Abstract
CD30 is a member of tumor necrosis factor (TNF) receptor superfamily that is expressed by activated T cells in the presence of interleukin-4 (IL-4). Although CD30 can mediate a variety of signals, CD30-deficient mice have impaired negative selection of T cells, suggesting that at least in the context of murine thymus, CD30 is a cell death–mediating molecule. The ligand for CD30 (CD30L) is a membrane-associated glycoprotein related to TNF, which is known to be expressed mainly by activated T cells and other leukocytes. However, the nature of CD30L-expressing cells involved in the interaction with CD30+ thymocytes is unclear. We report here that in postnatal human thymus the great majority of CD30+ cells are double positive (CD4+CD8+), activated, IL-4 receptor–expressing T cells which selectively localize in the medullary areas. Moreover, many medullary epithelial cells and Hassal's corpuscles in the same thymus specimens showed unusually high expression of CD30L in comparison with other lymphoid or nonlymphoid tissues. These findings provide additional information on the nature and localization of CD30+ thymocytes and show that epithelial cells are the major holder of CD30L in the thymic medulla.
CD30 IS A MEMBER of the tumor necrosis factor (TNF) receptor family1 that was originally identified as a surface antigen on Reed-Sternberg cells in Hodgkin's disease (HD).2,3 CD30 was subsequently found to be preferentially expressed by human activated T cells producing type 2 cytokines (Th2 cells) both in vitro4,5 and in vivo.6,7 The reason for this association has recently been clarified in mice, where CD30 expression seems to reflect the ability of CD4+ T cells to respond to interleukin-4 (IL-4).8
The ligand for CD30 (CD30L) is a membrane-associated glycoprotein related to TNF,9 which is known to be expressed mainly by activated T cells, as well as other leukocytes.9-11However, the physiological meaning of CD30/CD30L interactions is still unclear. In vitro studies have shown that CD30 can mediate a variety of activation and differentiation signals, a capacity that varies with cell type and origin. Engagement of CD30 on cell lines has been shown to induce immunoglobulin secretion in Epstein-Barr virus–transformed lymphoblastoid cells, proliferation in T-cell–like HD-derived cells, or cell death in anaplastic large-cell lymphoma cells.12Recently, it has been shown that the CD30-deficient mice contain elevated numbers of thymocytes and show a gross defect in negative but not positive selection, suggesting an important role for CD30/CD30L interactions in the deletion of autoreactive T cells.13However, the nature of CD30L-expressing cells in the thymus is unknown.
Here we show that CD30 is expressed on remarkable numbers of CD4+CD8+ (or more rarely CD4+CD8−), CD45RO+, IL-4 receptor (IL-4R)-expressing human medullary thymocytes. More importantly, both thymic epithelial cells (TEC) and Hassal's corpuscles in the medulla showed high CD30L expression. These data define the nature and localization of CD30+ thymocytes and identify the cells expressing CD30L in human thymus, thus providing indirect morphological support to the concept that negative selection might occur as a result of CD30/CD30L interactions in the thymic medulla.
MATERIALS AND METHODS
Antibodies.
Fluorescein isothiocyanate (FITC)- phyeoerythrin (PE)- and peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (Leu 4), anti-CD4 (Leu 3a), anti-CD8 (Leu 2a), and anti-CD45RO monoclonal antibodies (MoAbs) were purchased from Becton Dickinson (Mountain View, CA). FITC-conjugated anti-CD30 (Ber-H2) MoAb was purchased from Dako (Gastrup, Denmark). Anti–IL-4R MoAb was purchased from R & D Systems (Minneapolis, MN) and conjugated with Sulfo-NHS-LC-Biotin from Pierce (Rockford, USA). PE-conjugated streptavidin was purchased from Sigma Chemical Co (St Louis, MO). Anti-CD4 (Ancell Co, Bayport, MN), anti-CD30 (HRS4; Immunotech, Marseille, France), anti-CD30L (M81; Genzyme Diagnostics, Cambridge, MA), anti-pan-cytokeratin (C11; Sigma Immunochemicals, Milan, Italy), anti–IL-4R (25463.11; R & D Systems), and anti-TE4 (a generous gift of Dr B.F. Haynes (Duke University, Durham, NC) MoAbs were used for immunohistochemical studies. The mouse IgG2b used as isotype-matched control for CD30L MoAb was purchased from Southern Biotechnology Associated Inc (Birmingham, AL).
Tissues.
Normal postnatal thymus specimens were obtained from five children during corrective cardiac surgery at the Apuano Pediatric Hospital (Massa-Carrara, Italy). The five children were 5 days old, 7 days old, 5 months old, 7 months old, and 3 years old, respectively. Fetal thymus specimens were obtained from seven fetuses after voluntary or therapeutic abortions, four between gestation week 11 and 12 and three at gestation week 13. Tonsil fragments were obtained from two children undergoing tonsillectomy because of chronic tonsillitis. Lymph node fragments were obtained from biopsy specimens taken from two patients with nonspecific lymphoadenitis. Skin biopsy specimens were obtained from three patients suffering from atopic dermatitis. Kidney biopsy specimens were obtained from two patients with localized kidney tumor. Gut biopsy specimens were obtained from two patients suffering from Crohn's disease and one suffering from intestinal cancer. The procedures followed in the study were in accordance with the ethical standards of the responsible regional committee on human experimentation.
Cytofluorimetric analysis of thymocyte suspensions and separation of IL-4R+ and IL-4R− thymocytes.
Thymic tissue fragments were gently passed through a stainless-steel mesh to obtain single-cell suspensions from which mononuclear cells (MNC) were separated by centrifugation on Ficoll-Hypaque (Nycomed Pharma As., Oslo, Norway) gradient. Thymic MNC were resuspended in phosphate-buffered saline (PBS) containing bovine serum albumin (BSA) 0.5% and 0.02% sodium azide and then incubated with FITC-, PE-, or PerCP-conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD30, and anti-CD45RO MoAbs and biotinylated anti–IL-4R MoAb, followed by PE-conjugated streptavidin. Cell surface marker analysis was performed on a FACScan cytofluorimeter (Becton Dickinson). Separation of IL-4R+ and IL-4R− thymocytes was performed by high-gradient magnetic cell sorting.14 Briefly, MNC were incubated for 20 minutes with biotinylated anti–IL-4R MoAb, washed, and then incubated for an additional 20 minutes with MACS colloidal super-paramagnetic microbeads conjugated with streptavidin (MACS; Multifort, Milteny Biotec GmbH, Bergisch Gladbach, Germany). After washing, the cells were then separated on a MiniMACS column and inserted into a MiniMACS magnet. Negative and positive fractions were collected as IL-4R− and IL-4R+, respectively.
Establishment of TEC and kidney glomerular epithelial cell cultures.
Primary thymic stromal cell cultures were initiated by an explant technique from one postnatal (age, 5 months) and one fetal (age, 11 weeks) thymic sample according to the technique described by Fernandez et al.15 Briefly, small thymic fragments were anchored in 6-well plates (Costar, Cambridge, MA) and cultured in D-valine–containing Eagle's Minimal Essential Medium (GIBCO BRL Life Technologies, Ltd, Paisley, UK), supplemented with 10% inactivated fetal calf serum (FCS; GIBCO) (TEC medium). D-valine–containing medium was used to hinder the growth of fibroblasts. After 5 to 7 days at 37°C, the culture medium was replaced by fresh TEC medium. Explants were removed at day 14, the adherent cells detached by treatment with Puck's-modified solution containing trypsin and EDTA (GIBCO), and subcultured repeatedly in the TEC medium. After two passages, the epithelial nature of growing cells was assessed by immunostaining for pan-cytokeratin, as described below. Cloned TEC lines were obtained by seeding TEC at 1 cell/well in 96-well culture plates in TEC medium containing 25% culture supernatant from parental cells. At semiconfluency, growing clones were subcultured in standard TEC medium in 24-well plates, and subsequently transferred to 25-cm2 flasks.
Cultures of glomerular epithelial cells, obtained from macroscopically normal kidneys of patients with localized renal tumors undergoing nephrectomy, were also established.16 The cortex was separated from the medulla, minced, and glomeruli were isolated by a standard sieving technique through graded mesh size screens (60, 80, 150 mesh). The glomerular suspension was collected, washed, and incubated with 750 U/mL collagenase type IV at 37°C for 30 minutes. The glomeruli were then cultured in Dulbecco modified Eagle's medium (Sigma Immunchemicals) supplemented with 10% FCS, 5 μg/mL insulin, and 5 μg/mL transferrin. Glomeruli were maintained in culture with three changes of medium every week. Growing glomerular epithelial cells were characterized with the same anti-pan cytokeratin MoAb used to characterize TEC.
Cloning and sequencing of the CD30 probe.
mRNA was extracted from activated peripheral blood MNC and reversed to first-strand cDNA by oligo dT primer, using a first-strand synthesis kit (Stratagene Ltd, Cambridge, MA). Amplification of the first-strand products was performed in a Thermal cycler (Idaho Technology, Idaho Falls, ID). The samples were subjected to 30 cycles of amplification using 10 pmol of each primer (5′ GGAAGCGAATTCGGCAGAAGCTCCAC and 5′ CCACGATCACGGTGTCAGCCTTCATG) and 0.5 U of Taq DNA polymerase in 10-μL volume. The DNA fragment of 347 bp amplified by polymerase chain reaction was subcloned in pGEM-7 (Promega Co, Madison, WI) according to manufacturer's instructions. Sequencing of the amplified product was performed by the dideoxynucleotide chain-termination method,17 by using 35S dATP and sequenase enzyme (USB, Cleveland, OH).
In situ hybridization.
In situ hybridization was performed on frozen thymus sections by using CD30 probes. To do this, the plasmid containing the CD30 cDNA was linearized with SalI or SphI restriction enzymes, followed by phenol-chloroform extraction and ethanol precipitation. Thereafter, sense and antisense RNA probes were synthesized using SP6 or T7 RNA polymerases (Riboprobe Gemini System; Promega) in the presence of 35S alpha-thio-UTP (1,300 mCi/mmol; NEN Dupont, Paris, France). Frozen thymus sections were mounted onto gelatin-coated slides and fixed with 4% paraformaldehyde for 20 minutes at room temperature. Sections were subsequently treated with 0.2 N HCl for 20 minutes, pronase (0.125 mg/mL) for 10 minutes, 0.1 mol/L glycine for 30 seconds, and 4% paraformaldehyde for 20 minutes. Then, sections were rinsed with PBS, acetylated, and dehydrated in increasing ethanol concentrations. Thirty microliters of the hybridization solution (40% formamide, 4 × SSC, 10 mmol/L dithiothreitol, 1 × Denhardt's solution [Sigma], 10% dextran sulphate, 0.1 mg/mL sheared herring sperm DNA, and 1 mg/mL yeast tRNA), containing 8 × 105 cpm of 35S-labeled human CD30 RNA antisense probe, were applied to each section and covered with parafilm. Hybridization was performed at 52°C for 16 hours. Removal of the nonspecifically bound probe by RNAase digestion and autoradiography were performed, as detailed elsewhere.18Sections were subsequently counterstained with Mayer's hematoxylin and mounted with Kaiser's glycerol gelatin (Merck, Darmastadt, Germany). An average of five sections were analyzed for each tissue sample. Negative controls consisted of hybridization to a sense RNA probe. In same samples hybridized with anti-sense CD30 probe, immunostaining for CD30L was also performed. To do this, after hybridization with CD30 probe, RNA-ase digestion, and appropriate washings, sections were stained with the anti-CD30L MoAb and then subjected to autoradiography, as described above.
Immunohistochemistry.
Immunohistochemical staining was performed on 10-μm cryostat sections or cultured cells fixed in 4% paraformaldehyde for 20 minutes or in acetone for 10 minutes. Sections were subsequently exposed to 0.3% hydrogen peroxide-methanol solution to quench endogenous peroxidase activity. After a 30-minute preincubation with normal horse serum (Vectastain ABC kit; Vector Laboratories, DBA, Milan, Italy), sections were layered for 30 minutes with anti-CD4 (5 μg/mL), anti-CD30 (4 μg/mL), anti-CD30L (10 μg/mL), anti-cytokeratin (25 μg/mL), or anti–IL-4R (25 μg/mL) MoAbs, followed by biotinylated anti-mouse IgG horse Ab, and the avidin-biotin-peroxidase complex (Vectastain ABC kit), as described.19 As a peroxidase substrate, 3-amino-9-ethylcarbazole (AEC; Sigma) was used. Finally, sections were counterstained with Gill's hematoxylin (Merck) and mounted with Kaiser's glycerol gelatin. All incubations were performed at room temperature. As negative control, primary MoAb was replaced with an isotype-matched antibody with irrelevant specificity or mouse ascites fluid.
Double immunostaining.
Double immunostaining was performed by using the avidin-biotin-peroxidase system with two different substrates, as described.19 To identify CD30 and CD30L on the same specimen, the AEC (red color) and the Vector SG (bluish-grey) substrates were used, respectively. To identify CD30L and cytokeratin or CD30L and TE4 on the same specimen, the AEC and the Vector SG substrates were used, respectively. After double immunostaining, sections were counterstained with methyl green and mounted with Kaiser's glycerol gelatin.
Detection of apoptotic cells.
Apoptosis (DNA fragmentation) was detected by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) method, which has been shown to identify cells with DNA strand breaks in cryostat sections of normal lymphoid tissues, including murine thymus.20 In brief, after 10% formalin fixation and removal of endogenous peroxidase with 2% H2O2, the sections were incubated at 37°C for 1 hour in a solution containing TdT and digoxigenin-labelled dUTP. The sections were then treated with the peroxidase-labeled anti-digoxigenin Ab solution for 30 minutes. The reaction products were developed with AEC and counterstained with methyl green. As a negative control, PBS was substituted for TdT containing digoxigenin-labeled dUTP, which resulted in no staining. All the reagents were purchased from Oncor Ltd (Gaithersburg, MD).
RESULTS
Selective CD30 expression by a subset of activated medullary thymocytes.
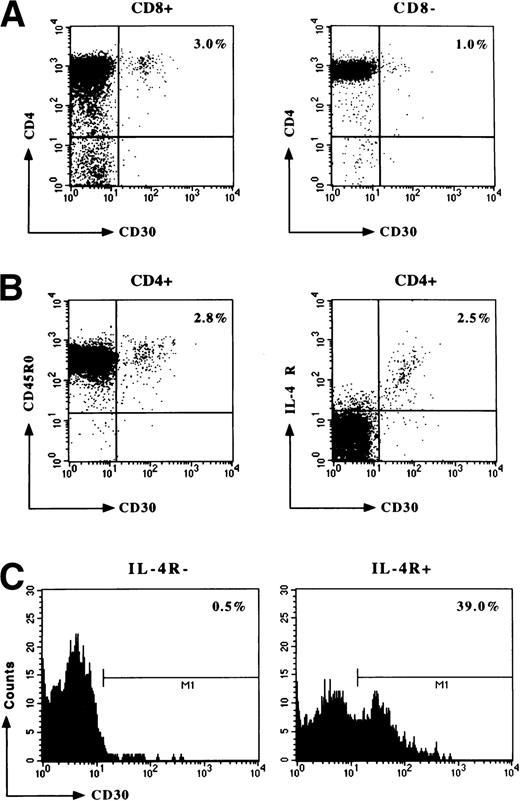
Fresh MNC suspensions from four fetal (between week 11 and 12 of gestation) and five postnatal (5 days to 3 years of age) thymuses were assessed by flow cytometry for CD30 expression. No CD30+ cells were observed in any of fetal thymuses, whereas small but detectable numbers of CD30+ cells were found in all postnatal thymuses examined, with percentages varying from 3.2 to 4.2 (mean values, 3.6 ± 0.1). The great majority of CD30+ cells were double-positive (CD4+CD8+) lymphocytes, the other being CD4+CD8− (Fig1A).Virtually all CD30+ T cells were CD45RO+ and showed the expression of IL-4R (Fig 1B). The consistent expression of IL-4R on CD30+ thymocytes was confirmed by fractionation experiments. When thymocyte suspensions were subdivided into IL-4R+ and IL-4R−, virtually all CD30+ cells were recovered in the IL-4R+ fraction (Fig 1C). Because IL-4 has been shown to be the most potent inducer of both IL-4R and CD30,8 21-24 these data may be consistent with the possibility that CD30+ thymocytes are activated T cells which are responding to IL-4.
Detection and characterization by flow cytometry of CD30+ T cells in postnatal thymus. Freshly isolated thymic MNC were resuspended in PBS containing 0.5% BSA and 0.02% sodium azide at the concentration of 1 × 106 cells/mL. Cells were then assessed for CD30, CD4, and CD8 expression (A), as well as for CD30, CD45RO, and IL-4R expression (B) by three-color flow cytometry. IL-4R+ were then separated from IL-4R− thymocytes by incubation of thymic MNC with biotin-conjugated anti–IL-4R MoAb, followed by addition of streptavidin-coated MACS colloidal supermagnetic microbeads, and the two subsets were then assessed for CD30 expression (C). A representative experiment is shown.
Detection and characterization by flow cytometry of CD30+ T cells in postnatal thymus. Freshly isolated thymic MNC were resuspended in PBS containing 0.5% BSA and 0.02% sodium azide at the concentration of 1 × 106 cells/mL. Cells were then assessed for CD30, CD4, and CD8 expression (A), as well as for CD30, CD45RO, and IL-4R expression (B) by three-color flow cytometry. IL-4R+ were then separated from IL-4R− thymocytes by incubation of thymic MNC with biotin-conjugated anti–IL-4R MoAb, followed by addition of streptavidin-coated MACS colloidal supermagnetic microbeads, and the two subsets were then assessed for CD30 expression (C). A representative experiment is shown.
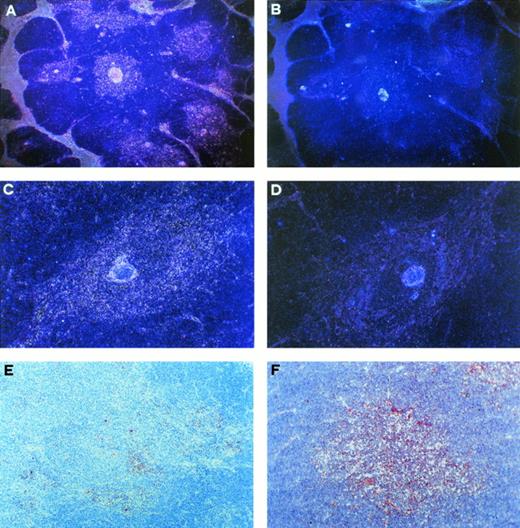
Localization of CD30+ and IL-4R+ cells in the medullary areas of postnatal thymus. (A) Autoradiograph of a thymus cryostat section hybridized with 35S-labeled antisense CD30 probe, showing positive signal in the medullary areas and along the septa (dark field, original magnification ×40). (B) Autoradiograph of a consecutive section hybridized with sense CD30 probe, showing virtually no signal (dark field, original magnification ×40). (C) Autoradiograph of a thymic medullary area hybridized with antisense CD30 probe showing high CD30 mRNA expression (dark field, original magnification × 100). (D) Autoradiograph of a consecutive section hybridized with sense CD30 probe showing no signal (dark field, original magnification ×100). (E) CD30 immunoreactive cells in the thymic medulla. Section was immunostained with anti-CD30 MoAb, using the avidin-biotin-peroxidase method, and the AEC substrate (red color, original magnification ×100). (F) IL-4R immunoreactive cells in the thymic medulla (red color, original magnification ×100).
Localization of CD30+ and IL-4R+ cells in the medullary areas of postnatal thymus. (A) Autoradiograph of a thymus cryostat section hybridized with 35S-labeled antisense CD30 probe, showing positive signal in the medullary areas and along the septa (dark field, original magnification ×40). (B) Autoradiograph of a consecutive section hybridized with sense CD30 probe, showing virtually no signal (dark field, original magnification ×40). (C) Autoradiograph of a thymic medullary area hybridized with antisense CD30 probe showing high CD30 mRNA expression (dark field, original magnification × 100). (D) Autoradiograph of a consecutive section hybridized with sense CD30 probe showing no signal (dark field, original magnification ×100). (E) CD30 immunoreactive cells in the thymic medulla. Section was immunostained with anti-CD30 MoAb, using the avidin-biotin-peroxidase method, and the AEC substrate (red color, original magnification ×100). (F) IL-4R immunoreactive cells in the thymic medulla (red color, original magnification ×100).
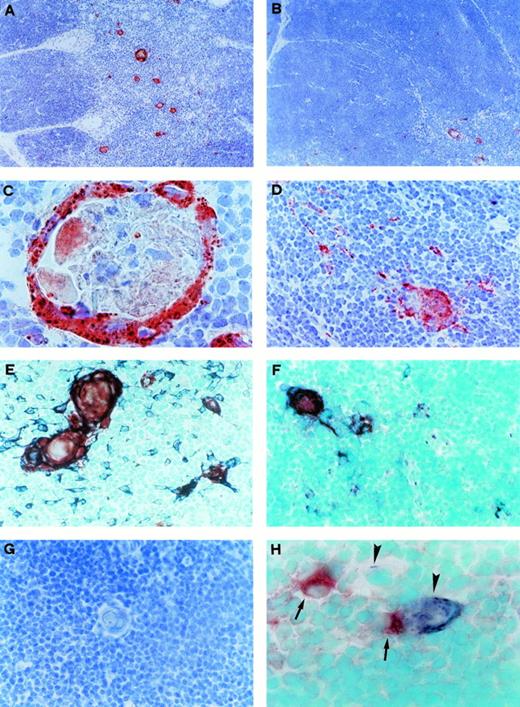
CD30L expression by Hassal's corpuscles and medullary TEC in postnatal thymus. (A) CD30L immunoreactivity in the thymic medulla. Section was immunostained with anti-CD30L MoAb, using the avidin-biotin-peroxidase method, and the AEC substrate (red color, original magnification ×100). (B) CD30L immunoreactivity (red color), which is clearly visible in a medullary area (bottom right), limited to a few scattered cells in the cortex (original magnification ×100). (C) Strong CD30L immunoreactivity in the outer part of a Hassal's corpuscle (original magnification ×1,000). (D) CD30L immunoreactivity in some medullary cells (original magnification ×250). (E) Double immunostaining for CD30L and cytokeratin in the medullary area. CD30L was identified by using the AEC substrate (red color) and cytokeratin by using the Vector SG substrate (bluish-grey color). Hassal's corpuscles and some cells staining for both cytokeratin and CD30L (purple-brown color), as well as many cells staining for cytokeratin alone, are visible (original magnification ×400). (F) Double immunostaining for CD30L (red color) and TE4 (bluish-grey color). Cells staining for both CD30L and TE4 (purple-brown color), as well as cells staining for TE4 alone, are visible (original magnification ×400). (G) Absence of immunostaining in a thymic medullary section where the anti-CD30L Ab was replaced by an isotype-matched control MoAb (original magnification ×400). (H) Double immunostaining showing distinct cellular distribution for CD30 (red color, arrows) and CD30L (bluish-grey color, arrowheads, original magnification ×1,000).
CD30L expression by Hassal's corpuscles and medullary TEC in postnatal thymus. (A) CD30L immunoreactivity in the thymic medulla. Section was immunostained with anti-CD30L MoAb, using the avidin-biotin-peroxidase method, and the AEC substrate (red color, original magnification ×100). (B) CD30L immunoreactivity (red color), which is clearly visible in a medullary area (bottom right), limited to a few scattered cells in the cortex (original magnification ×100). (C) Strong CD30L immunoreactivity in the outer part of a Hassal's corpuscle (original magnification ×1,000). (D) CD30L immunoreactivity in some medullary cells (original magnification ×250). (E) Double immunostaining for CD30L and cytokeratin in the medullary area. CD30L was identified by using the AEC substrate (red color) and cytokeratin by using the Vector SG substrate (bluish-grey color). Hassal's corpuscles and some cells staining for both cytokeratin and CD30L (purple-brown color), as well as many cells staining for cytokeratin alone, are visible (original magnification ×400). (F) Double immunostaining for CD30L (red color) and TE4 (bluish-grey color). Cells staining for both CD30L and TE4 (purple-brown color), as well as cells staining for TE4 alone, are visible (original magnification ×400). (G) Absence of immunostaining in a thymic medullary section where the anti-CD30L Ab was replaced by an isotype-matched control MoAb (original magnification ×400). (H) Double immunostaining showing distinct cellular distribution for CD30 (red color, arrows) and CD30L (bluish-grey color, arrowheads, original magnification ×1,000).
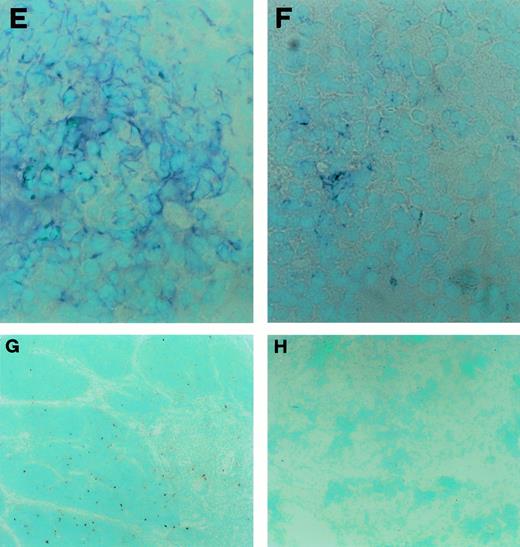
CD30L expression and detection of apoptotic cells in fetal and postfetal human thymuses. (A) Large numbers of cytokeratin-positive cells (bluish-grey color) in a fetal thymus specimen taken at week 11 of gestation (original magnification ×100). (B) Absence of TE4 and (C) absence of CD30L immunostaining (bluish-grey color) in the same fetal thymus (original magnification ×100). (D) Large numbers of cytokeratin-positive cells (bluish-grey color) in a fetal thymus taken at week 13 of gestation (original magnification ×400). (E) Several cells in a adjacent section staining positive for TE4 (bluish-grey color original magnification ×400). (F) A few cells in an adjacent section showing CD30L immunoreactivity (bluish-grey color, original magnification ×400). (G) Apoptotic cells detected by the TUNEL technique (red color) in a postnatal fetal thymus (original magnification ×100). (H) Absence of apoptotic cells in a fetal thymus taken at week 11 of gestation (original magnification ×100).
CD30L expression and detection of apoptotic cells in fetal and postfetal human thymuses. (A) Large numbers of cytokeratin-positive cells (bluish-grey color) in a fetal thymus specimen taken at week 11 of gestation (original magnification ×100). (B) Absence of TE4 and (C) absence of CD30L immunostaining (bluish-grey color) in the same fetal thymus (original magnification ×100). (D) Large numbers of cytokeratin-positive cells (bluish-grey color) in a fetal thymus taken at week 13 of gestation (original magnification ×400). (E) Several cells in a adjacent section staining positive for TE4 (bluish-grey color original magnification ×400). (F) A few cells in an adjacent section showing CD30L immunoreactivity (bluish-grey color, original magnification ×400). (G) Apoptotic cells detected by the TUNEL technique (red color) in a postnatal fetal thymus (original magnification ×100). (H) Absence of apoptotic cells in a fetal thymus taken at week 11 of gestation (original magnification ×100).
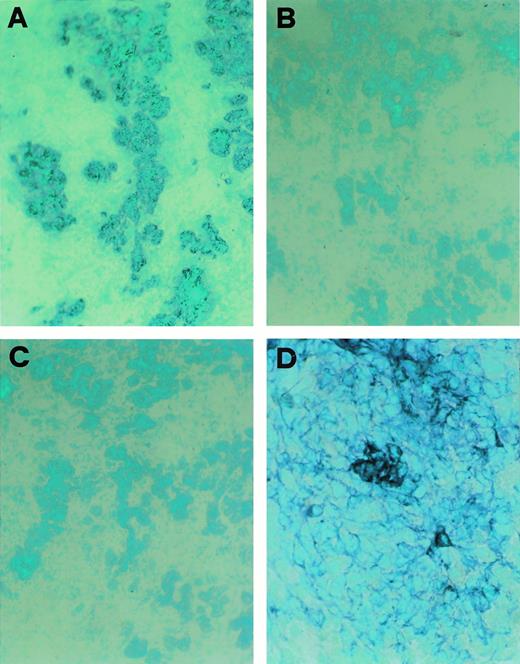
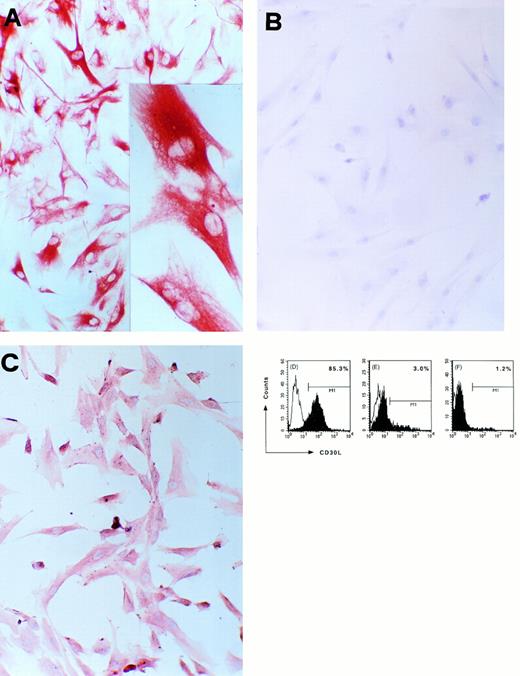
CD30L expression in a cultured TEC clone derived from postnatal human thymus. (A) Immunostaining for cytokeratin of cultured cells from a thymic clone; cells were fixed in acetone and stained by using the avidin-biotin-peroxidase method and the AEC substrate (red color, original magnification ×100); inset: high-power magnification of three cells showing intense cytokeratin immunoreactivity (original magnification ×250). (B) Absence of reactivity by the same cells stained with an isotype-matched control MoAb (original magnification ×100). (C) Immunostaining for CD30L of cultured epithelial cells from the same postnatal thymus clone; cells were fixed in 4% paraformaldehyde and staining was performed by using the avidin-biotin-peroxidase method and the AEC substrate (red color, original magnification ×100). (D) Detection of CD30L expression on cultured cells from the same postnatal thymus clone by flow cytometry. Cells (1 × 106/mL) were resuspended in PBS containing 0.5% BSA and 0.02% sodium azide and incubated with anti-CD30L (black area) or isotype-matched control (white area) MoAb, followed by FITC-conjugated anti-mouse IgG2b goat Ab. Absence of CD30L expression in cultured kidney glomerular epithelial cells (E) and in cultured T lymphocytes obtained from the same postnatal thymus (F).
CD30L expression in a cultured TEC clone derived from postnatal human thymus. (A) Immunostaining for cytokeratin of cultured cells from a thymic clone; cells were fixed in acetone and stained by using the avidin-biotin-peroxidase method and the AEC substrate (red color, original magnification ×100); inset: high-power magnification of three cells showing intense cytokeratin immunoreactivity (original magnification ×250). (B) Absence of reactivity by the same cells stained with an isotype-matched control MoAb (original magnification ×100). (C) Immunostaining for CD30L of cultured epithelial cells from the same postnatal thymus clone; cells were fixed in 4% paraformaldehyde and staining was performed by using the avidin-biotin-peroxidase method and the AEC substrate (red color, original magnification ×100). (D) Detection of CD30L expression on cultured cells from the same postnatal thymus clone by flow cytometry. Cells (1 × 106/mL) were resuspended in PBS containing 0.5% BSA and 0.02% sodium azide and incubated with anti-CD30L (black area) or isotype-matched control (white area) MoAb, followed by FITC-conjugated anti-mouse IgG2b goat Ab. Absence of CD30L expression in cultured kidney glomerular epithelial cells (E) and in cultured T lymphocytes obtained from the same postnatal thymus (F).
To provide additional information on the nature and localization of CD30+ T cells in the human thymus, in situ hybridization and immunohistochemical analyses were performed on the same postnatal thymus specimens. By in situ hybridization, CD30 expression was found in many cells scattered in the medullary areas and along the septa, whereas cortical areas showed little if any CD30 mRNA expression (Fig2A and B). By immunohistochemistry, CD30+ cells appeared to be selectively localized in the medullary areas, but their numbers were apparently lower than those revealed by in situ hybridization (Fig2C). Likewise, IL-4R–expressing cells were maximally detectable in the thymic medulla, their proportions being higher than those of CD30+ cells (Fig 2D). This was caused in part by the lack of CD30 expression by a proportion of IL-4R+ T lymphocytes, as shown by flow cytometry (Fig 1B and C), and in part by IL-4R expression by other cell types, possibly TEC.
High CD30L expression by medullary TEC and Hassal's corpuscles.
To establish whether cell types potentially able to interact with CD30+ medullary thymocytes were detectable, the presence of CD30L-expressing cells in the same thymus specimens was investigated. CD30L has been found to be expressed by a subset of activated macrophages and T lymphocytes,9 as well as by B cells,10 and granulocytes.11 Surprisingly, we found high CD30L expression in the outer wall of Hassal's corpuscles from all five thymuses examined, as well as in several TEC mainly localized in the medullary areas (Fig 3A, C, and D), whereas only a few scattered CD30L+ cells were found in the cortex (Fig 3B). As control, CD30L expression was assessed under the same experimental conditions in other lymphoid and nonlymphoid tissues, such as peripheral blood, tonsil, lymph nodes, skin, kidney, and gut. Although a few lymphoid cells in tonsils and lymph nodes showed slight CD30L staining, no similar CD30L immunoreactivity was found in any hematopoietic or epithelial cell from the different tissues examined (data not shown).
The epithelial nature of CD30L-expressing cells in the human thymus was confirmed by double immunostaining for CD30L and cytokeratin or TE4, an antigen selectively expressed by medullary and subcapsular cortical TEC.25 Indeed, all CD30L-reactive cells also stained positive for cytokeratin, but not all cytokeratin-positive cells stained positive for CD30L (Fig 3E). Likewise, all CD30L-expressing cells stained positively for TE4, but not vice versa (Fig 3F). No staining of medullary TEC or Hassal's corpuscles was found by using an isotype matched control MoAb (Fig 3G). More importantly, when double immunohistochemistry with anti-CD30 and anti-CD30L MoAb was performed, clear-cut separation of the two stainings in possibly interacting CD30+ T cells and CD30L+ TEC was observed (Fig 3H). By contrast, despite the presence of large numbers of cytokeratin-positive cells (Fig4A), neither TE4 nor CD30L expression was found in any of four fetal thymuses obtained before week 12 of gestation (Fig 4B and C). Of note, cells showing DNA strand breaks (apoptotic cells), as assessed by the TUNEL technique, were largely present in all postnatal thymuses (Fig 4G), but they could not be detected in fetal thymuses before week 12 of gestation (Fig 4H). However, in two of three fetal thymuses obtained at week 13 of gestation, which also contained large numbers of cells staining positively for cytokeratin (Fig 4D), TE4 immunoreactive cells (Fig 4E) and a few, but clearly distinguishable CD30L-reactive cells, were observed (Fig 4F). TEC cultures were also derived from one postnatal and one fetal thymic sample by an explant technique and repeated subculture in D-valine–containing medium as selective condition against fibroblast growth, and in the absence of exogenous growth factors. Some thymic stromal cell lines enriched in epithelial cells (80% to 100%, as determined by cytokeratin immunostaining) were obtained after repeated subculture from both postnatal and fetal thymuses. However, only two clones obtained from the postnatal thymus which showed positive staining with the anti-cytokeratin MoAb (Fig 5A), but not with an isotype-matched control Ab (Fig 5B), also stained positively with the anti-CD30L Ab (Fig 5C). Cytofluorimetric analysis revealed CD30L reactivity by the great majority of cells from the same TEC clones (Fig 5D), but neither by other TEC clones derived from the same line, nor by cultured kidney epithelial cells (Fig 5E), nor thymic T lymphocytes (Fig 5F), used as additional controls. Taken together, these findings suggest that CD30L expression is limited to a subset of medullary TEC, becomes detectable during the thymus development only after the week 12 of gestation, and precedes the appearance of apoptosis.
DISCUSSION
The results of the present study provide additional evidence for a role of CD30 expression by T cells in the outcome of differentiation and/or selection events in the thymus. A previous study had already reported spontaneous CD30 expression on small numbers of cells in the human thymic medulla.3 More recent data using Northern blot analysis have shown abundant expression of CD30 mRNA in the murine thymus.26 The mechanisms responsible for CD30 expression by T cells have recently been clarified. In activated human T cells, CD30 expression is associated with the production of Th2-type cytokines both in vitro4,5 and in vivo,6,7 and seems to be dependent on the presence of IL-4.21 In activated murine T cells, CD30 expression is mainly caused by the activity of IL-4.8 Because IL-4 strongly upregulates on murine T cells the expression of IL-4R,22-24 even in mice CD30 expression would be limited to T cells that express functional IL-4R. Thus, in both mice and humans CD30 expression seems to reflect the ability of T cells to respond to IL-4. The fact that virtually all CD30+ cells in the postnatal human thymus were CD45RO+ IL-4R+ (as shown by the cytofluorimetric analysis) and that CD30+ and IL-4R+ cells coexist in the thymic medulla (as shown by both in situ hybridization and immunohistochemistry) is in agreement with these findings. Based on these data, it is reasonable to speculate that after activation by autologous peptides, human thymocytes produce IL-4, which in turn upregulates in an autocrine or paracrine way the expression of both IL-4R22-24 and CD30.8,21 Thus, although it has been suggested that both positive and negative selection can occur in the cortex,27 the demonstration of IL-4R+ CD30+ thymocytes in the medullary areas may be rather consistent with the thought that negative selection takes place predominantly in the medulla.28
However, the most important finding emerging from this study is the identification of cells which, because of their ability to express the ligand for CD30, can represent the potential target for CD30+ thymocytes. Surprisingly and interestingly, CD30L was detected in the outer wall of Hassal's corpuscles and in medullary TEC, as clearly shown by the concomitant expression not only of cytokeratin, but also of TE4, an antigen selectively present in cortical subcapsular and medullary TEC.25 Of note, the expression of CD30L in both TEC and Hassal's corpuscles was unusually high in comparison with any other lymphoid and nonlymphoid tissue tested, suggesting a selective role for such a molecule in the thymic medulla.
The physiological meaning of CD30/CD30L interactions in the thymus is of great potential interest. In vitro studies have indeed showed that CD30 is able to mediate proliferation, differentiation, or even cell death, depending on both cell type and origin.12Interestingly, CD30-deficient mice have been shown to contain elevated numbers of thymocytes and exhibit a gross defect in negative but not positive selection, suggesting an important role for CD30/CD30L interactions at the thymic level in the deletion of autoreactive T cells.13 Because of the obvious difficulty to perform functional studies in humans, we could not directly prove that the interaction between CD30+ thymocytes and CD30L-expressing TEC in the thymic medulla is one of the effector mechanisms responsible for negative selection. However, the data here reported provide indirect morphological evidence in favor of this possibility. First, both TE4+ and CD30L+ cells were absent in fetal thymuses obtained before week 12 of gestation when apoptosis could not yet be observed. Cells staining positive for both TE4 and CD30L became detectable in fetal thymuses obtained at week 13 of gestation, which is consistent with the observation that human thymus is not differentiated fully before week 15 of gestation, when the cortico-medullary junctions and the first Hassal's corpuscles become visible.29 Finally, CD30L was widely expressed even at level of Hassal's corpuscles in all postnatal thymuses, where large numbers of apoptotic cells in both cortical and medullary areas could be observed.
An additional hypothesis that can be raised from the results of this study concerns the physiological meaning of Hassal's corpuscles. The function, if any, of these whorled structures is presently unknown. In a recent report, it has been shown that cross-linked CD30L can transduce a signal to the ligand-bearing cell, blurring the distinction between ligand and receptor.30 Thus, the demonstration of high CD30L expression in the outer wall of Hassal's corpuscles might provide the explanation of why these residues of degenerating TEC develop. It may indeed be suggested that CD30L-expressing Hassal's corpuscles reflect the high turnover of medullary TEC death because of reverse signaling provided by the interaction with CD30+ thymocytes.
ACKNOWLEDGMENT
The excellent technical assistance of Nadia Misciglia is acknowledged.
Supported by grants provided by Associazione Italiana Ricerca Cancro (AIRC), the Italian Ministry of Health (AIDS and Multiple Sclerosis Projects), and European Community (EC) (Biotech and FAIR Projects).
Address reprint requests to Sergio Romagnani, MD, Istituto di Medicina Interna e Immunoallergologia, Viale Morgagni 85, 50134-Firenze, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal