Abstract
Galectin-3, a lactose-binding mammalian lectin that is secreted from activated macrophages, basophils, and mast cells, was investigated with respect to its ability to activate the human neutrophil NADPH-oxidase. The galectin-3–induced activity was determined with in vivo exudated cells (obtained from a skin chamber) and compared with that of peripheral blood neutrophils. Galectin-3 was found to be a potent activator of the NADPH-oxidase only in exudated neutrophils and the binding of galectin-3 to the surface of these cells was increased compared with peripheral blood cells. Different in vitro priming protocols resulting in degranulation were used to mimic the exudation process in terms of increasing the receptor exposure on the cell surface. Galectin-3 could induce an oxidative response similar to that in exudated cells only after a significant amount of the intracellular organelles had been mobilized. This increase in oxidative response was paralleled by an increased binding of galectin-3 to the surface of the cells. The major conclusion of the study is that galectin-3 is a potent stimulus of the neutrophil respiratory burst, provided that the cells have first experienced an extravasation process. The results also imply that the neutrophil response to galectin-3 could be mediated through receptors mobilized from intracellular granules, and we report the presence of galectin-3–binding proteins in such organelles.
THE HUMAN neutrophils play a key role in the innate immune response to infection. They act at inflammatory sites, which they reach after targeting and extravasation from the peripheral blood stream where they are normally present. Upon interaction with invading microorganisms or inflammatory mediators they produce large amounts of toxic oxygen radicals (superoxide anion and hydrogen peroxide) by activation of the NADPH-oxidase. Although the mechanism by which such activation is induced and regulated in vivo remains unclear, multiple extracellular mediators have been found to activate the neutrophil NADPH-oxidase in vitro, and multiple intracellular signaling pathways have been implicated.1,2The degree of activation as well as the subcellular localization of the toxic oxygen radicals generated is determined by the identity of the agonist and the cell-surface receptor involved in the activation process.3 4
Most in vitro studies of neutrophil function have been performed using neutrophils isolated from peripheral blood, whereas activation in vivo occurs after extravasation into the tissue. Earlier published data show that exudated neutrophils are primed with respect to the function of the NADPH-oxidase, and as a consequence react with an enhanced response compared with peripheral blood neutrophils to a variety of mediators.5-7 Hence, to understand neutrophil activation in vivo, it is of paramount importance to examine exudated neutrophils.
Among known neutrophil-activating mechanisms is the binding of bacterial lectins to neutrophil cell-surface glycoproteins; these glycoproteins presumably act as cell-surface receptors whose activation often results in a signaling cascade that in turn may cause activation of the NADPH-oxidase.8 This raises the possibility that also endogenous mammalian lectins may play a role in neutrophil activation. There are many types of mammalian lectins thought to play various roles in inflammation,9,10 and one, galectin-3, has been found to bind to the surface of peripheral blood neutrophils11 and a variety of other immune cells.12,13 Galectin-3 has been reported to activate the NADPH-oxidase in peripheral blood neutrophils but only if the cells were also treated with cytochalasin B,14 a substance known to disrupt the microfilament system in the cell.15
Galectin-3, a member of a family of β-galactoside–binding proteins,12 is produced by activated macrophages. Thus, neutrophils that have extravasated into inflamed tissue might encounter galectin-3. To model this situation, we examined the ability of galectin-3 to activate the NADPH-oxidase in neutrophils that had been primed by in vivo exudation into a skin chamber. We found that galectin-3 is a potent activator of exudated neutrophils but not of peripheral blood neutrophils. Because the exudation-related priming apparently results in a dramatic increase in neutrophil responsiveness to galectin-3, we also examined the possibility that this is caused by mobilization of galectin-3 receptors from intracellular granules to the cell surface.
MATERIALS AND METHODS
Isolation of human neutrophils.
Exudated neutrophils were obtained from chambers placed on unroofed skin blister lesions on the volar surface of the forearms of healthy human volunteers as previously described.6 In each experiment, two chambers with three 0.6-mL wells covering the lesions were used. The chambers were filled with autologous serum, and the neutrophils were allowed to accumulate in the chambers for 24 hours. More than 95% of the cells obtained from the chambers were neutrophils.
Blood neutrophils were isolated from heparinized whole blood (obtained from the same person as was carrying the skin chambers) or from buffy coats from healthy blood donors, using dextran sedimentation and Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation.16 All cells were washed and resuspended (1 × 107/mL) in Krebs-Ringer phosphate buffer containing glucose (10 mmol/L), Ca2+ (1 mmol/L), and Mg2+ (1.5 mmol/L) (KRG, pH 7.3). This isolation procedure allows for cells to be isolated with minimal mobilization effects.17
Preparation of galectin-3.
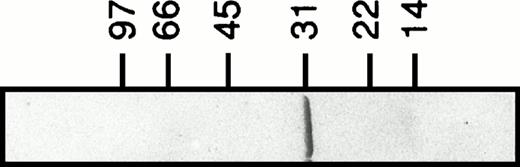
Recombinant human galectin-3 (apparent molecular weight of 31 kD; Fig 1) was produced inEscherichia coli and purified as previously described.18 The lectin was stored at 4°C in phosphate-buffered saline (PBS; pH 7.2) containing lactose (150 mmol/L). When used, the lectin preparation was applied to a gel-filtration column (PD10; Pharmacia) to remove lactose, and diluted to 400 μg/mL in PBS. The carboxyl-terminal domain fragment of galectin-3 was prepared by digesting recombinant lectin with collagenase as described.18 Labeling of galectin-3 with fluorescein isothiocyanate (FITC) was performed essentially according to Feizi et al.19
Recombinant human galectin-3. Galectin-3 (3 μg) produced in E coli and purified as described18 is shown on a Coomassie-stained SDS-polyacrylamide gel (5% to 20%).
Recombinant human galectin-3. Galectin-3 (3 μg) produced in E coli and purified as described18 is shown on a Coomassie-stained SDS-polyacrylamide gel (5% to 20%).
Neutrophil NADPH-oxidase activity.
The NADPH-oxidase activity was determined using a luminol/isoluminol enhanced chemiluminescence (CL) system.20 The CL activity was measured in a six-channel Biolumat LB 9505 (Berthold Co, Wildbad, Germany), using disposable 4-mL polypropylene tubes with a 0.95-mL reaction mixture containing 106 neutrophils. The tubes were equilibrated in the Biolumat for 5 minutes at 37°C, after which the stimulus (0.05 mL) was added. The light emission was recorded continuously. To quantify intracellularly and extracellularly generated reactive oxygen species, respectively, two different reaction mixtures were used. Tubes used for measurement of extracellular release of superoxide anion contained neutrophils, horseradish peroxidase (HRP; a cell impermeable peroxidase; 4 U), and isoluminol (a cell impermeable CL substrate; 2 × 10−5 mol/L).21 By a direct comparison of the superoxide dismutase (SOD) inhibitable reduction of cytochrome C and SOD inhibitable CL, 7.2 × 107 cpm were found to correspond to a production of 1 nmol of superoxide (a millimolar extinction coefficient for cytochrome C of 21.1 was used). Tubes used for measurement of intracellular generation of reactive oxygen species contained neutrophils, SOD (a cell impermeable scavenger for O2−; 50 U), catalase (a cell impermeable scavenger for H2O2; 2,000 U), and luminol (a cell permeable CL substrate; 2 × 10−5 mol/L).
Mobilization of subcellular organelles.
Three different protocols were used for mobilization of neutrophil subcellular organelles to the cell surface. The first cell population was merely incubated at 22°C for 60 minutes without additive.17 The second cell population was treated with the chemoattractant formyl-methionyl-leucyl-phenylalanine (fMLP). These cells were incubated at 15°C for 5 minutes after which fMLP (10−7 mol/L final concentration) was added and the incubation was continued for another 10 minutes. The cells were transferred to a heated water bath (37°C) and were allowed to incubate for 5 minutes. This treatment results in degranulation without any activation of the NADPH-oxidase.22 The third cell population was subjected to stimulation by ionomycin, a calcium ionophore.23 After preincubation of the neutrophils at 37°C for 5 minutes, ionomycin (5 × 10−7 mol/L final concentration) was added and the incubation was continued for 5 minutes. All cell populations were sedimented by centrifugation and the supernatants were collected for marker analysis. The pellets were suspended in KRG, washed once to remove any prestimulating agent, resuspended to 1 × 107 cells/mL in KRG and put on ice until use, either for cell-surface marker analysis or for NADPH-oxidase activation studies.
Marker analysis.
The mobilization of subcellular organelles was followed by measuring the exposure of complement receptors 1 and 3 (CR1 and CR3, respectively) on the neutrophil surface as well as determining the release of gelatinase, vitamin B12–binding protein and myeloperoxidase (MPO) into the supernatant. Exposure of CR1 was measured by labeling the cells with mouse anti-human CD35 (DAKO, Glostrup, Denmark; M0710; 10 μL to a cell pellet of 106cells) and subsequent binding of FITC-conjugated goat anti-mouse Ig (DAKO F0479; 1/2,000). To measure CR3 exposure, the cells were labeled with phycoerythrin-conjugated monoclonal antibodies (MoAbs) specific for CD11b (DAKO M741; 10 μL to a cell pellet of 106cells). The cells were examined by FACScan (Becton Dickinson, Mountain View, CA24). Vitamin B12–binding protein was determined with the cyanocobalamin technique as described by Gottlieb et al.25 Gelatinase and MPO were measured using enzyme-linked immunosorbent assay (ELISA) methods.26 27
Surface exposure of galectin-3–binding proteins was assessed in exudated cells as well as in the four different neutrophil populations described above (control, 22°C, fMLP, ionomycin). Paraformaldehyde fixed neutrophils were incubated with FITC-labeled galectin-3 (4 μg to a pellet of 106 cells) in the presence or absence of lactose (10 mmol/L) for 30 minutes on ice. After washing twice the cells were examined by FACScan.
Identification of galectin-3 receptor candidates.
Subcellular fractionation was performed according to the method described by Borregaard et al28 with some modifications. In short, peripheral blood neutrophils isolated from buffy coats were treated with the serine protease inhibitor diisopropylfluorophosphate (DFP; 8 μmol/L), disintegrated by nitrogen cavitation (Parr Instrument Co, Moline, IL), and the postnuclear supernatant was centrifuged on Percoll gradients. Plasma membranes were separated from secretory vesicles in a flotation gradient as previously described.29 Gelatinase granules were separated from the classical specific granules as described by Kjeldsen et al.30 The gradients were collected in 1.5-mL fractions by aspiration from the bottom of the centrifuge tube and the localization of subcellular organelles in the gradients was determined by marker analysis of the fractions.
Cytosol was prepared by centrifugation of cavitated neutrophils (as described above) at 100,000g for 90 minutes (4°C) and collection of the supernatant containing the cytosolic proteins.
Samples of the plasma membrane (γ2), the secretory vesicles (γ1), the gelatinase granules (β2), the specific granules (β1), and the azurophil granules (α), respectively, were diluted in nonreducing sample buffer, boiled for 5 minutes, and applied to the gels in volumes corresponding to the fractionated content of 5 × 106cells. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli31 in 5% to 20% gradient gels. After electrophoresis the proteins were transferred to polyvinylidene difluoride (PVDF) membranes using a Tris-Glycine buffer system.32 The membranes were blocked by incubation in PBS-Tween (0.05% vol/vol) containing gelatin (3% wt/vol) for 6 hours at room temperature before blotting with galectin-3 (8 μg/mL) in PBS-Tween containing gelatin (1% wt/vol) at 4°C overnight. The membranes were washed 5 × 5 minutes in PBS-Tween and incubated with anti–galectin-3 antibodies (anti–Mac-2 antibodies; culture supernatant from the hybridoma M3/38; 1/25) in PBS-Tween containing gelatin (1% wt/vol) for 6 hours at room temperature. After washing twice, the membranes were incubated in HRP-labeled rabbit anti-rat Ig antibodies (DAKO P0450; 1/1,000) for 1 hour at room temperature and developed by adding peroxidase substrate (VIP Kit; Vector Laboratories, Burlingame, CA).
Reagents.
The fMLP, FITC, ATP, EGTA, piperazine-N,N′-bis(2-ethane sulfonic acid), isoluminol, and luminol were obtained from Sigma (Sigma Chemical Co, St Louis, MO). The SDS was from Fluka Chemie AG (Buchs, Switzerland). Catalase, SOD, and HRP were purchased from Boehringer Mannheim (Mannheim, Germany). Dextran, Ficoll-Paque, and Percoll were from Pharmacia (Uppsala, Sweden). The molecular weight standard proteins were from Bio-Rad Laboratories (Richmond, CA). The [57Co]vitamin B12 was supplied by Amersham Laboratories (Amersham, Buckinghamshire, UK). Ionomycin was purchased from Calbiochem (La Jolla, CA). Antibodies for the gelatinase-ELISA were a kind gift from Drs Lars Kjeldsen and Niels Borregaard (Copenhagen, Denmark).
RESULTS
Galectin-3–induced NADPH-oxidase activity.
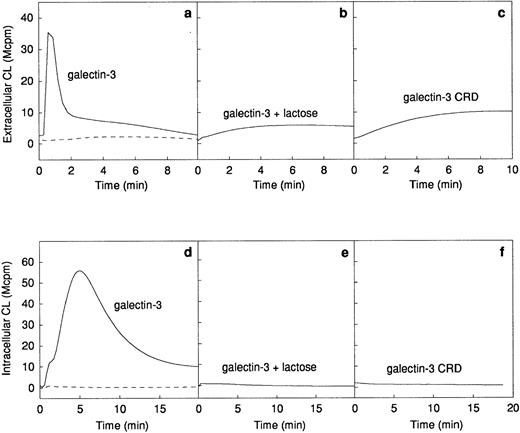
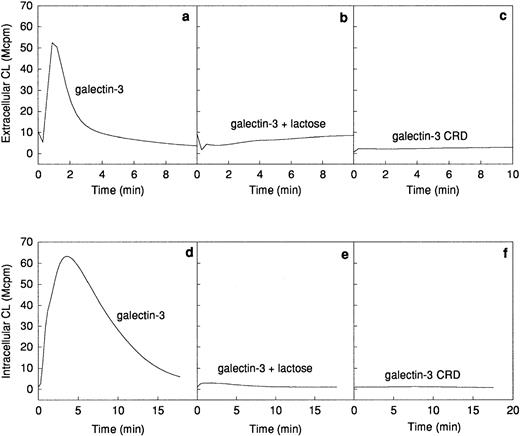
The ability of galectin-3 to induce superoxide anion production in human neutrophils was investigated. Galectin-3 was added to human neutrophils obtained from a skin chamber (exudated cells) or to neutrophils isolated from peripheral blood. The lectin induced a pronounced extracellular release (Fig 2a) as well as an intracellular production (Fig 2d) of superoxide anion in exudated neutrophils, whereas there was no response induced in peripheral blood cells (Fig 2a and d). The extracellular response induced by galectin-3 was around 25% of that induced by 10−7 mol/L fMLP (data not shown). To assess the influence of contaminating lipopolysaccharide in the galectin-3 preparation on the response, the lectin was preincubated for 10 minutes with polymyxin B.33 34 No alteration of the response was seen in the presence of polymyxin B, indicating that the neutrophil activity was induced by galectin-3 alone (data not shown).
Galectin-3–induced activation of the NADPH-oxidase in exudated neutrophils. The NADPH-oxidase activity in peripheral blood neutrophils (106 cells; dashed lines) or exudated neutrophils (106 cells; solid lines) was assessed. The figure shows the neutrophil responses to galectin-3 (20 μg/mL; a and d), galectin-3 (20 μg/mL) in the presence of lactose (10 mmol/L; b and e), and collagenase digested galectin-3 (CRD; 20 μg/mL; c and f). The extracellular release of superoxide anion (a through c) was measured in the presence of HRP (4 U) and isoluminol (5 × 10−5 mol/L) while the intracellular production of superoxide (d through f) was measured in the presence of SOD (50 U), catalase (2,000 U), and luminol (5 × 10−5 mol/L). Responses are given as chemiluminescence (CL) units in megacounts per minute (Mcpm).
Galectin-3–induced activation of the NADPH-oxidase in exudated neutrophils. The NADPH-oxidase activity in peripheral blood neutrophils (106 cells; dashed lines) or exudated neutrophils (106 cells; solid lines) was assessed. The figure shows the neutrophil responses to galectin-3 (20 μg/mL; a and d), galectin-3 (20 μg/mL) in the presence of lactose (10 mmol/L; b and e), and collagenase digested galectin-3 (CRD; 20 μg/mL; c and f). The extracellular release of superoxide anion (a through c) was measured in the presence of HRP (4 U) and isoluminol (5 × 10−5 mol/L) while the intracellular production of superoxide (d through f) was measured in the presence of SOD (50 U), catalase (2,000 U), and luminol (5 × 10−5 mol/L). Responses are given as chemiluminescence (CL) units in megacounts per minute (Mcpm).
The NADPH-oxidase was not activated by galectin-1, another member of the galectin family which is also implicated in inflammation35 (data not shown).
Features of galectin-3 required for activation of exudated neutrophils.
Galectin-3 consists of a C-terminal carbohydrate recognition domain (CRD, about 130 amino acids) with affinity for lactose and related saccharides, and an N-terminal region without known carbohydrate-binding activity (about 120 amino acids).12The lactose-binding activity of the CRD was required for galectin-3 activation of exudated neutrophils, because the galectin-3–induced NADPH-oxidase activity was inhibited by the addition of soluble lactose (Fig 2b and e). The N-terminal domain, previously shown to be required for aggregation and cooperative binding of galectin-3,18was also required for activation of the neutrophil NADPH-oxidase, illustrated by the fact that galectin-3 CRD, lacking the N-terminal domain, could not induce oxidase activation in exudated cells (Fig 2c and f).
Surface exposure of galectin-3–binding sites.
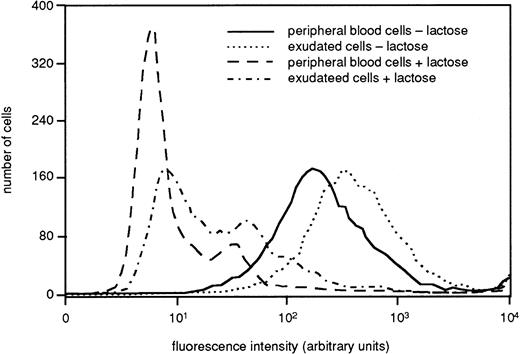
Many neutrophil receptors that are stored in the granules and secretory vesicles in peripheral blood cells are mobilized to the plasma membrane during the extravasation process.36 To determine if this applies also for galectin-3 attachment sites, we determined the binding of FITC-labeled galectin-3 to fixed intact cells by FACS analysis. The histogram in Fig 3 depicts a representative experiment (of three), showing a marked increase in galectin-3 binding to exudated cells as compared to peripheral blood cells (control), supporting that galectin-3–binding receptor structures may be stored intracellularly in resting cells with the potential to be upregulated to the cell surface upon cell activation. The sugar specificity of the binding was confirmed by the fact that it was inhibited by lactose (Fig3).
Presence of galectin-3–binding sites on the surface of resting and exudated neutrophils. Exudated cells were compared with resting peripheral blood cells regarding ability to bind galectin-3. The cells (106) were paraformaldehyde fixed, incubated with FITC-labeled galectin-3 in the presence or absence of lactose (50 mmol/L), and analyzed by flow cytometry. The figure shows a histogram from one representative experiment out of three.
Presence of galectin-3–binding sites on the surface of resting and exudated neutrophils. Exudated cells were compared with resting peripheral blood cells regarding ability to bind galectin-3. The cells (106) were paraformaldehyde fixed, incubated with FITC-labeled galectin-3 in the presence or absence of lactose (50 mmol/L), and analyzed by flow cytometry. The figure shows a histogram from one representative experiment out of three.
In vitro mobilization of subcellular organelles.
To examine the extent to which mobilization of intracellular granules to the cell surface might explain the increased responsiveness of the exudated neutrophils to galectin-3, a sequential mobilization of vesicles and granules was induced in vitro in peripheral blood neutrophils by three different priming protocols: 22°C treatment, fMLP treatment, and ionomycin treatment (see Materials and Methods for details).
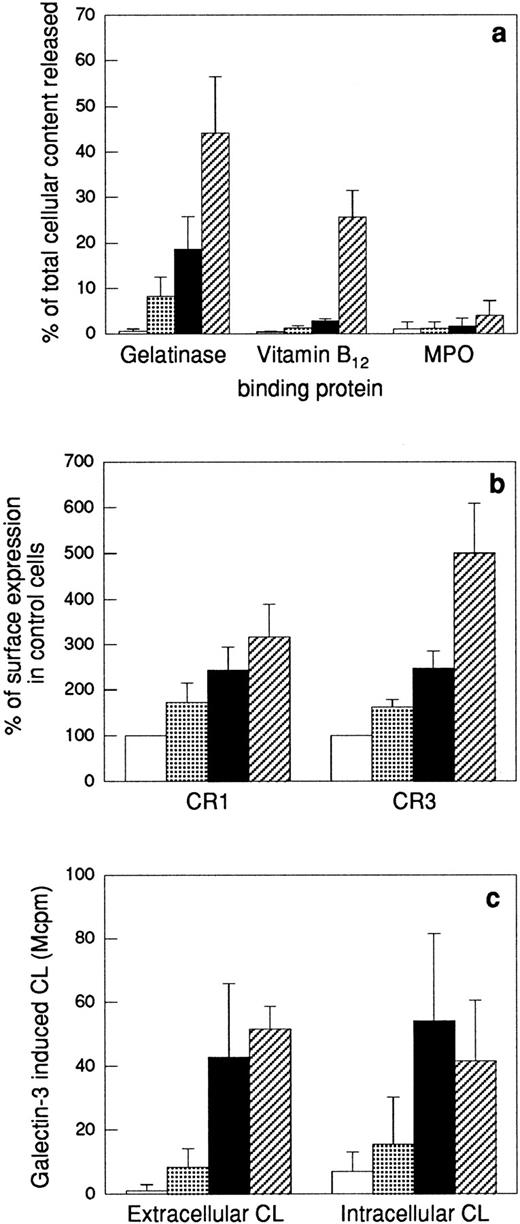
The upregulation of membrane proteins to the cell surface, the release of granule matrix proteins, as well as the ability of galectin-3 to activate the NADPH-oxidase in the different cell populations were determined (Fig4). The most easily mobilized of the neutrophil organelles, the secretory vesicles, are partly mobilized by in vitro “aging” of neutrophils17,37; ie, the secretory vesicles supply the plasma membrane with new receptors (shown for CR1 and CR3 in Fig 4b) during storage of the cells at 22°C without addition of any exogenous stimulus. In addition, a minor release of gelatinase was seen in these cells (Fig 4a). When this cell population was stimulated with galectin-3, a slight activation of the NADPH-oxidase was obtained (Fig 4c). In comparison, the fMLP-treated cells had further upregulated their CR1 and CR3 (Fig 4b) concomitant with a 20% release of the total cell content of gelatinase. Because the specific granule marker vitamin B12–binding protein was not released in these cells (Fig4a), the released gelatinase must be derived from gelatinase granules only, which thus have been mobilized to around 40% (as 50% of the neutrophil gelatinase is localized in these organelles38). The galectin-3–induced extracellular release as well as intracellular production of oxygen radicals in these cells was considerably increased compared with control cells (Fig 4c). Further release of gelatinase together with part of the specific granule marker vitamin B12–binding protein was achieved by stimulating the neutrophils with the calcium ionophore ionomycin (Fig 4a). When ionomycin-treated neutrophils were stimulated with galectin-3, the extracellular response was even higher than corresponding response in fMLP-treated cells, while the intracellular response was slightly decreased (possibly as a result of translocation of part of the specific granule localized NADPH-oxidase to the plasma membrane leading to a depletion of the intracellular oxidase pool) (Fig 4c).
Effect of different priming protocols on secretion or surface exposure of different granule markers, and on the oxidative response to galectin-3. Peripheral blood neutrophils were pretreated as stated in the Materials and Methods section. The top panel (a) shows release into the medium of markers for gelatinase granules (gelatinase), specific granules (gelatinase and vitamin B12–binding protein), and azurophil granules (MPO). The values are given as percent released marker of the total amount in control cells. The middle panel (b) shows the surface exposure of the membrane components CR1 (mobilized from the secretory vesicles) and CR3 (mobilized from secretory vesicles, gelatinase granules, and specific granules), calculated from the mean fluorescence intensity of each cell population and expressed in percent of the value obtained with control cells (□). The lower panel (c) shows the extracellular and intracellular production of superoxide anion in response to galectin-3 (20 μg/mL). The responses are measured as in Fig 2 and are given as chemiluminescence (CL) units in megacounts per minute (Mcpm). Data are given as mean + SD, n = 4. (▧), 22°C; (▪), fMLP; (▨), ionomycin.
Effect of different priming protocols on secretion or surface exposure of different granule markers, and on the oxidative response to galectin-3. Peripheral blood neutrophils were pretreated as stated in the Materials and Methods section. The top panel (a) shows release into the medium of markers for gelatinase granules (gelatinase), specific granules (gelatinase and vitamin B12–binding protein), and azurophil granules (MPO). The values are given as percent released marker of the total amount in control cells. The middle panel (b) shows the surface exposure of the membrane components CR1 (mobilized from the secretory vesicles) and CR3 (mobilized from secretory vesicles, gelatinase granules, and specific granules), calculated from the mean fluorescence intensity of each cell population and expressed in percent of the value obtained with control cells (□). The lower panel (c) shows the extracellular and intracellular production of superoxide anion in response to galectin-3 (20 μg/mL). The responses are measured as in Fig 2 and are given as chemiluminescence (CL) units in megacounts per minute (Mcpm). Data are given as mean + SD, n = 4. (▧), 22°C; (▪), fMLP; (▨), ionomycin.
The galectin-3–induced NADPH-oxidase responses in in vitro–primed neutrophils were inhibited by lactose, and required the presence of the N-terminal domain of galectin-3 (Fig 5).Hence, these responses are dependent on the same features of galectin-3 as are the responses of in vivo–exudated neutrophils.
Galectin-3–induced activation of the NADPH-oxidase in in vitro primed neutrophils. The figure shows the time courses of the CL responses in primed neutrophils (106 cells) induced by galectin-3 (20 μg/mL; a and d), galectin-3 in the presence of lactose (10 mmol/L; b and e), or collagenase digested galectin-3 (CRD; 20 μg/mL; c and f). The experiment was done with the primed cell population giving the largest response to galectin-3 (Fig 4), ie, ionomycin-treated cells for extracellular responses (a through c), and fMLP treated cells for intracellular responses (d through f). The responses were measured as in Fig 2 and are given as chemiluminescence (CL) units in megacounts per minute (Mcpm).
Galectin-3–induced activation of the NADPH-oxidase in in vitro primed neutrophils. The figure shows the time courses of the CL responses in primed neutrophils (106 cells) induced by galectin-3 (20 μg/mL; a and d), galectin-3 in the presence of lactose (10 mmol/L; b and e), or collagenase digested galectin-3 (CRD; 20 μg/mL; c and f). The experiment was done with the primed cell population giving the largest response to galectin-3 (Fig 4), ie, ionomycin-treated cells for extracellular responses (a through c), and fMLP treated cells for intracellular responses (d through f). The responses were measured as in Fig 2 and are given as chemiluminescence (CL) units in megacounts per minute (Mcpm).
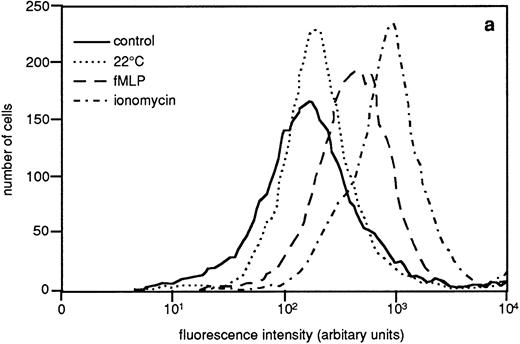
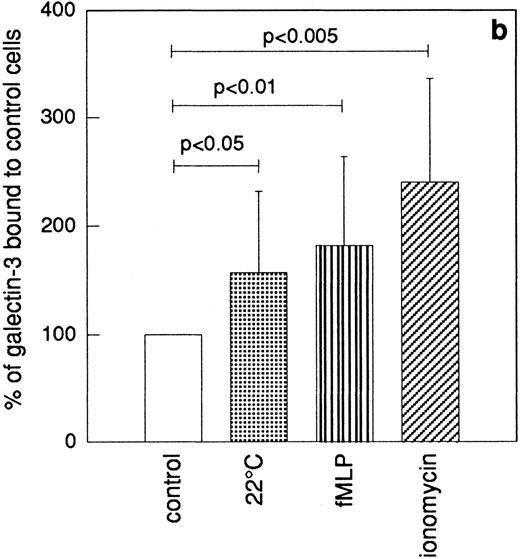
The granule marker expression/release induced by the sequential granule mobilization was paralleled by an increased binding of galectin-3 to the cell surface, shown by FACS analysis of cells labeled with FITC-galectin-3 (Fig 6). This binding of galectin-3 was inhibited by addition of lactose (data not shown).
Binding of galectin-3 to resting and in vitro primed neutrophils. In vitro–primed cells (see Materials and Methods) and resting peripheral blood cells (106) were paraformaldehyde fixed, incubated with FITC-labeled galectin-3, and analyzed by flow cytometry. Panel (a) shows a representative histogram while panel (b) depicts the relative amounts of bound galectin-3, calculated from the mean fluorescence intensity of each cell population and expressed in percent of the value obtained with control cells (□). The results are given as mean + SD, n = 8.
Binding of galectin-3 to resting and in vitro primed neutrophils. In vitro–primed cells (see Materials and Methods) and resting peripheral blood cells (106) were paraformaldehyde fixed, incubated with FITC-labeled galectin-3, and analyzed by flow cytometry. Panel (a) shows a representative histogram while panel (b) depicts the relative amounts of bound galectin-3, calculated from the mean fluorescence intensity of each cell population and expressed in percent of the value obtained with control cells (□). The results are given as mean + SD, n = 8.
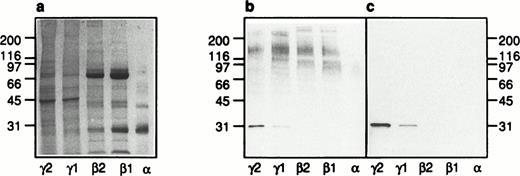
Identification of galectin-3–binding proteins in neutrophil organelles.
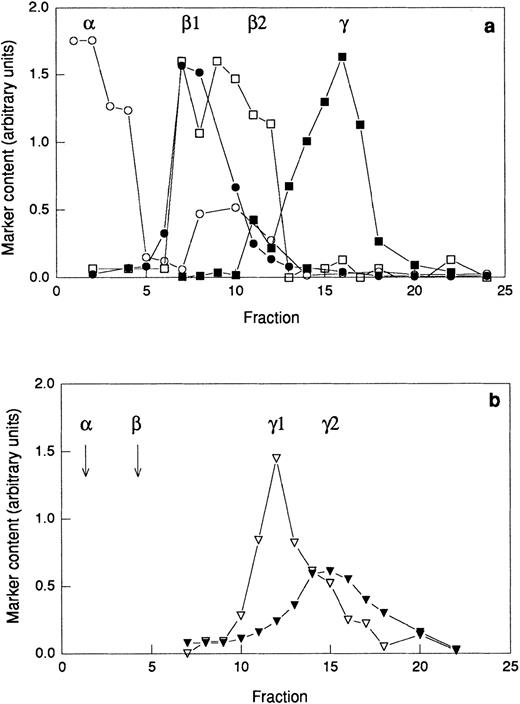
Provided that primed neutrophils are able to respond to galectin-3 due to the exposure of receptors mobilized from intracellular stores, galectin-3–binding residues could be expected to be found in such organelles. To investigate the presence of galectin-3–binding proteins in subcellular organelles, neutrophils were disintegrated and fractionated on discontinuous Percoll gradients. Two different gradients were used; the three-step gradient separates the gelatinase granules from the specific granules (Fig7a) whereas the flotation gradient allows separation of the plasma membrane from the secretory vesicles (Fig 7b). The azurophil granules were collected from the bottom of the three-step gradient (Fig 7a). Proteins from the different organelles and the cytosol were separated by SDS-PAGE (Fig 8a)and transferred to a blotting membrane. The blot was overlaid with galectin-3 and bound galectin-3 was detected using anti–galectin-3 and secondary antibody. Eight galectin-3–binding proteins were revealed (Fig 8b), and the binding was inhibited in the presence of lactose (Fig8c).
Distribution of marker molecules in discontinuous Percoll gradients. A postnuclear supernatant was divided in two and centrifuged on either a three-step Percoll gradient (a) or a flotation gradient (b) and fractions of 1.5 mL were collected from the bottom of the centrifuge tube. The fractions from the three-step gradient were analyzed for myeloperoxidase (marker for azurophil granules; α; ○), vitamin B12–binding protein (marker for the specific granules; β1; •), gelatinase (marker for specific and gelatinase granules; β1 and β2; □), and total alkaline phosphatase (marker for secretory vesicles and plasma membrane; γ1 and γ2; ▪). The fractions from the flotation gradient were analyzed for latent alkaline phosphatase (marker for the secretory vesicles; γ1; ▿) and nonlatent alkaline phosphatase (marker for the plasma membrane; γ2; ▾). Abscissa, fraction number; ordinate, amount of marker (arbitrary units). Samples for electrophoresis studies were prepared by pooling fractions from the three-step gradient (a) as follows: α, 1-3; β1, 6-8; β2 10-12; and from the flotation gradient (b) as follows: γ1, 10-13; γ2 15-18.
Distribution of marker molecules in discontinuous Percoll gradients. A postnuclear supernatant was divided in two and centrifuged on either a three-step Percoll gradient (a) or a flotation gradient (b) and fractions of 1.5 mL were collected from the bottom of the centrifuge tube. The fractions from the three-step gradient were analyzed for myeloperoxidase (marker for azurophil granules; α; ○), vitamin B12–binding protein (marker for the specific granules; β1; •), gelatinase (marker for specific and gelatinase granules; β1 and β2; □), and total alkaline phosphatase (marker for secretory vesicles and plasma membrane; γ1 and γ2; ▪). The fractions from the flotation gradient were analyzed for latent alkaline phosphatase (marker for the secretory vesicles; γ1; ▿) and nonlatent alkaline phosphatase (marker for the plasma membrane; γ2; ▾). Abscissa, fraction number; ordinate, amount of marker (arbitrary units). Samples for electrophoresis studies were prepared by pooling fractions from the three-step gradient (a) as follows: α, 1-3; β1, 6-8; β2 10-12; and from the flotation gradient (b) as follows: γ1, 10-13; γ2 15-18.
Subcellular localization of galectin-3–binding proteins in neutrophils. The organelles were obtained by fractionation of disintegrated peripheral blood neutrophils on a three-step Percoll gradient (α, β1, and β2)30 and a flotation gradient (γ1 and γ2),29 respectively (Fig 7). A Coomassie-stained SDS-polyacrylamide gel (5% to 20%; a) with separated proteins from the neutrophil plasma membrane (γ2), secretory vesicles (γ1), gelatinase granules (β2), specific granules (β1), and azurophil granules (α), respectively, corresponding to the fractionated content of 5 × 106cells, is shown together with corresponding Western blots (b and c). The blots were incubated with galectin-3 (8 μg/mL) in the absence (b) or presence (c) of lactose (100 mmol/L), followed by incubation with antibodies directed against galectin-3 (anti–Mac-2 antibodies; culture supernatant from the hybridoma M3/38; 1/25) and finally with HRP-labeled rabbit anti-rat Ig antibodies (DAKO P0450; 1/1,000). The blots were developed with peroxidase substrate (VIP Kit; Vector). Molecular sizes are given in kilodaltons.
Subcellular localization of galectin-3–binding proteins in neutrophils. The organelles were obtained by fractionation of disintegrated peripheral blood neutrophils on a three-step Percoll gradient (α, β1, and β2)30 and a flotation gradient (γ1 and γ2),29 respectively (Fig 7). A Coomassie-stained SDS-polyacrylamide gel (5% to 20%; a) with separated proteins from the neutrophil plasma membrane (γ2), secretory vesicles (γ1), gelatinase granules (β2), specific granules (β1), and azurophil granules (α), respectively, corresponding to the fractionated content of 5 × 106cells, is shown together with corresponding Western blots (b and c). The blots were incubated with galectin-3 (8 μg/mL) in the absence (b) or presence (c) of lactose (100 mmol/L), followed by incubation with antibodies directed against galectin-3 (anti–Mac-2 antibodies; culture supernatant from the hybridoma M3/38; 1/25) and finally with HRP-labeled rabbit anti-rat Ig antibodies (DAKO P0450; 1/1,000). The blots were developed with peroxidase substrate (VIP Kit; Vector). Molecular sizes are given in kilodaltons.
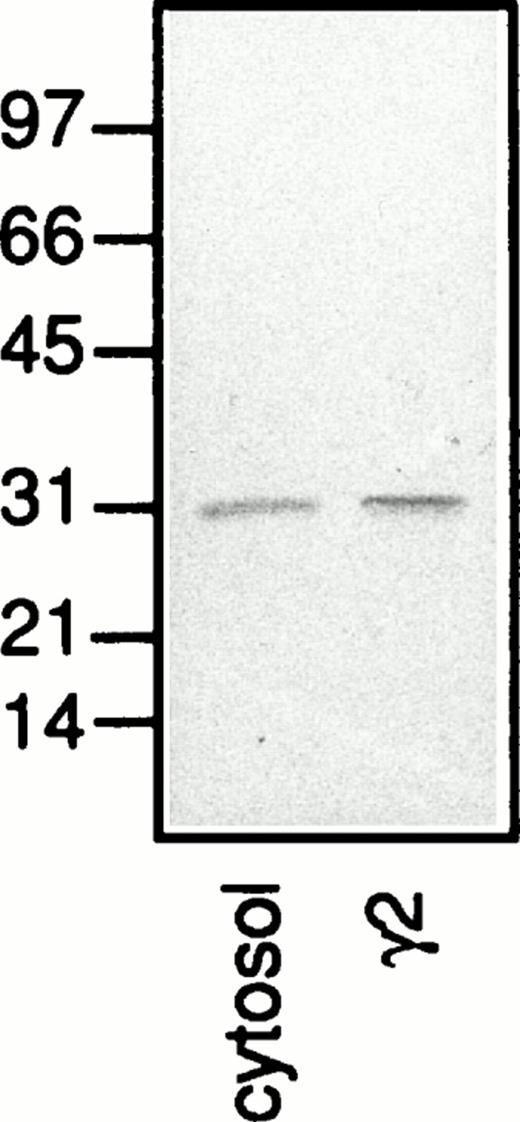
A protein with an apparent molecular weight of 31 kD which was mainly detected in the plasma membrane fraction (Fig 8b; γ2) and stained also in the presence of lactose (Fig 8c; γ2) was identified as galectin-3 based on the fact that this protein was detected also when no galectin-3 but only the primary and secondary antibodies were added to the Western blot (Fig 9; γ2). Because the neutrophil cytosol contains galectin-3 (Fig 9; cytosol), we hypothesize that the lectin is bound to the plasma membrane after disintegration of the cells and that it follows the membrane through the fractionation procedure. This hypothesis gains support from the fact that only a small amount of the 31-kD protein is found in the secretory vesicles (Fig 8; γ1), although these organelles are plasma membrane derived.39
A Western blot with separated proteins from neutrophil plasma membrane (γ2) and cytosol, corresponding to the fractionated content of 5 × 106 and 1 × 106 cells, respectively. The blot was incubated with antibodies directed against galectin-3 (anti–Mac-2 antibodies; culture supernatant from the hybridoma M3/38; 1/25) followed by HRP-labeled rabbit anti-rat Ig antibodies (DAKO P0450; 1/1,000), after which the blot was developed with peroxidase substrate (VIP Kit; Vector). Molecular sizes are given in kilodaltons.
A Western blot with separated proteins from neutrophil plasma membrane (γ2) and cytosol, corresponding to the fractionated content of 5 × 106 and 1 × 106 cells, respectively. The blot was incubated with antibodies directed against galectin-3 (anti–Mac-2 antibodies; culture supernatant from the hybridoma M3/38; 1/25) followed by HRP-labeled rabbit anti-rat Ig antibodies (DAKO P0450; 1/1,000), after which the blot was developed with peroxidase substrate (VIP Kit; Vector). Molecular sizes are given in kilodaltons.
The plasma membrane fraction (γ2) contains one major galectin-3–binding protein of approximately 150 kD. The secretory vesicles also contain a 150-kD protein in addition to four proteins of 95, 107, 120, and >200 kD. The gelatinase (β2) and specific (β1) granules stain equally for galectin-3–binding proteins, exhibiting a 150- and a 120-kD protein in addition to a 110- and a >200-kD protein. The >200-kD protein in the secretory vesicles differs in molecular weight from that in the gelatinase/specific granules. No galectin-3–binding proteins were detected in the azurophil granules (α).
DISCUSSION
We show that galectin-3 is capable of inducing a respiratory burst in neutrophils, provided that the cells have experienced the extravasation process. That exudated cells are primed is evident, not only in response to galectin-3, but to various inflammatory mediators, chemoattractants, and other ligands.6,7 40-42 The need for prior extravasation to achieve a responding cell (eg, to galectin-3) may be a regulatory mechanism, granting a cellular response (such as release of toxic oxygen radicals) only at sites where it is functional and necessary, eg, in inflamed or infected areas. Activation of neutrophils at other sites could result in an undesirable release of oxygen metabolites and proteases that would risk damage to healthy tissue.
During the extravasation process, a number of different inflammatory mediators are generated5,43 and most of these substances have been shown to induce in vitro priming of neutrophils.44 Although the molecular mechanism(s) responsible for induction of the primed state is unclear, a review of the current literature makes it obvious that several pathways/mechanisms work in parallel.17,37,44-46 Suggested mechanisms include signaling events such as tyrosine phosphorylation,46 intracellular free calcium increases,44 and activation of phospholipases.45 The exposure of new receptors is another attractive model as molecular base for an augmented response. We know from an earlier study36 that intracellular organelles are mobilized to the cell surface during extravasation, resulting in an increased exposure of various receptors. Human neutrophils contain at least four such organelles: the azurophil granules, serving primarily an intracellular role, and the specific granules, the gelatinase granules and the secretory vesicles, functioning as secretory organelles.39 These secretory organelles are mobilized during in vivo exudation; the secretory vesicles are completely mobilized together with 40% of the gelatinase granules and 20% of the specific granules while virtually all azurophil granules are retained.36 The secretory organelles all function as easily accessible reservoirs of plasma membrane proteins, including various receptors,38,39 which have been shown to occur in increased amounts on the surface of exudated cells.7,36 42
By use of in vitro priming protocols we also investigated whether the primed response to galectin-3 in exudated cells could be accounted for by receptor mobilization. Indeed, the ability to respond to galectin-3 paralleled the mobilization state in the in vitro–primed cells, and, although correlationally, our data suggest that galectin-3–binding receptors would reside in the gelatinase and possibly also the specific granules in unperturbed (resting) cells. This conclusion is based on the following: strong oxidative responses were induced by galectin-3 only after a significant portion of the gelatinase granules (40%) had been mobilized to the cell surface, and the extracellular response was further enhanced after the specific granules (25%) and additional gelatinase granules (calculated to about 20%) had been mobilized (Fig4).
There are additional facts supporting receptor upregulation as a probable cause of the primed response to galectin-3. The study by Yamaoka et al14 shows that galectin-3–induced NADPH-oxidase activity in peripheral blood neutrophils was dependent on the pretreatment of the cells with cytochalasin B, a fungal metabolite that blocks the polymerization of contractile microfilaments15 thereby facilitating degranulation.47 Further, when resting, exudated, and in vitro–primed neutrophils were labeled with fluorescent galectin-3, the amount of bound lectin increased with increasing granule mobilization. However, we want to emphasize that we cannot at this time conclude whether the galectin-3–induced activation is dependent on a certain density/avidity of attachment sites, allowing for aggregation of the lectin to occur, or whether a specific receptor structure has to appear on the cell surface to make the cells responsive.
Our data suggest that the potential galectin-3 receptor(s) should be found in intracellular organelles in resting cells, and we could also identify galectin-3–binding glycoproteins in the secretory vesicles, gelatinase granules, specific granules, and in the plasma membrane. It remains to be determined whether these galectin-3–binding proteins are similar to any earlier identified galectin-3–binding protein14,48,49 and if they have signaling capacity. The fact that galectin-3 binds to many different proteins (of which the majority probably will lack signaling capacity) makes it hard to determine binding characteristics (number of binding sites and affinity) of the signaling receptor, even after the identity of the protein has been established. Yamaoka et al14 found two galectin-3–binding proteins after cell-surface iodination of resting neutrophils (not treated with cytochalasin B). One of them was suggested to be the nonspecific cross-reacting antigen, NCA-160. Interestingly, the NCA-160 is localized mainly in the specific granules in resting neutrophils50 and is thus a possible activation-mediating receptor.
Galectin-3 was found to be present in human neutrophils, detected in the plasma membrane and cytosol fractions (Figs 8 and 9). We hypothesize that galectin-3 is present in the cytosol in intact neutrophils, but that it attaches to the outside of the plasma membrane–derived vesicles after disintegration of the cells. Galectin-3 is probably not bound to any higher extent to the plasma membrane of intact cells, since the secretory vesicles, which are derived from the plasma membrane and largely contain the same membrane markers, do not contain any substantial amounts of the lectin (Fig 8).
Finally, we conclude that two different signals are induced by galectin-3 in neutrophils, one that induces assembly of the NADPH-oxidase in the plasma membrane (giving extracellular release of oxygen radicals) and one that induces assembly of the NADPH-oxidase in the specific granule membrane (giving rise to intracellularly produced oxygen radicals).4,51,52 Whether these signals are generated by a single galectin-3 receptor, or if two different receptors are involved, remains to be determined. Recent evidence from both plant and animal cells suggest a role for intracellular reactive oxygen species as intracellular messengers, mediating activation of transcription factors and protein phosphorylation events.53How the galectin-3–induced intracellular oxygen radicals may take part in a signal transduction cascade that leads to functional effects in the cell is an intriguing question for future studies.
ACKNOWLEDGMENT
The skillful technical assistance of Lisbeth Björck and Marie Samuelsson is gratefully acknowledged.
Supported by the Swedish Medical Research Council, the King Gustaf V 80-Year Foundation, the Fredrik and Ingrid Thurings Foundation, the Lars Hierta Foundation, the Swedish Society for Medical Research, the Tore Nilsson Foundation, the Swedish Society for Medicine, the Anna-Greta Crafoord Foundation for Rheumatological Research, and the Swedish Association Against Rheumatism.
Address reprint requests to Anna Karlsson, PhD, The Phagocyte Research Laboratory, Department of Medical Microbiology and Immunology, Guldhedsgatan 10, S-413 46 Göteborg, Sweden.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal