Abstract
NF-E2 binding sites, located in distant regulatory sequences, may be important for high level α- and β-globin gene expression. Surprisingly, targeted disruption of each subunit of NF-E2 has either little or no effect on erythroid maturation in mice. For p18 NF-E2, this lack of effect is due, at least in part, to the presence of redundant proteins. For p45 NF-E2, one possibility is that NF-E2–related factors, Nrf-1 or Nrf-2, activate globin gene expression in the absence of NF-E2. To test this hypothesis for Nrf-2, we disrupted the Nrf-2 gene by homologous recombination. Nrf-2–deficient mice had no detectable hematopoietic defect. In addition, no evidence was found for reciprocal upregulation of NF-E2 or Nrf-2 protein in fetal liver cells deficient for either factor. Fetal liver cells deficient for both NF-E2 and Nrf-2 expressed normal levels of α- and β-globin. Mature mice with combined deficiency of NF-E2 and Nrf-2 did not exhibit a defect in erythroid maturation beyond that seen with loss of NF-E2 alone. Thus, the presence of a mild erythroid defect in NF-E2–deficient mice is not the result of compensation by Nrf-2.
HIGH-LEVEL EXPRESSION from the β-like globin genes depends on far upstream regulatory sequences known as the locus control region (LCR).1-3 The β-globin LCR acts, in part, by establishing an open chromatin domain over the entire β-globin cluster.3,4 However, the complete mechanism of action of the LCR remains elusive. The β-globin LCR contains four core elements, which are marked by erythroid specific DNase I hypersensitive sites (HS1 to HS4). Individual core elements do not retain the activity of the full LCR, but are sufficient to confer high level, tissue-specific expression on a linked β-globin gene in transgenic mice.5-7 These core elements contain numerous binding sites for erythroid-specific and ubiquitous transcription factors, including NF-E2, GATA, and GGTGG/CACC motifs. In contrast to other binding motifs, the distribution of NF-E2 binding sites in globin regulatory sequences is limited to core-elements in the β-globin LCR and the α-globin positive regulatory element (PRE).8,9This distribution, along with the evolutionary conservation of these sites,10-14 suggests an integral role for NF-E2 sites in LCR function.
NF-E2 is a heterodimeric member of the basic-leucine zipper (BZIP) transcription factor family, composed of 45- and 18-kD subunits (p45 and p18 NF-E2).15-18 Targeted disruption of p45 NF-E2 causes a maturation defect in megakaryocytes, severe thrombocytopenia, and perinatal mortality.19 NF-E2 per se is not required for terminal erythroid differentiation; targeted disruption of each subunit has either little (p45), or no (p18) effect on erythroid maturation.19-21 For p18 NF-E2, this lack of effect can be attributed to the presence of redundant proteins. For p45 NF-E2, the role of redundant factors is less clear.
p45 NF-E2 is a member of the cap'n collar (CNC) subfamily of BZIP transcription factors, named for the prototype protein CNC inDrosophila.22 These proteins share a conserved region of 43 amino acids, which lies immediately N-terminal of the BZIP domain. This region is required for activation of globin gene expression by NF-E2.23 Besides NF-E2, CNC family members in man and mouse include NF-E2 related factors-1 and -2 (Nrf-1/LCR-F1 and Nrf-2).24-27 Both Nrf-1/LCR-F1 and Nrf-2 have been shown to activate reporter genes in transient assays.25,26 Nrf-2 is the homolog of a chicken protein, erythroid protein with CNC homology (ECH).28 Interestingly, ECH, rather than a homolog of NF-E2, was the predominant isolate from an anemic chicken erythrocyte library screened with the BZIP domain of murine NF-E2. We hypothesized that the mild erythroid defect in NF-E2–deficient mice might reflect the ability of Nrf-2 to compensate for the loss of NF-E2. To test this hypothesis, we examined the effect of combined NF-E2 and Nrf-2 deficiency on globin gene expression in mice.
MATERIALS AND METHODS
Cloning of Nrf-2.
A partial cDNA clone of human Nrf-2 was provided by S. Ruben (Human Genome Systems, Rockville, MD). This was used to screen a murine pre-B–cell library and obtain a full-length cDNA clone. The murine Nrf-2 cDNA was used to screen a 129 strain (CCE) embryonic stem cell (ES) library in EMBL3. Three genomic clones were obtained, of which two showed evidence of rearrangement. The third clone was 17.5 kb and contained exons 2-5 of the Nrf-2 gene.
Targeting of the Nrf-2 gene.
A 1.9-kb Cla I-Hind III fragment from the second intron to the fifth exon and a 4.5-kb Hind III-Eco RV fragment from the fifth exon into 3′ flanking sequences were subcloned into targeting vector PPNT (gift from S. Orkin, Children's Hospital, Boston, MA).29 The targeting vector was linearized at a unique Not I site and transfected into RW4 ES cells (Genome Systems Inc, St Louis, MO). ES cells were cultured on SNLH9 feeder cells,30 and the media was supplemented with 1,000 U/mL of leukemia inhibitory factor (LIF) (ESGRO; Life Technologies, Gaithersburg, MD). Clones were selected in G418 (Geneticin, 350 μg/mL active, Life Technologies, and 1(1-2-deoxy-2-fluoro-β-Darabinofuransyl)-5-iodouracil (FIAU, 0.2 μmol/L), and screened for targeted disruption of Nrf-2 by Southern blot analysis. The probe used in the Southern analysis was a 0.7-kb Cla I-Bgl II fragment of exon 2 and intron 2, which was 5′ of the homologous sequences used in the targeting construct. Six clones with homologous recombination of the Nrf-2 gene were karyotyped. Four clones with a normal karyotype were injected into C57BL/6J blastocysts and implanted into pseudopregnant foster mothers, as previously described.31 Male chimeras were mated with C57BL/6J females and germline transmission confirmed by agouti color in the F1 animals. Agouti offspring were tested for mutation of Nrf-2 by Southern blot analysis of tail DNA.
Breeding of mice with combined deficiency of NF-E2 and Nrf-2.
Nrf-2 (-/-) mice on a mixed 129/SvJ-C57BL/6J background were mated to inbred 129/Sv NF-E2 (+/-) mice (gift from S. Orkin).19 Compound heterozygotes from this mating were bred to obtain double homozygous mutant embryos, for analysis, at a frequency of 1/16. Nrf-2 (-/-)/NF-E2 (+/-) mice, generated from the mating of compound heterozygotes, were bred to obtain double homozygous progeny at a frequency of 1/4.
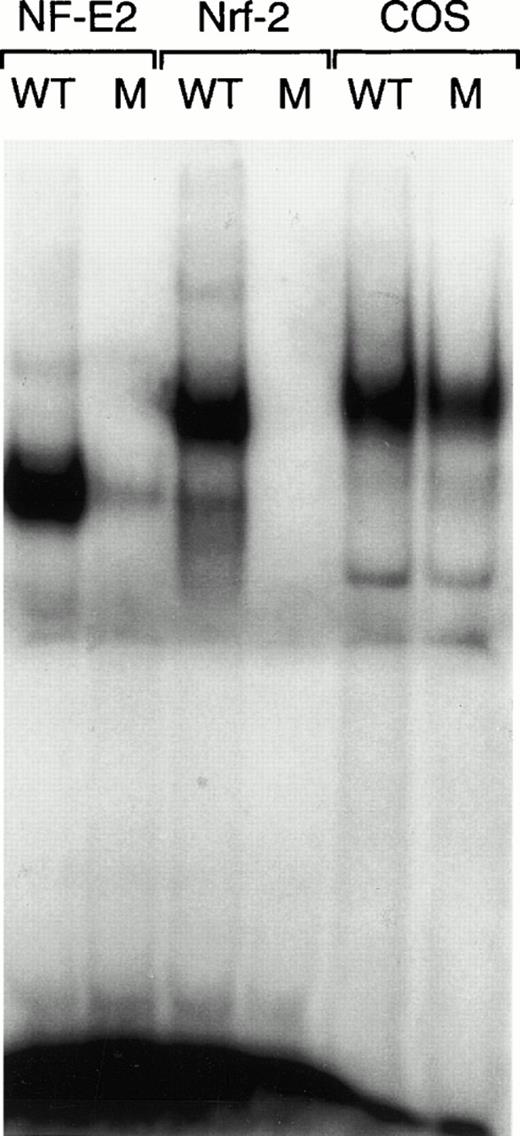
Nrf-2/p18 heterodimers have the same DNA binding specificity as NF-E2. A gel mobility shift assay was performed on in vitro–translated p45/p18 NF-E2 (lanes 1 and 2) and Nrf-2/p18 heterodimers (lanes 3 and 4) and on nuclear extract from untransfected COS cells, as a source of AP-1 binding activity (lanes 5 and 6). The discriminatory mutant probe (M) differs from wild-type (WT) at one nucleotide.39
Nrf-2/p18 heterodimers have the same DNA binding specificity as NF-E2. A gel mobility shift assay was performed on in vitro–translated p45/p18 NF-E2 (lanes 1 and 2) and Nrf-2/p18 heterodimers (lanes 3 and 4) and on nuclear extract from untransfected COS cells, as a source of AP-1 binding activity (lanes 5 and 6). The discriminatory mutant probe (M) differs from wild-type (WT) at one nucleotide.39
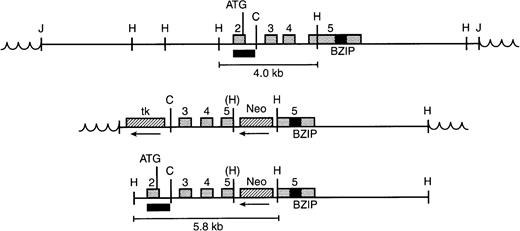
Gene targeting strategy. (Top) Genomic clone containing exons 2-5 of Nrf-2 in EMBL3. Restriction sites indicated are Hind III (H) and Cla I (C). The exon 2 probe used to screen Southern blots is shown by a black box. The junction (J) with vector sequences (wavy lines) is shown, as is the germline Hind III fragment detected with the exon 2 probe. (Middle) Targeting vector showing insertion of the Neomycin resistance gene (Neo) into the fifth exon, in the reverse orientation relative to Nrf-2. An inactivated Hind III site is indicated by parentheses. The thymidine kinase gene (tk) is also in reverse orientation and lies outside the region of homology. (Bottom) Structure of the targeted allele after homologous recombination. The thymidine kinase gene has been lost as a result of homologous recombination. A rearranged 5.8-kb Hind III fragment is shown.
Gene targeting strategy. (Top) Genomic clone containing exons 2-5 of Nrf-2 in EMBL3. Restriction sites indicated are Hind III (H) and Cla I (C). The exon 2 probe used to screen Southern blots is shown by a black box. The junction (J) with vector sequences (wavy lines) is shown, as is the germline Hind III fragment detected with the exon 2 probe. (Middle) Targeting vector showing insertion of the Neomycin resistance gene (Neo) into the fifth exon, in the reverse orientation relative to Nrf-2. An inactivated Hind III site is indicated by parentheses. The thymidine kinase gene (tk) is also in reverse orientation and lies outside the region of homology. (Bottom) Structure of the targeted allele after homologous recombination. The thymidine kinase gene has been lost as a result of homologous recombination. A rearranged 5.8-kb Hind III fragment is shown.
Targeting of the Nrf-2 gene by homologous recombination. (A) Targeted disruption of one Nrf-2 allele. A representative Southern blot is shown hybridized with the Nrf-2 exon 2 probe (top) or a Neomycin resistance cDNA probe (bottom). The germline Hind III band of 4.0 kb is present in all clones. The 5.8-rearranged band, specific for homologous recombination, was present in about 60% of the clones. (B) Southern blot of tail DNA from the first F2 litter with controls. (C) Targeted disruption of both Nrf-2 alleles. A Southern blot is shown hybridized with the Nrf-2 exon 2 probe (top) or a hygromycin resistance cDNA probe (bottom). The second allele, disrupted by the hygromycin resistance gene, gives a 6.0-kb rearranged Hind III band and the appearance of a doublet on shorter exposures.
Targeting of the Nrf-2 gene by homologous recombination. (A) Targeted disruption of one Nrf-2 allele. A representative Southern blot is shown hybridized with the Nrf-2 exon 2 probe (top) or a Neomycin resistance cDNA probe (bottom). The germline Hind III band of 4.0 kb is present in all clones. The 5.8-rearranged band, specific for homologous recombination, was present in about 60% of the clones. (B) Southern blot of tail DNA from the first F2 litter with controls. (C) Targeted disruption of both Nrf-2 alleles. A Southern blot is shown hybridized with the Nrf-2 exon 2 probe (top) or a hygromycin resistance cDNA probe (bottom). The second allele, disrupted by the hygromycin resistance gene, gives a 6.0-kb rearranged Hind III band and the appearance of a doublet on shorter exposures.
In vitro differentiation of Nrf-2 (-/-) embryoid bodies.
The targeting construct was modified by substituting a hygromycin resistance gene for neomycin resistance. This construct was transfected into ES clone 5.18 with one targeted Nrf-2 allele. ES clones were selected in hygromycin (350 μg/mL; Boehringer Mannheim, Indianapolis, IN), G418 (350 μg/mL), and gancyclovir (2 μmol/L), and resistant clones screened for targeted disruption of both Nrf-2 alleles by Southern blot analysis. For the in vitro differentiation assay, ES clones were adapted to growth without feeder cells on gelatin-coated plates in the presence of LIF.32 Gelatin-adapted ES cells were plated at various concentrations in 1.1% methylcellulose (Stem Cell Technologies Inc, Vancouver, Canada), Iscove's modified Dulbecco's medium (IMDM), 15% fetal bovine serum, iron-saturated transferrin (300 μg/mL; Boehringer Mannheim), insulin (10 μg/mL; Sigma, St Louis, MO), hemin (10 μg/mL; Sigma), monothioglycerol (450 μmol/L), erythropoietin (EPO, 1 U/mL; Amgen, Thousand Oaks, CA), murine interleukin-3 (IL-3, 25 U/mL; Collaborative Biomedical Products, Bedford, MA), and murine stem cell factor (SCF, 25 ng/mL; R & D Systems, Minneapolis, MN). Embryoid bodies were assessed by light microscopy for evidence of hemoglobinization and by benzidine staining as previously described.33
Analysis of mice.
Peripheral blood (150 μL) was obtained from anesthetized mice by retro-orbital puncture using heparinized microhematocrit tubes. One tube was used to determine the hematocrit. Red and white blood cell counts were determined on 20 μL of blood using an automated cell counter (Multisizer II; Coulter, Miami, FL). Hemoglobin concentration was determined on 10 μL of blood by Drabkin's method.34 Platelet counts were determined on 20 μL of blood, by phase-contrast microscopy. Peripheral blood smears were treated with Wright-Giemsa stain and erythrocyte morphology examined by light microscopy. Bone marrow hematopoietic progenitors were cultured in methylcellulose with IL-3, IL-6, SCF, and (+/-) EPO (MethoCult M3434, and M3534; Stem Cell Technologies Inc). Colony-forming unit-erythroid (CFU-E) were quantitated 3 days after plating and colony-forming cells (CFC) (colony-forming unit-granulocyte [CFU-G], colony-forming unit–granulocyte-macrophage [CFU-GM], and colony-forming unit–macrophage [CFU-M]) and burst-forming unit–erythroid (BFU-E) were quantitated 8 days after plating the cells.
RNA analysis.
Total RNA was extracted from the bone marrow of Nrf-2 (+/+) and Nrf-2 (-/-) mice by the method of Chomczynski and Sacchi35(RNazol B, Tel-Test). Northern blot analysis was performed on bone marrow RNA by the method of Sambrook et al.36 The blot was probed first with full-length Nrf-2 cDNA, stripped, then reprobed with a human beta-actin cDNA probe (Clontech, Palo Alto, CA). Total RNA was extracted from fetal livers of day 13.5 to 15.5 embryos and analyzed by ribonuclease protection assay, as previously described.23 The β-globin, α-globin, and β-actin riboprobes protected 245, 128, and 150 bp fragments, respectively.23 37 Test samples fell within standard curves for all three probes.
Protein assays.
In vitro transcription and translation of p18 and p45 NF-E2 and Nrf-2 was performed with the rabbit reticulocyte lysate (Promega, Madison, WI) and [35S]methionine in accordance with the manufacturer's instructions. NF-E2 and Nrf-2 were expressed in the in vitro transcription/translation vector pCITE4a (Novagen, Madison, WI), as previously described.23 COS cell nuclear extracts were made by the method of Dignam.38 Gel mobility shift assays were performed as previously described.23 DNA sequence of the wild-type NF-E2 probe was GGAACCTGTGCTGAGTCACTGGAGG. Sequence of the mutant NF-E2 probe was GGAACCTGTTCTGAGTCACTGGAGG.39 Gel shift probes were labeled with deoxycytidine triphosphate (dCTP) and Klenow enzyme (Boehringer Mannheim).
Immunoprecipitations were performed in radioimmune precipitation assay (RIPA) lysis buffer (150 mmol/L NaCl, 50 mmol/L Tris [pH 8.0], 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], and 0.5% deoxycholate) by the method of Harlow and Lane.40 Fetal livers were harvested from day 13.5 to 15.5 embryos and processed separately. A single cell suspension of fetal liver cells (FLCs) was made by repeated passage of livers through a 21-gauge needle. FLCs were washed and resuspended in methionine-free Dulbecco's modified Eagle medium (DMEM). FLCs were starved for 1 hour (37°C, 5% CO2), followed by addition of [35S] methionine to a final concentration of 100 μCi/mL, and 4 more hours of culture. FLCs were washed once in phosphate-buffered saline (PBS) and resuspended in 0.5 mL of RIPA buffer with protease inhibitors. The lysate was incubated, while rocking, at 4°C for 30 minutes and centrifuged for 15 minutes at RCFmax=156,800 in a TLA-100.2 rotor (Beckman). The supernatant was precleared and incubated for 1 hour at 4°C with specific antibodies, followed by Protein A-Sepharose (Pharmacia, Uppsala, Sweden). Anti-p45 NF-E2 antibody was raised to a synthetic peptide (NVPSETSFEPQAPTPY) as previously described.23Anti-Nrf2 antibody was raised to a synthetic peptide near the N-terminus (LQKEQEKAFFAQFQLDE) by the same method (Rockland Inc, Boyertown, PA). Anti-Sp1 antibody was raised to a synthetic peptide corresponding to amino acid residues 436-454 of human Sp1 (Santa Cruz Biotechnology, Santa Cruz, CA).
RESULTS
Gene targeting of Nrf-2.
Like p45 NF-E2, Nrf-2 is able to form a heterodimer with p18 NF-E2 and bind NF-E2 sites (Fig 1). NF-E2 binding sites consist of an extended AP-1 site, such that a mutation 5′ of the AP-1 core specifically interferes with NF-E2 binding.39 This specificity is conferred by the small subunit of NF-E2, p18 NF-E2.18 Nrf-2/p18 heterodimers exhibit this same binding specificity suggesting that, in cells expressing both proteins, NF-E2 and Nrf-2 could have overlapping effects on gene expression. The slower mobility of Nrf-2/p18 relative to NF-E2 raises the possibility that Nrf-2/p18 heterodimers could be present in a slowly migrating complex in extracts from fetal liver cells.20 This complex, which includes proteins from the AP-1 family, is unaffected by the loss of NF-E2.
Effect of gene targeting on Nrf-2 transcript size. Northern blot analysis of total RNA from bone marrow (Nrf-2 (+/+), 13.0 μg RNA; Nrf-2 (-/-), 9.6 μg RNA). The blot was probed with full-length Nrf-2 cDNA (top) or β-actin cDNA as a control (bottom).
Effect of gene targeting on Nrf-2 transcript size. Northern blot analysis of total RNA from bone marrow (Nrf-2 (+/+), 13.0 μg RNA; Nrf-2 (-/-), 9.6 μg RNA). The blot was probed with full-length Nrf-2 cDNA (top) or β-actin cDNA as a control (bottom).
To determine the significance of Nrf-2 for globin gene expression, we inactivated the Nrf-2 gene by homologous recombination. The murine Nrf-2 gene contains five exons (Fig 2). The large terminal exon encodes two-thirds of the protein, including the BZIP domain. To inactivate the Nrf-2 gene, a neomycin resistance cassette was inserted in reverse orientation into exon 5. This mutation introduced stop codons in all three reading frames and prevented translation of the essential dimerization and DNA binding domains. The frequency of homologous recombination in embryonic stem (ES) cells was approximately 60% (Fig 3A). Single knock-out ES cells were injected into C57BL/6J strain blastocysts and implanted into pseudopregnant females.31 After passage of the mutation in the germline, heterozygous mice were mated, producing viable homozygous progeny (Fig 3B). In a sample of 164 progeny, Nrf-2 (-/-) mice were present in the predicted Mendelian proportion, indicating that Nrf-2 deficiency is not associated with embryonic or neonatal lethality. Nrf-2–deficient mice had no apparent developmental defects. NF-E2–deficient mice are severely thrombocytopenic due to a defect in megakaryocyte maturation.19 In contrast, the hematologic parameters of Nrf-2–deficient mice were normal, including the platelet count (Nrf-2 (+/+) mice, 1.24 ± 0.32 × 106/μL [n = 12]; Nrf-2 (-/-) mice, 1.33 ± 0.34 × 106/μL [n = 12]). Bone marrow hematopoietic progenitors (CFU-E, BFU-E, and CFC) were also present in normal numbers in adult Nrf-2 (-/-) mice (data not shown).
To verify that the Nrf-2 gene had been disrupted, we performed Northern analysis of bone marrow RNA from wild-type and Nrf-2 (-/-) mice. Nrf-2 transcripts from Nrf-2 (-/-) cells migrated slower than those from wild-type cells, consistent with insertion of the neomycin resistance gene (Fig 4). RNA polymerase chain reaction (PCR) and sequencing confirmed that this insertion resulted in multiple in-frame stop codons.
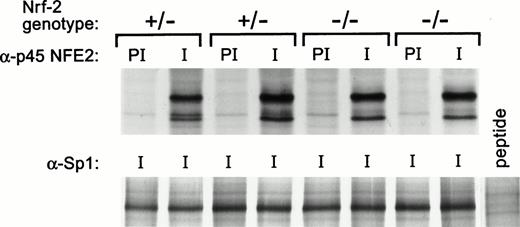
Next, we considered the possibility that expression of NF-E2 and Nrf-2 might be cross-regulated, as previously shown for GATA-1 and GATA-2.41 To examine this, we immunoprecipitated p45 NF-E2 from the fetal liver cells of Nrf-2 heterozygous and homozygous knock-out embryos. No evidence was found for a change in p45 NF-E2 protein levels in Nrf-2–deficient erythroid cells (Fig 5). Conversely, Nrf-2 protein was undetectable in both wild-type and NF-E2–deficient fetal liver cells (data not shown). Thus, if NF-E2 and Nrf-2 complement one another, this occurs without a demonstrable change in expression of either protein.
NF-E2 expression in Nrf-2 (+/−) and Nrf-2 (-/-) fetal liver cells. NF-E2 was immunoprecipitated from fetal liver cells of day 13.5 to 15.5 embryos as described in Materials and Methods. Four fetal livers are shown from the embryos of one litter. Fourteen fetal livers from two litters were studied, and these results are representative. Nrf-2 genotype is indicated at the top with preimmune (PI) and immune (I) antisera. The extracts were serially immunoprecipitated with anti-Sp1 antibody (Santa Cruz) as a control. Sp1 peptide was added to one reaction as a negative control.
NF-E2 expression in Nrf-2 (+/−) and Nrf-2 (-/-) fetal liver cells. NF-E2 was immunoprecipitated from fetal liver cells of day 13.5 to 15.5 embryos as described in Materials and Methods. Four fetal livers are shown from the embryos of one litter. Fourteen fetal livers from two litters were studied, and these results are representative. Nrf-2 genotype is indicated at the top with preimmune (PI) and immune (I) antisera. The extracts were serially immunoprecipitated with anti-Sp1 antibody (Santa Cruz) as a control. Sp1 peptide was added to one reaction as a negative control.
In vitro differentiation of Nrf-2 (-/-) ES cells.
Because compensatory mechanisms may exist in vivo that do not exist in vitro, we also studied the effect of Nrf-2 deficiency on erythroid differentiation in vitro. Through the use of a second gene targeting vector, which contained a hygromycin resistance gene instead of a neomycin resistance gene, we disrupted the second Nrf-2 allele at approximately a 60% frequency (Fig 3C). ES cells were adapted to growth without feeders and allowed to differentiate in vitro, as previously described. 32 Embryoid bodies derived from wild-type or Nrf-2 (-/-) ES cells showed no impairment in their ability to undergo terminal erythroid differentiation (Fig 6).
In vitro differentiation of Nrf-2 (-/-) embryoid bodies. (A) Light microscopy of two Nrf-2 (-/-) embryoid bodies, one showing terminal erythroid differentiation. (B) Light microscopy of Nrf-2 (-/-) embryoid bodies after staining with benzidine.33
In vitro differentiation of Nrf-2 (-/-) embryoid bodies. (A) Light microscopy of two Nrf-2 (-/-) embryoid bodies, one showing terminal erythroid differentiation. (B) Light microscopy of Nrf-2 (-/-) embryoid bodies after staining with benzidine.33
Immunoprecipitation experiments with Nrf-2 (-/-) ES cells confirmed that insertion of neomycin and hygromycin resistance genes functionally inactivated the Nrf-2 gene. Antibody raised to an N-terminal epitope of Nrf-2 (amino acids 46-62) efficiently precipitated in vitro translated Nrf-2, as well as Nrf-2 protein expressed in COS cells (Fig 7A and B). Endogenous Nrf-2 protein, which has not been previously shown, was present in low amount in wild-type ES cells (Fig 7C). Nrf-2 (-/-) ES cells did not express detectable full-length Nrf-2. Furthermore, there was no evidence of a premature termination product, suggesting that a truncated protein, if expressed, was not stable.
Absence of Nrf-2 protein in Nrf-2 (-/-) embryonic stem (ES) cells. (A) Immunoprecipitation of [35S]-labeled Nrf-2 from in vitro transcription/translation reaction showing the starting material, immunoprecipitation with preimmune serum, and immunoprecipitation with anti-Nrf2 antibody. The 98-kD marker is indicated. Nrf-2 migrates at 96 kD.26 (B) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 1 × 107 COS cells transfected with vector alone, or with Nrf-2 cDNA in COS expression vector pEUK-1 (Clontech). (C) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 4 × 107 Nrf-2 (+/+) ES cells and Nrf-2 (-/-) ES cells.
Absence of Nrf-2 protein in Nrf-2 (-/-) embryonic stem (ES) cells. (A) Immunoprecipitation of [35S]-labeled Nrf-2 from in vitro transcription/translation reaction showing the starting material, immunoprecipitation with preimmune serum, and immunoprecipitation with anti-Nrf2 antibody. The 98-kD marker is indicated. Nrf-2 migrates at 96 kD.26 (B) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 1 × 107 COS cells transfected with vector alone, or with Nrf-2 cDNA in COS expression vector pEUK-1 (Clontech). (C) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 4 × 107 Nrf-2 (+/+) ES cells and Nrf-2 (-/-) ES cells.
Combined deficiency of NF-E2 and Nrf-2.
To determine the effect of combined deficiency of Nrf-2 and NF-E2 on globin gene expression, mice deficient in the two factors were interbred. Compound heterozygotes from the first round of breeding were mated to obtain mice deficient in both factors. Fetal liver cells from embryos of this mating were examined for globin gene expression by ribonuclease protection assay. Day 13.5 to 15.5 embryos deficient for both NF-E2 and Nrf-2 expressed normal levels of α-and β-globin (Fig 8). Mature mice deficient for both factors showed the same hematologic profile as mice deficient for NF-E2 alone (Table 1). NF-E2–deficient mice are mildly anemic, possibly due to hemorrhage or defective erythroid maturation.20 However, combined deficiency of NF-E2 and Nrf-2 was not associated with a more severe erythroid defect.
Globin gene expression in embryos with combined deficiency of NF-E2 and Nrf-2. Compound heterozygous mice were mated and total RNA was extracted from the fetal livers of day 13.5 to 15.5 embryos. Genomic DNA was extracted from the heads of the embryos and analyzed for NF-E2 and Nrf-2 genotype. β- and α-globin and β-actin expression was analyzed by ribonuclease protection assay (1 μg RNA). The size of the protected transcripts was 245, 128, and 150 bp, respectively. As indicated at the top, comparisons were made between samples from the same litter. A standard curve was performed with RNA from murine erythroleukemia cells for each of the probes.
Globin gene expression in embryos with combined deficiency of NF-E2 and Nrf-2. Compound heterozygous mice were mated and total RNA was extracted from the fetal livers of day 13.5 to 15.5 embryos. Genomic DNA was extracted from the heads of the embryos and analyzed for NF-E2 and Nrf-2 genotype. β- and α-globin and β-actin expression was analyzed by ribonuclease protection assay (1 μg RNA). The size of the protected transcripts was 245, 128, and 150 bp, respectively. As indicated at the top, comparisons were made between samples from the same litter. A standard curve was performed with RNA from murine erythroleukemia cells for each of the probes.
Hematologic Profile of Mice With Combined Deficiency of Nrf-2 and NF-E2
| No. . | Genotype (Nrf2/NF-E2) . | Hct (%) . | Hb (g/dL) . | RBC (×106/μL) . | WBC (×103/μL) . | Platelets (×106/μL)-150 . |
|---|---|---|---|---|---|---|
| 15 | (+/+)/(+/+) | 46.9 ± 2.8-151 | 15.0 ± 0.8 | 7.7 ± 1.0 | 4.4 ± 1.2 | 1.25 |
| 8 | (−/−)/(+/+) | 46.1 ± 1.7 | 15.2 ± 0.7 | 7.6 ± 0.5 | 5.1 ± 2.3 | ND-152 |
| 5 | (−/−)/(+/−) | 46.2 ± 3.1 | 14.9 ± 1.1 | 8.0 ± 0.5 | 4.3 ± 2.4 | ND |
| 8 | (+/+)/(−/−) | 42.1 ± 4.5 | 13.0 ± 1.5 | 7.7 ± 1.0 | 4.4 ± 1.1 | 0.04 |
| 4 | (+/−)/(−/−) | 40.5 ± 3.3 | 11.9 ± 1.1 | 7.5 ± 0.8 | 4.6 ± 2.3 | ND |
| 4 | (−/−)/(−/−) | 41.8 ± 4.4 | 12.3 ± 1.5 | 7.9 ± 1.2 | 5.6 ± 1.4 | 0.03 |
| No. . | Genotype (Nrf2/NF-E2) . | Hct (%) . | Hb (g/dL) . | RBC (×106/μL) . | WBC (×103/μL) . | Platelets (×106/μL)-150 . |
|---|---|---|---|---|---|---|
| 15 | (+/+)/(+/+) | 46.9 ± 2.8-151 | 15.0 ± 0.8 | 7.7 ± 1.0 | 4.4 ± 1.2 | 1.25 |
| 8 | (−/−)/(+/+) | 46.1 ± 1.7 | 15.2 ± 0.7 | 7.6 ± 0.5 | 5.1 ± 2.3 | ND-152 |
| 5 | (−/−)/(+/−) | 46.2 ± 3.1 | 14.9 ± 1.1 | 8.0 ± 0.5 | 4.3 ± 2.4 | ND |
| 8 | (+/+)/(−/−) | 42.1 ± 4.5 | 13.0 ± 1.5 | 7.7 ± 1.0 | 4.4 ± 1.1 | 0.04 |
| 4 | (+/−)/(−/−) | 40.5 ± 3.3 | 11.9 ± 1.1 | 7.5 ± 0.8 | 4.6 ± 2.3 | ND |
| 4 | (−/−)/(−/−) | 41.8 ± 4.4 | 12.3 ± 1.5 | 7.9 ± 1.2 | 5.6 ± 1.4 | 0.03 |
Age range, 2 to 4 months.
Mean, n = 2 for platelet counts.
Standard deviation.
Not determined in this experiment.
DISCUSSION
NF-E2 binding sites contribute to the activity of erythroid-specific elements in the β-globin LCR and the α-globin PRE. Evidence exists that NF-E2 itself is a regulator of globin gene expression through these sites. Murine erythroleukemia cells deficient in p45 NF-E2 have a defect in globin gene expression, which can be rescued by introduction of p45 NF-E2.42 43 However, the lack of a significant defect in vivo raises the possibility that there are other activators of globin gene expression that function through these sites. We tested the hypothesis that Nrf-2 compensates for the loss of NF-E2 by generating mice with combined deficiency of NF-E2 and Nrf-2.
Mice deficient in Nrf-2 had no apparent abnormalities. This result, which confirms the findings of Chan et al,44 indicates that Nrf-2 is not required for terminal erythroid differentiation. This result was expected, as Nrf-2–deficient mice still express normal levels of p45 NF-E2. No evidence was found for reciprocal upregulation of expression of NF-E2 or Nrf-2 in fetal liver cells deficient for either protein. Moreover, mice deficient in both NF-E2 and Nrf-2 failed to show an erythroid defect beyond that seen with NF-E2 deficiency alone. These results indicate that the mild erythroid phenotype seen in NF-E2–deficient mice is not the result of compensation by Nrf-2. Recent experiments indicate that the function of Nrf-2 may not be in hematopoiesis, but in the induction of antioxidant enzymes through NF-E2–like binding sites.45
Because Nrf-2 does not compensate for deficiency of NF-E2 in mice, what accounts for the mild erythroid defect? One possibility is that other members of the CNC family can substitute for NF-E2. Targeted disruption of Nrf-1/LCR-F1 is not associated with a defect in erythroid maturation in chimeric mice.33 The effect of combined NF-E2 and Nrf-1/LCR-F1 deficiency on erythropoiesis will be difficult to assess due to the early embryonic death of Nrf-1/LCR-F1 knock-out mice. The description of two new CNC-related proteins, Bach-1 and Bach-2, further complicates resolution of this issue.46
A second possibility is that redundancy exists at the level of cis-acting sequences, bypassing the need for NF-E2 sites or proteins that bind to these sites. Ablation of individual core elements HS2 or HS3 is associated with a modest 30% decrease in β-globin expression.47,48 However, because NF-E2 sites are associated with each of the three most active core elements (HS2-4), these experiments do not address the requirement for NF-E2 sites in the LCR. In contrast to the β-globin LCR, there appears to be a single core element upstream of the α-globin cluster (HS-40 in man, HS-26 in mouse). 9,13,49,50 Although this element is not sufficient for normal regulation of α-globin expression in transgenic mice, it does seem to be required for expression of the endogenous α-globin gene.51 It will be interesting to determine the role of tandem NF-E2 sites present in this element on α-globin expression.
Finally, studies in thalassemic patients suggest that a mechanism may exist to compensate for a balanced defect in globin gene expression. Whereas patients with β-thalassemia trait and a normal complement of α-globin genes have microcytic anemia, patients who coinherit β-thalassemia trait and deletion of two α-globin genes (out of four α-globin genes) are not anemic and have near normal red blood cell indices.52-54 Similarly, a balanced defect in globin gene expression may exist in NF-E2–deficient mice. NF-E2 deficiency in cell lines is associated with a defect in both α-and β-globin expression.42,43 Moreover, studies in NF-E2–deficient mice suggest the presence of an ongoing compensatory process. Erythrocytes in NF-E2–deficient mice are hypochromic and microcytic, despite the presence of adequate iron stores.20 Spleen weight is increased fivefold to sixfold as a consequence of expanded, and possibly ineffective, erythropoiesis. In contrast to GATA-1 deficiency, which causes a maturation arrest at the proerythroblast stage,55 or erythroid Krüppel-like factor (EKLF) deficiency, which causes a marked imbalance in the ratio of α- to β-globin expression,56,57 this phenotype is consistent with a specific and balanced defect in α- and β-globin expression. Further studies are needed to test this hypothesis.
The contribution of NF-E2, or other proteins that bind to NF-E2 sites, to high level globin gene expression remains an important question in tissue-specific gene regulation. Identification of the proteins that activate globin gene expression through these sites will be a necessary step towards understanding the mechanism of action of the β-globin LCR. Our results indicate that Nrf-2 does not make an important contribution to globin gene expression in NF-E2–deficient mice. Several alternative hypotheses are under consideration.
ACKNOWLEDGMENT
The authors thank Melanie Loyd, Teresa Bean, and Shirley Steward for expert technical assistance. The authors also thank Steve Ruben (Human Genome Systems) for the human Nrf-2 cDNA clone and Stuart Orkin for sending NF-E2–deficient mice in advance of publication.
Supported by National Institutes of Health (NIH) Cancer Center Support CORE Grant No. P30 CA21765 (Bethesda, MD) and the American Lebanese Syrian Associated Charities (ALSAC) (Memphis, TN).
Address reprint requests to Paul A. Ney, MD, Department of Biochemistry, Room 4064, Thomas Tower, 332 N Lauderdale, Memphis, TN 38101.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




![Fig. 7. Absence of Nrf-2 protein in Nrf-2 (-/-) embryonic stem (ES) cells. (A) Immunoprecipitation of [35S]-labeled Nrf-2 from in vitro transcription/translation reaction showing the starting material, immunoprecipitation with preimmune serum, and immunoprecipitation with anti-Nrf2 antibody. The 98-kD marker is indicated. Nrf-2 migrates at 96 kD.26 (B) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 1 × 107 COS cells transfected with vector alone, or with Nrf-2 cDNA in COS expression vector pEUK-1 (Clontech). (C) Immunoprecipitation of [35S]-labeled Nrf-2 from whole cell extracts of 4 × 107 Nrf-2 (+/+) ES cells and Nrf-2 (-/-) ES cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3459/3/m_blod40940007w.jpeg?Expires=1767739390&Signature=MiVJi1LWdcr3AgU62vDJKvsL-5cdgCWacbhCvRqmwoXFwmHEKHGpj7pS4oM6sCncT73nU-v3DSW~XN3ILrkM1kkbj94WsBMnM~ZFoec~jgM-yeE06xMoPCdoJBZDCx7ya3TmdkS33L49GynBJU05O5RwHSDMSTdwFRmEFzdOORx7gbp8tI2TKdxBBtpbZcDUaPOF1epkXPAZDRdx53kWM5xCCbQ~8yjiIjeum1Ht~mEtWDXMIcH9tbEEkclHTdXH3nlFx0Hy3IjUvqa~KrF~1hU8pXfiFcrxbMuSMPATAwdYyH80-OJRUnLId~ShRdEVGttdPOmf0e41M6UcrOGGmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal