Abstract
A primitive human hematopoietic myeloid progenitor cell line, KG1a, characterized by high expression of the CD34 surface antigen has been observed to extend long, thin pseudopodia. Once extended, these pseudopods may take on one of two newly described morphologies, tenupodia or magnupodia. Tenupodia are very thin and form in linear segments. They adhere to the substrate, can bifurcate multiple times, and often appear to connect the membranes of cells more than 300 μm apart. Magnupodia are much thicker and have been observed to extend more than 330 μm away from the cell. Magnupods are flexible and can exhibit rapid dynamic motion, extending or retracting in a few seconds. During retraction, the extended material often pools into a bulb located on the pod. Both morphologies can adhere to substrates coated with fibronectin, collagen IV, and laminin as well as plastic. The CD34 and CD44 antigens are also present on the surface of these podia. Primary human CD34+ cells from fetal liver, umbilical cord blood, adult bone marrow, and mobilized peripheral blood extend these podia as well. The morphology that these pseudopods exhibit suggest that they may play both sensory and mechanical roles during cell migration and homing after bone marrow transplantation.
MANY CELL TYPES have been observed to exhibit dynamic formation and retraction of surface extensions as they migrate. These pseudopodia may play either a sensory or mechanical role and are typically classified by their morphology. Some examples are the bulbous lobopodia used by the parasitic amoeba Entamoeba histolytica and the broad, flat lamellipodia produced by the amoeba Acanthamoeba castellani.1 Mammalian cells also exhibit pseudopods of the lamellipodia variety along with accompanying microspikes that are fine hair-like extensions about 0.1 to 0.2 μm in diameter and up to 20 μm in length. T lymphocytes are known to project uropods that mediate cell-cell interaction,2whereas neurites form longer microspikes known as filopodia that are critical to the growth cone guidance process.3-6 The filopodia extended by neurites can be up to 50 μm long.7Platelets also extend active membrane processes in which dynamic membrane ruffling is accompanied by the deployment of microspikes.8
The acute myelogenous leukemia cell line, KG1a, was first isolated in 1980.9 A prominent phenotypic abnormality of these cells is their inability to differentiate into functionally mature cells, causing them to remain in an early or primitive state of development.10 Because of their primitive nature, KG1a cells were used in a strategy to develop antibodies that recognized immature hematopoietic cells.11 The result was the first antibody against the cell surface marker CD34, which has since become the marker of choice in progenitor cell selection for hematopoietic studies as well as enrichment protocols for stem cell therapies.12
The migratory and homing characteristics of hematopoietic stem cells (HSCs) are of significant current interest, because these processes play a major role in hematopoietic engraftment and recovery after bone marrow transplantation. Transplantation of cells characterized by the CD34 surface antigen induces hematopoietic reconstitution in patients undergoing myeloablation. The homing of these cells to the marrow is a complex and poorly understood process that likely involves chemokines for navigation13 as well as adhesive interactions14-17 to guide them to their appropriate niches within the bone marrow. Of the many adhesive interactions likely involved in this process, the CD44 antigen is known to bind to fibronectin, a common extracellular matrix component.18 In addition to forming adhesive attachments, migration also requires the cell to deform, extending cytoplasmic projections and generating contractile forces as it moves.19,20 Although in principle CD34+ cells must also undergo the same physical processes required for motion, little is known about how they actually accomplish this task. The discovery of the mobilization of HSCs into circulation,21 enabling the collection of an engrafting cell population by leukophoresis, further illustrates the importance of understanding the migratory characteristics of primitive hematopoietic cell populations.
We have examined the migration characteristics of KG1a cells in culture using time-lapse fluorescent microscopy. What we report here is the characterization of two new morphologies of thin (about 1 μm or less) pseudopods, tenupodia, and magnupodia displayed by these cells. Tenupodia (from Latin meaning thin or tenuous) are very thin and form in linear segments. They adhere to the substrate, can bifurcate multiple times, and often appear to connect the membranes of cells more than 300 μm apart. Magnupodia (from Latin meaning large or long) are slightly thicker and have been observed to extend more than 330 μm away from the cell. Magnupods are flexible and can exhibit rapid dynamic motion, deploying or retracting in a few seconds. As they retract, the extended material often pools into a bulb located on the pod. Both morphologies can adhere to substrates coated with fibronectin, collagen IV, and laminin as well as tissue culture-treated plastic. We have observed the deployment of these podia during in vitro cell migration, suggesting that they perform both sensory and mechanical functions. These observations may have implications as to the role podia play in engraftment after bone marrow transplantation.
MATERIALS AND METHODS
Cell line.
The human hematopoietic cell line KG1a was obtained from the ATCC (Rockville, MD). Cells were received cryopreserved and then rapidly thawed and suspended in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO-BRL, Grand Island, NY) containing 4 mmol/L L-glutamine, 1.5 g/L sodium bicarbonate, and 20% fetal bovine serum. The line was maintained in an incubator at 37°C, 95% humidity, and 5% CO2. Passage was performed every 3 to 4 days (as recommended by the ATCC). Propidium iodide (Boehringer Mannheim, Indianapolis, IN) was used to check for cell viability following the manufacturer’s recommendations.
Primary cell sources.
Human CD34+ cells were obtained from several sources. CD34+ cells from mobilized peripheral blood (mPB) were obtained from donors that were mobilized with a combination of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) cytokines. The patients’ peripheral blood was leukaphoresed and the mononuclear cells were isolated. CD34 enrichment was performed with an immunomagnetic separation column (AmCell, Sunnyvale, CA). Approximately 85% purity of CD34+ cells was typically achieved. The cells were then cryopreserved in liquid nitrogen. After rapid thawing at 37°C, they were suspended in Meylocult H5100 media (Stem Cell Technologies Inc, Vancouver, British Columbia, Canada) containing 12.5% fetal calf serum and 12.5% horse serum (without additional hematopoietic growth factors) and allowed to recover for 6 to 12 hours before staining.
Purer CD34+ preparations from fetal liver (FL), umbilical cord blood (UCB), and adult bone marrow (aBM) were obtained using flow cytometric sorting (FACStar Plus; Becton Dickinson, San Jose, CA). The fluorescein isothiocyanate (FITC)-conjugated CD34 antibody used was anti-HCPA-2 (clone 8G12; Becton Dickinson). These cells are suspended in the same Meylocult media and are used fresh, immediately after sorting. The number of viable cells was increased by sorting out dead cells based on their light scattering characteristics.
Cell preparation.
For visualization, the cells were stained with the fluorescent membrane dye, PKH26 (Zynaxis, Malvern, PA), which excites at a wavelength of 551 nm and emits at 567 nm. The staining procedure incorporates aliphatic reporter molecules into the cell membrane so the fluorescence detected depends on the amount of membrane in any given location.22 23 Cells were stained following the manufacturer’s guidelines. Briefly, approximately 1 million cells were suspended in 1 mL of diluent C. PKH26 was diluted in diluent C to a ratio of 8 μL dye to 1 mL of diluent C. The diluted dye was combined with the cells (final concentration, 4 μmol/L) for a period of 6 minutes, after which an equal volume of Dulbecco’s phosphate-buffered saline (PBS; GIBCO-BRL) containing 1% Leptalb-7 (Intergen, Purchase, NY) was added to stop the staining reaction. After 1 minute, an equal volume of fresh medium was added. The cells were then spun down at 400g for 10 minutes and washed two additional times in PBS or fresh media.
Immunostaining for surface antigens was performed using the following technique. KG1a cells were washed in PBS (GIBCO-BRL) and then resuspended in a isotonic, 295 mOsm sucrose solution to avoid salt crystal formation as the media dried. Fifty microliters of the cell suspension was allowed to dry on a glass slide. Drying was performed to preserve the fragile pod structures, because conventional fixing protocols resulted in damaged and missing podia. Then, 50 μL of 1:100 dilution of either CD34 (phycoerythrin-conjugated anti-HCPA-2; Becton Dickinson) or CD44 (phycoerythrin conjugated; Pharmingen, San Diego, CA) antibody in the isotonic sucrose solution was applied to the dried cells and allowed to incubate at 4°C overnight before imaging the next day. As a negative control, isotype control antibodies conjugated to phycoerythrin (mouse IgG2b for CD44 and mouse IgG1 for CD34; Pharmingen) were used in the same procedure.
Time-lapse image acquisition.
Cells were imaged using an inverted Diaphot 300 fluorescent microscope (Nikon Inc, Melville, NY) with 20×, 40×, and 100× objectives. PKH26 images were obtained using fluorescent filter set 41003 (Chroma Technologies, Brattleboro, VT). Digitized images were acquired with a cooled CCD camera (Photometrics Inc, Tucson, AZ) and stored on an SGI O2 workstation (Silicon Graphics, Mountain View, CA). All acquisition and processing functions were controlled by Isee software (Inovision Corp, Durham, NC), which provided complete automation for the system.
Image processing.
Podia are not clearly visible using bright field, phase contrast, or Hoffman modulation contrast microscopy at lower magnification, but are readily detectable using the fluorescent membrane dye PKH26 or with a higher power, 100× objective. The fluorescent podia are very dim relative to the bright cells from which they emanate. For this reason, the contrast of the fluorescent images was enhanced so that the podia became visible. With the contrast enhanced, the image of the cell is degraded and small pieces of membrane fragments and debris created during the staining process appear as bright objects in the image.
Environmental conditions and cell manipulation.
The cells were kept in a constant 37°C environment by an incubator surrounding the microscope. The incubator consisted of a large, sealed, custom fabricated plexiglas box heated by a warm air blower controlled by a PID temperature controller (Cole-Parmer, Vernon Hills, IL). Control of pH to approximately 7.4 was achieved by flushing the plexiglas box with CO2. A controller constantly sensed the CO2 in the box and maintained its concentration at 5%. Media evaporation was reduced to approximately 2% per day by filling adjacent wells with water and wrapping the multiwell plates with parafilm (American National Can, Greenwich, CT).
Cells were plated at different densities on various substrates in plastic tissue culture dishes, 96-well plates (Costar, Cambridge, MA), and glass microscope slides and coverslips. Other surfaces tested were human fibronectin-coated glass coverslips, 35-mm plastic dishes, and 96-well plates coated with mouse collagen IV, mouse laminin, and human fibronectin (Becton Dickinson, Bedford, MA).
Other experiments involved the manipulation of the cells once they deployed their pods. A micromanipulator system (Eppendorf, Hamburg, Germany) was used to position a standard 1.1-mm (outside diameter) capillary tube in the cell well. The tube was bent at a 90° angle approximately 18 mm from the end (so it could fit into the deep, small diameter wells) by heating it with a bunsen burner. This process prohibited the use of micropipette tips, because the heat caused the thin glass tip to melt. Through the use of a syringe, media was gently drawn into the pipette and then expelled causing the podia to experience mechanical forces due to the fluid motion.
RESULTS
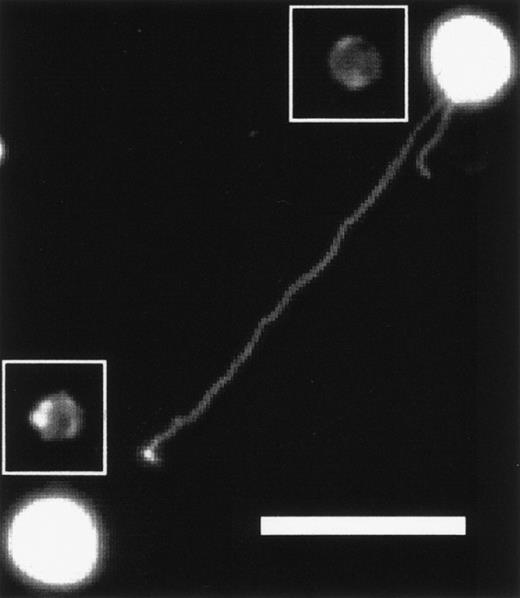
When KG1a cells are plated and allowed to incubate for approximately 1 hour at 37°C, they begin to deploy long, thin pods. The KG1a cell shown in Fig 1 deployed a magnupod that extended approximately 94 μm away from the cell towards a neighboring cell. Magnupods tend to float in the medium and, if there is any fluid motion, will extend downstream, whereas the cell remains adhered to the substrate. This phenomena is most obvious when a 10-μL drop of cell suspension is placed on a glass slide. As the media evaporates, fluid moves towards the edge of the drop due to capillary flow causing a radial current.24 The cells near the edge deploy magnupods that point radially outward.
KG1a cell projecting a magnupod that is approximately 94-μm long toward a neighboring cell. The surface was coated with fibronectin and a 20× objective was used. The scale bar is 50-μm long. The contrast of the image has been enhanced so that the podia are visible. Unenhanced images of the cells have been inserted into the figure so the actual size of the cells is more obvious.
KG1a cell projecting a magnupod that is approximately 94-μm long toward a neighboring cell. The surface was coated with fibronectin and a 20× objective was used. The scale bar is 50-μm long. The contrast of the image has been enhanced so that the podia are visible. Unenhanced images of the cells have been inserted into the figure so the actual size of the cells is more obvious.
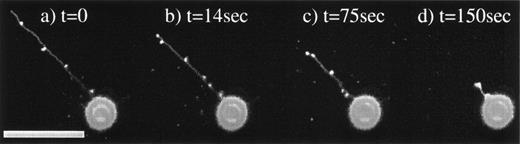
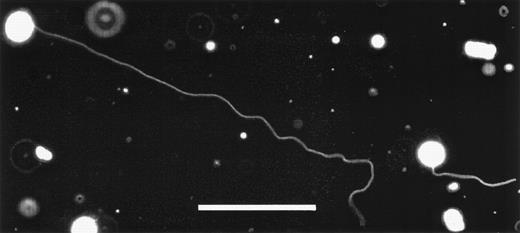
Magnupods are capable of rapidly retracting as seen in the time sequence of Fig 2a through d. Shorter magnupods (<20 μm) can retract into the cell body in a matter of seconds, whereas the longer podia pool the membrane into bulbs that form along the length of the pod. The cell shown in Fig 2 was observed to retract its magnupod at a rate of 0.4 μm/s. Extension and retraction of magnupods is not a continuous process. Once the magnupod has extended, it is not uncommon for it to remain deployed for several hours. The stimulus responsible for the extension or retraction of magnupodia is unknown at this time. Other interesting features of the magnupod morphology are that they can extend to great distances away from the cell, as seen in Fig 3, which shows a magnupod with a total length of greater than 330 μm. The tip of the magnupod is out of focus, indicating that it is floating freely above the surface. However, magnupods are also capable of forming attachments to the substrate. These can be identified by applying mechanical forces with a micropipette. The attachment was determined by using the micropipette to induce fluid flow around the cell. The cell and the pod deform and may float away, except where it is anchored to the substrate (data not shown). However, these attachments are not continuous along the magnupod, suggesting a nonuniform distribution of adhesion complexes.
KG1a cell retracting a magnupod. Four images were taken at various time intervals. Image (a) was taken at t = 0, (b) at t = 14 seconds, (c) at t = 75 seconds, and (d) at t = 150 seconds. The corresponding lengths of the magnupod are 77, 58, 42, and 14 μm, respectively, resulting in a retraction rate of approximately 0.4 μm/s. The scale bar is 50-μm long.
KG1a cell retracting a magnupod. Four images were taken at various time intervals. Image (a) was taken at t = 0, (b) at t = 14 seconds, (c) at t = 75 seconds, and (d) at t = 150 seconds. The corresponding lengths of the magnupod are 77, 58, 42, and 14 μm, respectively, resulting in a retraction rate of approximately 0.4 μm/s. The scale bar is 50-μm long.
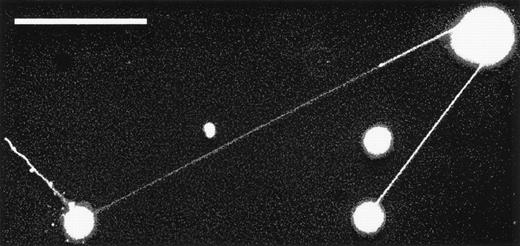
Two KG1a cells displaying extremely long magnupodia. The total length of the podia extended by the cell on the left is over 330-μm long. The tip of one magnupod is out of focus, indicating that it is floating above the plane of focus. The scale bar in this image is 100-μm long. Enhancing the contrast of this image so that the podia become visible has also caused membrane fragments and debris to appear as bright objects.
Two KG1a cells displaying extremely long magnupodia. The total length of the podia extended by the cell on the left is over 330-μm long. The tip of one magnupod is out of focus, indicating that it is floating above the plane of focus. The scale bar in this image is 100-μm long. Enhancing the contrast of this image so that the podia become visible has also caused membrane fragments and debris to appear as bright objects.
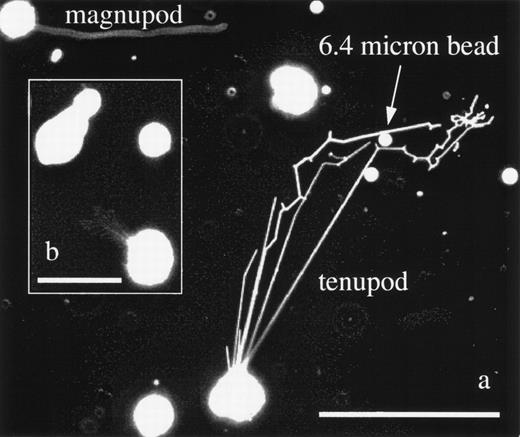
Tenupodia are the second new pod morphology found on KG1a cells. Tenupodia are morphologically substantially different from magnupodia and they exhibit different behavior. An example of tenupod formation is given in Fig 4. Figure 4a shows a KG1a cell that is sprouting tenupods on a laminin-coated substrate (at the top is a second KG1a cell projecting a magnupod). The tenupod appears as linear segments that deploy from a common point of origin on the cell surface. They extend away from the cell body and may branch or turn at specific points. One of the tenupods, upon encountering a 6.4-μm plastic bead, navigates around it by bifurcating. This bifurcation behavior is also seen in Fig 4b, in which a cluster of tenupods extend away from a single cell. Tenupodia are more prevalent on surfaces coated with fibronectin, laminin, and collagen IV than on glass or plastic, suggesting that interactions with the surface play an important role in determining podia morphology. Finally, tenupods have been observed to connect the membranes of two different cells located more than 300 μm apart. A typical example of this behavior is seen in Fig 5, which shows tenupods extended in a linear fashion directly to the neighboring cell. In contrast, multiple tenupods from the cell in Fig 4a deployed along a more circuitous route as they converged on a region to the upper right of the cell, changing direction in a seemingly nonrandom manner, as if some factor were acting to bias the direction of their extension. Taken together, these observations suggest that tenupodia sense and respond to environmental cues. Capabilities such as these would allow tenupods to home in on a distant source that was secreting a soluble factor or to follow a trail of adhesion molecules bound to the extracellular matrix.
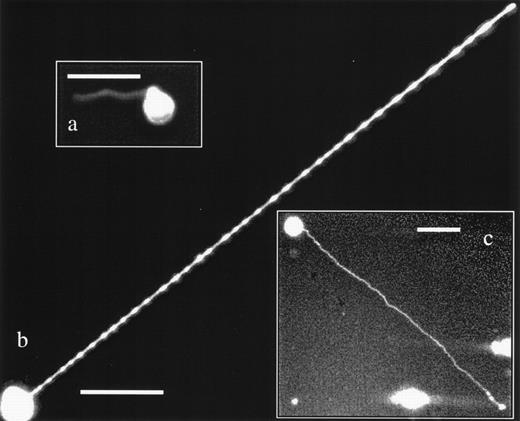
(a) KG1a cells displaying both podia morphologies. The cell in the upper left corner has extended a 114-μm long magnupod, whereas the other cell has deployed multiple tenupodia. One tenupod has extended in the direction of a 6.4-μm plastic bead before bifurcating and heading off to the right of the image, where it appears to have located whatever factor was attracting it. The scale bar is 100-μm long. (b) This inset image shows a KG1a cell with a highly branched tenupod network that has extended toward a neighboring KG1a cell that was migrating via a lamellapodia. The scale bar is 50-μm long.
(a) KG1a cells displaying both podia morphologies. The cell in the upper left corner has extended a 114-μm long magnupod, whereas the other cell has deployed multiple tenupodia. One tenupod has extended in the direction of a 6.4-μm plastic bead before bifurcating and heading off to the right of the image, where it appears to have located whatever factor was attracting it. The scale bar is 100-μm long. (b) This inset image shows a KG1a cell with a highly branched tenupod network that has extended toward a neighboring KG1a cell that was migrating via a lamellapodia. The scale bar is 50-μm long.
Tenupods can extend toward other cells and appear to connect the membranes. In this image, tenupodia connect the membranes of 3 neighboring cells. The longest tenupod is 310-μm long. The scale bar is 100-μm long.
Tenupods can extend toward other cells and appear to connect the membranes. In this image, tenupodia connect the membranes of 3 neighboring cells. The longest tenupod is 310-μm long. The scale bar is 100-μm long.
Primary human CD34+ cells also exhibit the same podia formation behavior as the KG1a cells. The images in Fig 6a through c show CD34+cells from FL, UCB, and aBM displaying both magnupodia and tenupodia. Mobilized peripheral blood CD34+ cells also behave in a similar manner (data not shown). In more than 40 experiments with primary cells, we have observed podia formation in greater than 75% of the experiments. Magnupod and tenupod formation by the primary cells was not as consistent as with the KG1a cell line, possibly due to donor variability25 or sensitivity to cell processing, preparation, and culture conditions. Interestingly, these podia morphologies appear to be common among all the sources of primary CD34+ cells we have tested thus far.
Primary human CD34+ cells also display magnupodia and tenupodia. (a) A CD34+ cell from an umbilical cord blood source displaying a magnupod. (b) A human fetal liver CD34+ cell with a very long tenupod. (c) aBM CD34+ cell deploying a long magnupod. Scale bars in all images are 30-μm long.
Primary human CD34+ cells also display magnupodia and tenupodia. (a) A CD34+ cell from an umbilical cord blood source displaying a magnupod. (b) A human fetal liver CD34+ cell with a very long tenupod. (c) aBM CD34+ cell deploying a long magnupod. Scale bars in all images are 30-μm long.
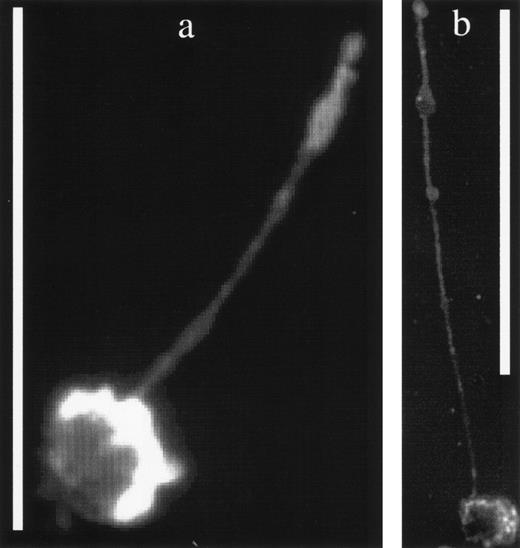
The CD34 and CD44 surface antigens are also associated with podia formation in KG1a cells, as shown in Fig 7a and b. The magnupod in Fig 7a has stained brightly for the CD34 antigen, whereas staining for CD44, a known surface adhesion molecule, has also shown its presence on the surface of the podia in Fig 7b. Negative controls were always dim and no detectable nonspecific binding was observed. The fact that these podia were detected using antibody staining and also on unstained cells at high magnification indicates that they are not an artifact of the PKH26 membrane dye staining. At the time of observation, approximately 95% of the KG1a cells were viable. Primary cells were not checked for viability. KG1a cells that were killed by heating to 65°C for 15 minutes did not deploy any podia, showing that, as expected, their extension is an active process.
(a) A KG1a cell displaying a magnupod that has been stained with an antibody against the CD34 surface marker. The scale bar is 50-μm long. (b) A similar KG1a cell stained for the presence of the CD44 antigen on its surface. The scale bar is 100-μm long. Note that the antibody staining of the cell bodies in these fluorescent images is not as bright as the PKH26 staining, resulting in more uniformly enhanced images.
(a) A KG1a cell displaying a magnupod that has been stained with an antibody against the CD34 surface marker. The scale bar is 50-μm long. (b) A similar KG1a cell stained for the presence of the CD44 antigen on its surface. The scale bar is 100-μm long. Note that the antibody staining of the cell bodies in these fluorescent images is not as bright as the PKH26 staining, resulting in more uniformly enhanced images.
DISCUSSION
Many cell types exhibit dynamic surface extensions when they migrate or change shape. Such extensions are capable of dynamic formation and retraction with typical velocities for microspikes and lamellipodia on the order of 0.1 μm/sec.1 In neurites, filopodia are believed to play a role in the progression of axon elongation by aiding the assembly of microtubules.26 These filopodia extend from the lamellipodial region, acting as radial sensors, and are crucial to growth cone navigation.3-6 They have also been found to carry receptors for certain cell adhesion molecules.27Mature blood cells can also deploy cytoplasmic extensions. Recently, structures termed uropods have been found on T lymphocytes2that form during lymphocyte-endothelial cell interaction. T cells have been found to use uropods to contact and communicate directly with other T cells. Uropod development was promoted by physiologic factors such as chemokines. Pseudopodia, therefore, can display a variety of dynamic and morphological properties while performing a spectrum of functions in many different cell types related to migration and communication.
We have discovered that cells of the primitive human hematopoietic myeloid progenitor cell line, KG1a, are capable of extending two fundamentally new pseudopodia morphologies. These podia (1) exhibit dynamic extension and retraction behavior, (2) can adhere to the substrate they are migrating over, (3) appear to be guided by environmental stimuli, and (4) have antigens for CD34 and CD44 antibodies on their surfaces. However, magnupodia and tenupodia are distinctly different morphologies exhibited by KG1a cells and may therefore perform specialized migratory functions that are unique to primitive hematopoietic cells, such as homing.
Magnupods have morphological similarities with the long (50 μm), thin (0.2 μm) filopodia found on neurites.28,29 However, they differ in that magnupod extension occurs to much greater distances (>300 μm, or roughly 30 cell diameters) from the main cell body, and they exhibit rapid dynamic behavior, retracting more than eight times faster than neurite filopodia. Magnupods may remain extended for several hours, whereas neurite filopodia extend and retract continuously. It has been suggested that the switch between neurite filopodia extension and retraction reflects a shift in the actin polymerization and depolymerization rates.29 This difference in dynamic characteristics may indicate structural as well as functional differences between filopodia and magnupodia. Whereas filopodia appear to act primarily as sensory appendages in neurites, magnupods may play a more mechanical role in migration, similar to lamellipodia. Magnupods can adhere to the substrate at specific points, and recently it has been reported that the migratory properties of KG1a cells are enhanced when they are crawling over a substrate coated with fibronectin.30 One may speculate that a deployed magnupod drifts freely until its surface adhesion complexes encounter suitable binding sites on the substrate. A retraction would then drag the cell body towards the site of adhesion. Additionally, a retraction may also be hindered by multiple point attachments, with the result being that the podia material pools into bulbs along the length of the magnupod. This dynamic behavior makes the magnupod morphology uniquely different from filopodia.
Tenupodia, on the other hand, may perform a more sensory type role. They are thinner and probably do not have the structural characteristics necessary to drag a cell. A peculiar feature of this morphology is that they extend in perfectly linear segments and often in a line directly toward another cell. If the tenupod stops growing, it will likely bifurcate or turn about a fixed point and continue its linear propagation until it encounters its target. This type of guidance may be mediated by diffusible factors released by cellular targets.31,32 It is estimated that the domain that a single cell can effectively communicate in by using a soluble signal is about 250 μm in size and that the communication within this domain takes place in 10 to 30 minutes.33 These theoretically derived estimates correlate well with the times and distances that tenupods travel to reach or connect with a neighboring cell. Uropods also exhibit similar sensory properties in that they have been observed to contact, capture, and recruit additional T cells during lymphocyte-endothelial interaction.2 Although a precedent has been set for this type of behavior, uropodia appear to be wider and extend only a few cell diameters away from the cell, nowhere near the extension distances of tenupods. Ultrastructural analysis of antigen-presenting dendritic cells indicates that these cells also deploy extensions that are much longer than the cellular body.34 However, these observations show that the extension constituted a major part of the cellular volume, whereas the volume of tenupods is insignificant compared with the cell. This high surface area to volume ratio could also make the tenupod highly sensitive to environmental signals.5 Tenupods are also more prevalent when the cells are cultured on surfaces coated with extracellular matrix components such as fibronectin, laminin, or collagen IV. This indicates that adhesion and contact guidance play a role in their formation and morphology. Whether it is via soluble signals, surface adhesion, or both, environmental interactions clearly influence the morphology of tenupods, suggesting that the function they perform is sensory in nature.
The CD44 antigen has been implicated as playing an important role in homing and hematopoiesis35,36 due to its ability to bind to a variety of extracellular matrix components, including fibronectin and collagen.18,37 Recently, it has been suggested that the CD34 antigen also participates in adhesive interactions thorough an indirect mechanism, signaling changes in the surface profile of other adhesion molecules.38 Furthermore, there are intracellular stores of CD34 protein that can be translocated to the plasma membrane in response to extracellular signals.39 Compositional data such as these indicate that a relationship between key surface markers, homing-related adhesive proteins, and pseudopod morphology may exist.
Perhaps the most interesting finding is that these podia are found on primary human hematopoietic cells. In addition to the CD34+KG1a cells, primary human CD34+ cells from FL, UCB, aBM, and mPB that we have tested as well as mouse fetal liver cells all display these new pseudopod morphologies. Therefore, these new morphologies are not a particular property of the KG1a cell line. It should also be noted that KG1a cells and primary human CD34+ cells extend lamellipodia-type processes during migration. When imaged in time-lapse mode, these cells also exhibit the classical crawling behavior as described in the literature.1,19 40 Of the plated cells, only about 1% to 10% actually deploy magnupods or tenupods at any given time.
Given that (1) known pseudopodia types perform specific functions in communication and migration, (2) primitive hematopoietic cells extend long thin pseudopods, and (3) important activities of primitive hematopoietic cells are communication and migration, it is possible that the morphology and physiologic activity of magnupodia and tenupodia are related to the function they perform while the cell is homing after transplantation. These data may significantly impact our thinking about how primitive hematopoietic cells migrate and the role of cytoskeletal structures in the clinically important process of stem cell engraftment.
ACKNOWLEDGMENT
The authors acknowledge the assistance of Dr Anthony D. Ho, Dr Ping Law, Dennis Young, Sandy Peterson, Dr Ewa Carrier, Jody Donahue, and Dr Shiang Huang of the USCD Bone Marrow Transplant Program in supplying primary human and mouse cells and Dr Duk-Jae Oh and Mahshid Palsson of the UCSD Bioengineering Department for maintaining the cell lines and assisting with cell preparation.
Supported by Grant No. NAG9-652 from NASA, a grant from the Charles H. Stern and Anna S. Stern Foundation (La Jolla, CA), and National Institutes of Health Grant No. RO1HL59234-01.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bernhard O. Palsson, PhD, Department of Bioengineering, University of California San Diego, 9500 Gilman Dr, Mail Code 0412, La Jolla, CA 92093-0412.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal