processes of these expanded populations of B-1 cells can lead to autoimmune disease.
ACKNOWLEDGMENT
We thank Dr T. Ushijima, National Institute for Cancer Research, Japan for helpful discussion and M. Ohara for comments on the manuscript.
Supported in part by CREST (Core Research for Evolutional Science and Technology) of Japan Science and Technology Corporation and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Toshikazu Shirai, MD, PhD, Department of Pathology, Juntendo University School of Medicine, 2-1-1, Hongo, Bunkyo-ku, 113-8421, Tokyo, Japan; e-mail:toshirai@med.juntendo.ac.jp).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal