Abstract
Thrombin-treated tumor cells induce a metastatic phenotype in experimental pulmonary murine metastasis. Thrombin binds to a unique protease-activated receptor (PAR-1) that requires N-terminal proteolytic cleavage for activation by its tethered end. A 14-mer thrombin receptor activation peptide (TRAP) of the tethered end induces the same cellular changes as thrombin. Four murine tumor cells (Lewis lung, CT26 colon CA, B16F10 melanoma, and CCL163 fibroblasts) contain PAR-1, as detected by reverse transcriptase-polymerase chain reaction (RT-PCR). B16F10 cells did not contain the two other thrombin receptors, PAR-3 and glycoprotein Ib. TRAP-treated B16F10 tumor cells enhance pulmonary metastasis 41- to 48-fold (n = 17). Thrombin-treated B16F10 cells transfected with full-length murine PAR-1 sense cDNA (S6, S7, S14, and S22) enhanced their adhesion to fibronectin 1.5- to 2.4-fold (n = 5, P < .04), whereas thrombin-treated wild-type cells do not. S6 (adhesion index, 1.5-fold) and S14 (index, 2.4-fold) when examined by RT-PCR and Northern analysis showed minimal expression of PAR-1 for S6 over wild-type and considerable expression for S14. Immunohistochemistry showed greater expression of PAR-1 for S14 compared with wild-type or empty-plasmid transfected cells. In vivo experiments with the thrombin-treated S14 transfectant showed a fivefold to sixfold increase in metastases compared with empty-plasmid transfected thrombin-treated naive cells or S6 cells (n = 20, P = .0001 to .02). Antisense had no effect on thrombin-stimulated tumor mass. Thus, PAR-1 ligation and expression enhances and regulates tumor metastasis.
THROMBIN TREATMENT of tumor cells induces a metastatic phenotype in an experimental murine pulmonary system.1-3 Thrombin-treated tumor cells increase their adhesion to platelets, endothelial cells, fibronectin, and von Willebrand factor (vWF) twofold to threefold in vitro2,4,5and enhance their murine pulmonary metastasis 10- to 160-fold in vivo.1,2 Tumor cells bind 125I-thrombin in a saturation-dependent manner.5
The expression of the seven transmembrane spanning, protease-activated thrombin receptor (PAR-1)6,7 has been identified in 7 different human tumor cell lines by reverse transcriptase-polymerase chain reaction (RT-PCR),5,8 and in nontumor cells by responsiveness to the thrombin receptor activation peptide, SFLLRN.6 9-11
Because thrombin can bind to three different receptors (glycoprotein [GP] Ib,12,13 PAR-1,6 7 and PAR-314), we designed experiments to determine which thrombin receptor(s) was responsible for induction of the metastatic phenotype.
In the present report, we focus on a murine B16F10 melanoma tumor cell line that only contains PAR-1. We report that ligation of PAR-1 with an SFLLRN-containing 14-mer peptide enhances experimental pulmonary metastasis 41- to 48-fold. Transfection of PAR-1 into B16F10 cells enhances thrombin-treated tumor cell adhesion to fibronectin as high as 2.5-fold in vitro and pulmonary metastasis as high as fivefold in vivo, compared with nontransfected thrombin-treated tumor cells.
MATERIALS AND METHODS
Tumor Cell Lines, Tissue Culture Media, and Reagents.
CT26, N-nitroso-N-methyl urethane–induced mouse undifferentiated colon carcinoma cells were obtained through the courtesy of Dr M.H. Goldrosen (Roswell Park Memorial Institute, Buffalo, NY). B16F10 mouse melanoma cells were obtained through the courtesy of Dr I.J. Fidler (University of Texas, M.D. Anderson Cancer Center, Houston, TX). The B16F10 variant that we used was selected for its relatively weak in vivo metastatic effect to be rigorous in selecting against a thrombin-responsive effect and providing for a more clear cut difference between naive and thrombin-treated cells. Lewis lung and CCL163 (fibroblast) mouse cell lines were obtained from the American Type Culture Collection (Rockville, MD). All cell lines were grown in tissue culture media with supplements, as described previously.1,2 15-17 Human thrombin (3,000 U/mg) was obtained from Sigma (St Louis, MO).
In vivo metastatic studies.
Experiments were performed as described previously.1,2,15,16 Tumor cells were removed from subconfluent tissue culture dishes with 0.02% EDTA, washed in phosphate-buffered saline (PBS)-Ca2+-Mg2+ and examined for viability with trypan blue. Tumor cells (1 × 106) were suspended and incubated with or without thrombin or thrombin receptor activation peptide SFLLRN or SFLLRNPNDKYEPF in PBS-bovine serum albumin (BSA)-Ca2+-Mg2+-0.1% BSA, incubated for 1 hour at 37°C, sedimented, and washed twice in PBS-Ca2+-Mg2+, and 50,000 viable tumor cells were injected intravenously into the tail vein of syngeneic C57Bl/6J mice (Taconic Farms, Germantown, NY) in a volume of 200 μL. Control and experimental mice were injected alternately to avoid bias of possible changes in in vivo metastatic potential after in vitro storage at 4°C. Animals were killed on day 21 or 35 and the lungs were removed and fixed in 10% neutral-buffered formalin for 40 hours before macroscopic enumeration of tumor nodule number and volume. Pulmonary tumor mass can vary considerably from experiment to experiment1,2,15 16 due to metastatic tumor cell heterogeneity of tissue culture lines, viable cell number injected, and duration of in vivo time of exposure to tumor cells before measuring tumor metastasis. Accordingly, experiments have been reported with varying tumor burdens. As will be noted, similar PAR-1–induced effects were noted, despite the variability of pulmonary tumor burden.
Thrombin receptor (PAR-1) cDNA-cloned transfection studies.
Bluescript plasmid containing mouse thrombin receptor (PAR-1) was kindly provided by Dr S. Coughlin (University of California, San Francisco, CA). The sense and antisense transfectants were constructed by inserting an approximately 1.5-kb Xba I fragment containing the complete coding sequence derived from pBluescript into the expression vector pCDNA3 (InVitrogen, Carlsbad, CA) in the appropriate direction (after excision of a pcDNA3 ATG site upstream of the 5′ end of the insert). Transfection of B16F10 cells was performed by the calcium phosphate method.18 Stable transfectants were selected for Geneticin resistance with G418 and cloned by limiting dilution. The expression of PAR-1 in transfected B16F10 cells was studied by Northern blotting using the PAR-1 cDNA fragment of pcDNA3 labeled with 32P-dCTP.18
Immunocytochemistry.
An affinity-purified rabbit antirat antibody (Ab; TR-R9) raised against an extracellular amino acid sequence of PAR-1, SFFLRNPSE, was kindly supplied by Dr M. Runge (University of Texas Medical Branch, Galveston, TX). B16F10 cells were subcultured onto chamber slides (Lab-Tek, Naperville, IL), fixed with 4% paraformaldehyde, rinsed with PBS, and stained for PAR-1 using 2 μg/mL of rabbit anti–PAR-1, goat antirabbit biotinylated secondary antibody, and avidin-biotin-horseradish peroxidase (HRP) complex reagent (Vector Labs, Burlingham, CA), as previously described.19 Slides were counterstained with Mayer’s Hematoxylin and visualized and photographed with an Olympus BX50 light microscope (Hitech Instruments, Edgemont, PA). Controls used rabbit isotype nonimmunized serum and absence of primary Ab.
Examination of the expression of thrombin receptors (PAR-1, PAR-3, and GPIbα) and murine HPRT house-keeping gene by RT-PCR.
RT-PCR was used as described previously8 for identification of PAR-1, PAR-3, and GPIbα. The RNAs tested were treated with deoxyribonuclease I (Perkin Elmer Cetus, Branchburg, NJ) to eliminate contaminating DNA. Two sets of primers were used for the PAR-1 (Genbank accession no. L03529): 5′-GGTGTGCTACACGTCCATCAT at bp 890-910 and 3′-AGCACAAGATGCTGTAGAGGT at bp 1191-1171, designed to give a 301-bp product; and 5′-GGTCTGCTACACGTCCATCAT at bp 900-920 and 3′-AGCACAAGATGCTGTAGAGGT at bp 1169-1190, designed to give a 290-bp product. One microgram of total RNA was converted to cDNA with 10 U/μL Moloney murine leukemia virus (MMLV) reverse transcriptase, 100 nmol/L dNTP, 100 pmol/L of random hexamer, and 2 μL of 10× RT buffer at 37°C for 30 minutes in a total volume of 20 μL. One tenth of the synthesized cDNA was used as template in the PCR reaction containing 2 U of Taq DNA polymerase, 50 nmol/L each of dNTPs, and 50 pmol/L of primers in a total volume of 50 μL. Amplification cycles consisted of 1 minute at 94°C, 1 minute at 55°C, and 1 minute at 72°C for 30 cycles. RT-PCR products were analyzed in 2% agarose gel electrophoresis.
Two sets of primers were used for endogenous murine PAR-3 (GenBank accession no. U92972): 5′-GTGTACCATCCAACATCGTGACC at bp 472-494 and 3′-AAACCATGACCCACACTATGCC at bp 813-792, designed to give a 341-bp product; and 5′-GGCTCACCCTTTCACATACCAG at bp 734-755 and 3′-AGTTGGCATGGTGGATTACGAG at bp 1131-1110, designed to give a 397-bp product.
Primers used for murine GPIbα were constructed from the cloned cDNA sequence kindly made available to us by Dr J. Ware (Scripps Research Institute, La Jolla, CA; unpublished data): 5′-ACCGAGTCATAGCCTGGAAGA at bp 31-51 and 3′-CCAGGTACAGATAAGTGAGGTGAG at bp 321-298, designed to give a 290-bp product.
Primers for murine hypoxanthine phosphoribosyltransferase (HPRT; GenBank acession no. J00423) were 5′-GGTGGAGATGATCTCTCAAC at bp 439-458 and 3′-TCCAGTTTCACTAATGACAC at bp 723-704, designed to give a 284-bp product. PCR for HPRT was performed in the same PCR reaction to serve as an internal control.
Tumor cell adhesion studies.
Fibronectin dissolved in PBS-Ca2+-Mg2+ buffer was applied to Falcon 3913 96-well microtiter plates (Becton Dickinson, Oxnard, CA) at 5 μg/well overnight. Wells were washed in buffer + 0.1% BSA and then incubated with 2 × 104 B16F10 cells pretreated with either thrombin or buffer for 30 minutes at 37°C, followed by washing to remove thrombin. A thrombin dose-response curve showed a 1.5-fold enhancement at 0.05 U/mL and a twofold to 2.5-fold enhancement at 0.1 to 0.5 U/mL. Experiments were therefore performed at 0.5 U/mL. Suspended cells were then applied to fibronectin-coated plates for 1 hour at 37°C. Nonadherent cells were washed away with buffer and adherent cells enumerated by either (1) microscopic quantitation of cells eluted with trypsin-EDTA or (2) absorbance of added calcium dye, calecin AM (C-1430; 15 μmol/L in PBS), a fluorogenic esterase substrate used for detection of live cells (Molecular Probes, Portland, OR). After 30 minutes of exposure to tumor cells at 37°C, fluorescence was monitored with excitation at 485 nm and emission at 538 nm with an automated fluoresence multiwell plate scanner (Fluoroskan II, Helsinki, Finland). Background fluorescence determined in the absence of cells was negligible (∼1%).
RESULTS
Previous studies from our laboratory have demonstrated the presence of PAR-1 in 6 different human tumor cell lines that enhanced their adhesion to platelets after treatment with the 14-mer thrombin receptor activation peptide, SFLLRNPNDKYEPF.8 Other studies demonstrated enhanced experimental murine pulmonary metastasis after treatment of syngeneic B16F10 melanoma and CT26 colon carcinoma with thrombin.2 We therefore elected to determine whether the murine cell lines expressed the PAR-1 receptor and whether it is responsible for thrombin-induced enhanced tumor adhesion in vitro and metastasis in vivo.
Expression of PAR-1 in murine tumors.
PAR-1 mRNA was found in 4 different murine cell lines tested by RT-PCR: Lewis lung, CT26, B16F10, and CCL163 (data not shown). B16F10 cells were selected for further study.
Absence of other known thrombin receptors, PAR-3, and GPIb in B16F10 cells.
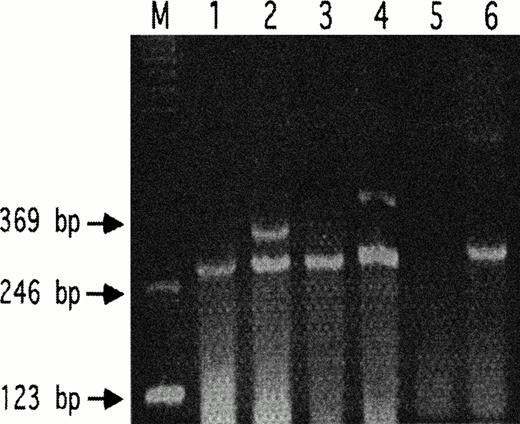
PAR-3 was absent in B16F10 cells using two sets of primers spanning bp 472-1131. GPIbα was absent using primers spanning bp 31-321 (Fig 1). We therefore focused on PAR-1.
Absence of PAR-3 and GPIb in B16F10 cells. RT-PCR are shown for wild-type B16F10 (lanes 1, 3, and 5) and mouse spleen (lanes 2, 4, and 6). HPRT was used as an internal standard in lanes 1 through 4 (expected product, 284 bp). Lanes 1 and 2 demonstrate the respective absence and presence of PAR-3 in B16F10 versus mouse spleen for the 341-bp PAR-3 primer product. Similar results are shown for lanes 3 and 4 using PAR-3 primers with the expected 397-bp product. Lanes 5 and 6 demonstrate the respective absence and presence of GPIb versus mouse spleen for the expected 290-bp product.
Absence of PAR-3 and GPIb in B16F10 cells. RT-PCR are shown for wild-type B16F10 (lanes 1, 3, and 5) and mouse spleen (lanes 2, 4, and 6). HPRT was used as an internal standard in lanes 1 through 4 (expected product, 284 bp). Lanes 1 and 2 demonstrate the respective absence and presence of PAR-3 in B16F10 versus mouse spleen for the 341-bp PAR-3 primer product. Similar results are shown for lanes 3 and 4 using PAR-3 primers with the expected 397-bp product. Lanes 5 and 6 demonstrate the respective absence and presence of GPIb versus mouse spleen for the expected 290-bp product.
Effect of PAR-1 TRAP on pulmonary metastasis.
Responsiveness to the PAR-1 ligand TRAP was next demonstrated in Table 1. A 44- to 48-fold increase in tumor mass was induced by the 14 mer and 6 mer TRAP, respectively, with two different control tumor burdens. Note the enhancement of both nodule number as well as volume in the two experiments. A similar sixfold to 17-fold enhancement of tumor mass was noted with 14 mer TRAP treatment of CT26 cells (data not shown).
Effect of Thrombin and TRAP of the PAR-1 Thrombin Receptor on Pulmonary Metastasis of B16F10 Melanoma
| Group . | Median No. of Nodules (range) . | Median Nodule Volume (μL; range) . | Tumor Mass (μL) . | Fold Increase . | P . |
|---|---|---|---|---|---|
| Experiment 1 (n = 12) | |||||
| Control | 1.5 (0-7) | 0.6 (0-6) | 0.9 | ||
| Thrombin | 7 (2-10) | 5.7 (1-15) | 40.0 | 44 | .02 |
| 100 μmol/L 13-mer control peptide | 2.5 (0-9) | 2.3 (0-15) | 5.8 | NS | |
| 100 μmol/L 14-mer TRAP | 5.5 (0-14) | 7.9 (0-39) | 43.5 | 48 (8)§ | .02 (0.03)* |
| Experiment 2 (n = 5) | |||||
| SALLRN | 7 (2-14) | 19.9 (2-59) | 139 | ||
| SFLLRN | 26 (13-31) | 219 (131-353) | 5,694 | 41 | .04 |
| Group . | Median No. of Nodules (range) . | Median Nodule Volume (μL; range) . | Tumor Mass (μL) . | Fold Increase . | P . |
|---|---|---|---|---|---|
| Experiment 1 (n = 12) | |||||
| Control | 1.5 (0-7) | 0.6 (0-6) | 0.9 | ||
| Thrombin | 7 (2-10) | 5.7 (1-15) | 40.0 | 44 | .02 |
| 100 μmol/L 13-mer control peptide | 2.5 (0-9) | 2.3 (0-15) | 5.8 | NS | |
| 100 μmol/L 14-mer TRAP | 5.5 (0-14) | 7.9 (0-39) | 43.5 | 48 (8)§ | .02 (0.03)* |
| Experiment 2 (n = 5) | |||||
| SALLRN | 7 (2-14) | 19.9 (2-59) | 139 | ||
| SFLLRN | 26 (13-31) | 219 (131-353) | 5,694 | 41 | .04 |
TRAP refers to 14 mer-SFLLRNPNDKYEPF or 6 mer-SFLLRN. Fifty thousand viable B16F10 cells were treated with either buffer, 1 U mL thrombin, 100 μmol/L TRAP, or 100 μmol/L control peptides, 13 mer SDEALPLGSPRCD, or 6 mer SALLRN for 60 minutes at 37°C. Cells were then washed three times with PBS-Ca-Mg-0.1 BSA before intravenous injection into the tail vein of syngeneic mice. Pulmonary metastases were examined macroscopically on day 21 for experiment 1 and on day 35 for experiment 2. n refers to number of animals in each subset of the experiment. P value refers to significance of the fold increase in tumor mass compared with control, determined by Wilcoxon Rank Sum test. The SEM is given.
Abbreviation: NS, not significant.
The number in parentheses refers to the increase over control peptide, with P value.
Transfection studies.
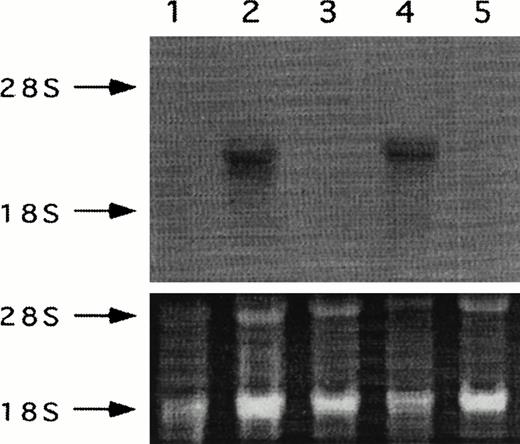
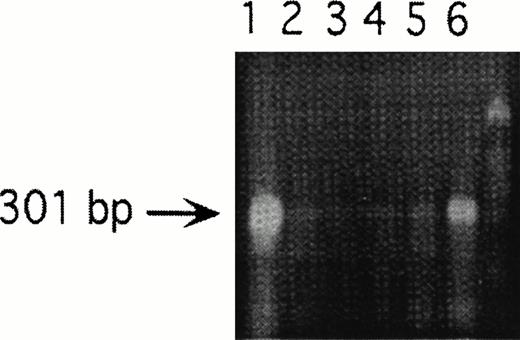
Studies were next designed to determine whether thrombin-enhanced tumor metastasis correlated with PAR-1 expression. This was implemented by modulating PAR-1 expression with PAR-1 transfection of sense and anti-sense constructs. Six sense transfectants (S4, S5, S6, S7, S14, and S22) were constructed (data not shown). S6 and S14 were selected for in vivo studies because of the greater expression of PAR-1 in S14 compared with S6, as suggested by RT-PCR (data not shown) and confirmed by Northern analysis (Fig 2). Four antisense transfectants were constructed (A2, A12, A18, and A57). A12, also shown in Figs 2 and 3, was selected for further in vitro and in vivo studies because of its potent inhibitory effect on PAR-1 mRNA concentration (Fig 3).
Northern analysis of PAR-1 sense and antisense transfectants of B16F10 cells probed with 32P-dCTP–labeled thrombin receptor cDNA. Lane 1, wild-type B16F10; lane 2, antisense A12; lane 3, S6; lane 4, S14; and lane 5, mock-plasmid. Respective ethidium bromide stained ribosomal RNA is given for each lane.
Northern analysis of PAR-1 sense and antisense transfectants of B16F10 cells probed with 32P-dCTP–labeled thrombin receptor cDNA. Lane 1, wild-type B16F10; lane 2, antisense A12; lane 3, S6; lane 4, S14; and lane 5, mock-plasmid. Respective ethidium bromide stained ribosomal RNA is given for each lane.
RT-PCR of endogenous PAR-1 after transfection with antisense constructs, demonstrating relative loss of endogenous PAR-1. Lane 1, pCDNA3 containing PAR-1 cDNA; lanes 2, 3, 4, and 5, antisense constructs A2, A12, A18, and A57, respectively; lane 6, wild-type B16; and unmarked lane 7, molecular weight markers. A12 (lane 3) was used for the in vitro and in vivo antisense experiments.
RT-PCR of endogenous PAR-1 after transfection with antisense constructs, demonstrating relative loss of endogenous PAR-1. Lane 1, pCDNA3 containing PAR-1 cDNA; lanes 2, 3, 4, and 5, antisense constructs A2, A12, A18, and A57, respectively; lane 6, wild-type B16; and unmarked lane 7, molecular weight markers. A12 (lane 3) was used for the in vitro and in vivo antisense experiments.
Immunocytochemistry.
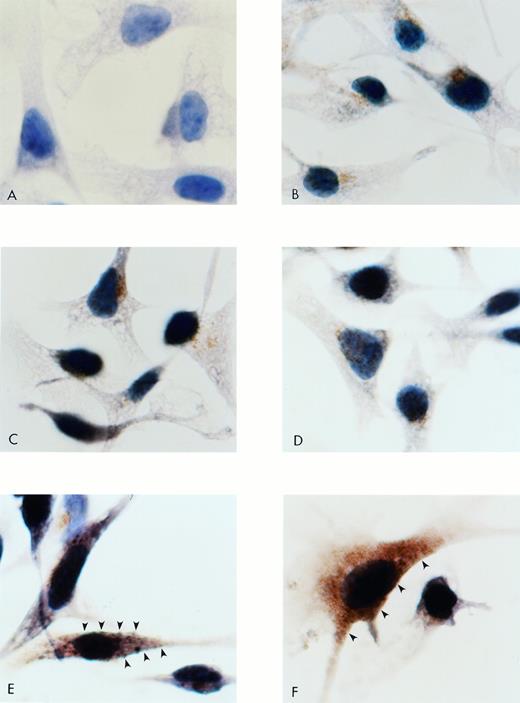
Figure 4 demonstrates intense membrane and intracytoplasmic staining for PAR-1 cells, compared with wild-type B16F10, mock-transfected, antisense, and negative control cells.
Immunocytochemistry for PAR-1 in B16F10 cells transfected with S14 PAR-1, antisense A12, and mock plasmid. Cells were subcultured onto chamber slides, fixed, washed, and stained with a rabbit antirat PAR-1 Ab. PAR-1 binding was detected with a goat antirabbit biotinylated Ab, followed by avidin-biotin-HRP and appropriate chromagen. Cells were counterstained with hematoxylin. (A) Negative control with absence of primary Ab. (B) Wild-type B16F10. (C) Mock plasmid-transfected cells. (D) Antisense A12. (E and F) S14 cells. Note membrane (arrows) as well as intracytoplasmic positive brown staining of PAR-1. Original magnification × 1,500.
Immunocytochemistry for PAR-1 in B16F10 cells transfected with S14 PAR-1, antisense A12, and mock plasmid. Cells were subcultured onto chamber slides, fixed, washed, and stained with a rabbit antirat PAR-1 Ab. PAR-1 binding was detected with a goat antirabbit biotinylated Ab, followed by avidin-biotin-HRP and appropriate chromagen. Cells were counterstained with hematoxylin. (A) Negative control with absence of primary Ab. (B) Wild-type B16F10. (C) Mock plasmid-transfected cells. (D) Antisense A12. (E and F) S14 cells. Note membrane (arrows) as well as intracytoplasmic positive brown staining of PAR-1. Original magnification × 1,500.
Adhesion of transfected cells to fibronectin.
Human thrombin-treated tumor cells enhance their adhesion to the adhesive ligands: fibronectin and vWF as well as platelets and endothelial cells.2-5 We therefore elected to determine whether naive B16F10 cells that do not enhance their adhesion to fibronectin with thrombin treatment would become thrombin-responsive to fibronectin adhesion after PAR-1 transfection. Figure 5 demonstrates the relative increase in adhesion to fibronectin after treatment of transfected B16F10 cells with thrombin. Note the induction of thrombin-responsive adhesion of PAR-1 sense transfectants, S6, S14, S7, and S22. No effect was noted with wild-type, pcDNA3 (empty plasmid), or antisense constructs.
Adhesion of transfected B16F10 cells to fibronectin. Tumor cells were treated with thrombin (0.5 U/mL). Bars refer to the mean ± SD of six to eight experiments performed in triplicate. WT, wild-type; PL, mock transfection with empty plasmid; AS12, antisense transfectant; S6-S22, sense transfectants. Difference between mock transfection and sense transfectants were significant at the P< .004 to .04 level (Student’s t-test).
Adhesion of transfected B16F10 cells to fibronectin. Tumor cells were treated with thrombin (0.5 U/mL). Bars refer to the mean ± SD of six to eight experiments performed in triplicate. WT, wild-type; PL, mock transfection with empty plasmid; AS12, antisense transfectant; S6-S22, sense transfectants. Difference between mock transfection and sense transfectants were significant at the P< .004 to .04 level (Student’s t-test).
Effect of transfected PAR-1 on pulmonary metastasis.
Experiments were next conducted with PAR-1 transfectants of B16F10 to determine whether PAR-1 was rate-limiting for tumor metastasis.
Thrombin-treated mock transfectants increased tumor mass 266-fold by increasing the number as well as volume of nodules in the lung (Table 2). Similar effects were noted with the thrombin-treated S6 transfectant, with low PAR-1 mRNA, which increased tumor mass 200-fold. However, the thrombin-treated S14 transfectant increased tumor mass 1,226-fold, approximately fivefold to sixfold greater than thrombin-treated mock or S6 transfectants. No effect was noted with the thrombin-treated antisense transfectant, which served as an additional control.
Effect of Transfected Thrombin Receptor PAR-1 on Pulmonary Metastasis of B16F10 Murine Tumor Cells
| N . | Group . | Median Nodule No. (range) . | Median Nodule Volume (μL; range) . | Median Tumor Mass (μL) . | Fold Increase* . | P† . |
|---|---|---|---|---|---|---|
| 20 | Mock | 1.0 (0-5) | 0.35 (0-5) | 0.35 | ||
| 20 | Mock + T | 8.3 (0-13) | 11.2 (2-71) | 93 | 266 | .005 |
| 10 | AS | 2 (0-5) | 1.1 (0-4) | 2.2 | ||
| 10 | AS + T | 2 (0-5) | 0.75 (0-4) | 1.5 | ||
| 10 | Sense 6 | 0 (1-8) | 0 (0-7) | 0 | ||
| 10 | Sense 6 + T | 6.5 (2-15) | 10.7 (0.1-43) | 70 | 200 | .02 |
| 20 | Sense 14 | 0 (0-4) | 0 (0-10) | 0 | ||
| 20 | Sense 14 + T | 13.8 (2-23) | 31.1 (1-192) | 429 | 1,226 | .0001 |
| N . | Group . | Median Nodule No. (range) . | Median Nodule Volume (μL; range) . | Median Tumor Mass (μL) . | Fold Increase* . | P† . |
|---|---|---|---|---|---|---|
| 20 | Mock | 1.0 (0-5) | 0.35 (0-5) | 0.35 | ||
| 20 | Mock + T | 8.3 (0-13) | 11.2 (2-71) | 93 | 266 | .005 |
| 10 | AS | 2 (0-5) | 1.1 (0-4) | 2.2 | ||
| 10 | AS + T | 2 (0-5) | 0.75 (0-4) | 1.5 | ||
| 10 | Sense 6 | 0 (1-8) | 0 (0-7) | 0 | ||
| 10 | Sense 6 + T | 6.5 (2-15) | 10.7 (0.1-43) | 70 | 200 | .02 |
| 20 | Sense 14 | 0 (0-4) | 0 (0-10) | 0 | ||
| 20 | Sense 14 + T | 13.8 (2-23) | 31.1 (1-192) | 429 | 1,226 | .0001 |
Experimental details are the same as in Table 1, with pulmonary metastasis examined on day 21.
Abbreviations: Mock, vector alone; T, thrombin (1 U/mL); AS, antisense 12 transfectant.
Increase compared with control mock transfectant.
P value refers to respective thrombin-induced increases between mock or sense transfections determined by Wilcoxon Rank Sum test.
DISCUSSION
These data clearly define for the first time a role for PAR-1 in thrombin-enhanced tumor cell metastasis and adhesion.1-5 8This is documented by (1) enhancement of B16F10 pulmonary metastasis in vivo after treatment of B16F10 cells with the 14-mer PAR-1 thrombin receptor activation peptide SFLLRNPNDKYEPF; (2) enhanced stimulation of thrombin-induced pulmonary metastasis in transfectants overexpressing PAR-1, but not with control transfectants (empty plasmid or antisense); and (3) induction of thrombin-induced B16F10 adhesiveness to fibronectin in naive cells not responding to thrombin after transfection with PAR-1.
A role for PAR-1 in thrombin-induced tumor cell mitogenesis is supported by (1) the absence of the thrombin-induced adhesive effect on wild-type B16F10 and CT26 cells,2 despite thrombin-enhanced pulmonary metastasis; (2) increased pulmonary nodule volume in PAR-1–dependent pulmonary metastatic studies, suggesting that thrombin acts as a growth factor; and (3) thrombin-induced mitogenesis of fibroblasts and smooth muscle cells.20-25
One should consider how an initial thrombin-responsive cohort of cells could lead to a larger pulmonary tumor volume 21 days after introduction into the animal. This is likely due to the initial advantage of enhanced adhesion and mitogenesis in the cohort with respect to establishment of lung colonies. An additional possibility could be the persistence of thrombin responsive genes in daughter cells of the second generation. Preliminary data show that this may be the case with 3 tumor cell lines studied (unpublished data).
It should be recognized that PAR-1 per se does not induce a metastatic phenotype, but that other malignant and/or invasive properties are required. For example, we have observed that nonmetastatic thrombin-treated 3T3 cells (which contain PAR-1) as well as 3T3 cells transfected with PAR-1 do not induce pulmonary metastasis after intravenous injection into syngeneic mice (unpublished data). It is likely that PAR-1 contributes to metastasis by augmentation of already established metastatic properties of malignant cells capable of undergoing this event. It is possible that this may in part be due to the induction of thrombin-responsive genes in malignant cells. A precedent for such an association has been reported for a human PC-3 prostatic cancer cell line in which the urokinase gene is induced,26 resulting in an enzyme attributed to be required for tumor cell invasion.27,28 An alternative and/or additional possibility would be stimulation of cell-cycle pathways leading to mitogenesis, as supported by induction of c-fos and c-jun,29,30 stimulation of tyrosine kinase phosphorylation,8 and activation of the MAP-kinase pathway31 after PAR-1 stimulation.
A role for PAR-1 in tumor cell adhesion is supported by the induction of thrombin-responsive adhesiveness to fibronectin in PAR-1–transfected naive cells. The mechanism of thrombin-enhanced adhesion of human tumor cells has been examined by our group5 with respect to measurement of adhesive ligand integrin receptor density before and after thrombin stimulation. No enhancement of receptor density was noted for fibronectin, laminin, collagen, vitronectin, vWF, intercellular adhesion molecule-1 (ICAM-1), ICAM-2, fibrinogen, C3b, or factor X in an SK-melanoma-28 human cell line responsive to thrombin-enhanced adhesion to endothelial cells,5 platelets,2 fibronectin, and vWF (unpublished data). These observations are compatible with a receptor change in conformation rather than density. The mechanism of this interaction remains to be determined.
These data also support the concept that PAR-1 is both required and rate-limiting for thrombin-induced adhesion and metastasis, because (1) B16F10 cells do not contain detectable PAR-3 or GPIb; (2) B16F10 cells transfected with PAR-1 became thrombin-responsive for adhesion to fibronectin in vitro; and (3) B16F10 cells transfected with PAR-1 increased thrombin-enhanced metastatic potential fivefold to sixfold. This is compatible with the concept that downstream events induced by ligation of PAR-1 are in excess compared with endogenous PAR-1 availability for cell signaling.
Thus, ligation of PAR-1 on tumor cells induces a metastatic phenotype. Because most tumors are capable of generating thrombin, presumably via activation of plasma coagulation factor X by constitutively expressed tissue factor and/or a tumor-associated cysteine protease,17,32 an autocrine more malignant phenotype can be induced. In this regard it is of interest that expression of tissue factor on melanoma cells correlates with hematogenous metastasis33-35; and enzymatically active thrombin is present on surgically removed tumor specimens, including malignant melanoma, by immunohistochemical analysis.36
Of clinical relevance are the observations that low-grade intravascular coagulation has been observed in most patients with solid tumors.37-40 Indeed, fibrinopeptide A levels are elevated in 60% of cancer patients at time of presentation, with increasing levels noted with progressive disease and persistent elevation associated with a poor prognosis.40 It is therefore proposed that agents designed to inhibit PAR-1 ligation and/or thrombin generation at the site of the tumor may prove helpful in containing the metastatic and/or malignant process.
M.L.N. and K.C. contributed equally to this work.
Supported by Grant No. DHP-59 from the American Cancer Society (S.K.), Merit Review Award 001 from the New York Department of Veterans Affairs (M.L.N.), the Helen Polonsky Research Fund, and the Dorothy and Seymour Weinstein Platelet Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
NOTE ADDED IN PROOF
Subcutaneous injection of 5 × 104 thrombin-treated (0.5 U/mL) PAR-1 transfected S14 cells into C57BL/6J mice resulted in a 17.7-fold increase in nodule volume compared to mock-transfected cells at 17 days (1,171 v 66 mm3, n = 6, P= .04). Thus, thrombin directly stimulates tumor growth in vivo.
Author notes
Address reprint requests to Simon Karpatkin, MD, Department of Medicine, Professor of Medicine, New York University Medical Center, 550 First Ave, New York, NY 10016; e-mail:simon.karpatkin@MCFPO.med.nyu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal