Abstract
The truncated Aα-chain of fibrinogen Marburg is partly linked with albumin by a disulfide bond. Based on the recovery of the first six amino acid residues assigned to the subunit polypeptides of fibrinogen (the Aα-and γ-chains) and albumin, 0.33 mol of albumin was estimated to be linked to 1 mol of the Marburg fibrinogen. When the Marburg fibrinogen was clotted with thrombin-factor XIIIa-Ca2+, various αmγnheteromultimers were produced, and part of the albumin was cross-linked to the γ-chain. Acid-solubilized Marburg fibrin monomer failed to form large aggregates that could be detected by monitoring turbidity at A350, but it was able to enhance tissue-type plasminogen-activator–catalyzed plasmin generation, though not as avidly as the normal control, indicating that the double-stranded protofibrils had, to some extent, been constructed. This idea seems to be supported by normal factor XIIIa–catalyzed cross-linking of the fibrin γ-chains. However, the cross-linked Marburg fibrin, being apparently fragile and translucent, was highly resistant against plasmin, and its subunit components were considerably retained for 48 hours as noted by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Although the exact mechanisms are still unclear, the albumin-incorporated factor XIIIa–cross-linked Marburg fibrin seems to have undergone a critical structural alteration(s) to acquire resistance against plasmin. This aquisition of plasmin resistance may be contributed to the postoperative pelvic vein thrombosis and recurrent pulmonary embolisms in the patient after caesarian section for her first delivery at the age of 20 years.
CONGENITALLY ABNORMAL fibrinogens with a cysteine (Cys) substitution have been shown to be linked with either albumin as reported for fibrinogens Nijmegen (Bβ Arg44 to Cys),1 Ijmuiden (Bβ Arg14 to Cys),1Dusart2 and Chapel Hill III (Aα Arg554 to Cys),3 and Fukuoka (Bβ Gly15 to Cys),4 or a single Cys molecule as was described in fibrinogen Osaka II (γ Arg275 to Cys).5 Otherwise, the aberrant polypeptide subunits may be disulfide linked with their counterpart in the same molecule as shown in fibrinogens Metz6 and Kawaguchi.7 Any abnormal fibrinogens with a Cys substitution seem to be loaded with a severe structural alteration due to disulfide-linked additives. In particular, the fibrinogen-associated albumin could affect the fibrin polymerization by steric hindrance, and thus interpretation for the structure-function relationship in such abnormal molecules is most difficult.
Fibrinogen Marburg is a homozygous dysfibrinogen lacking amino acid residues Aα 461-610 due to premature appearance of a stop codon TAA for AAA coding for A lysine (Lys) 461 of human fibrinogen. Because of this truncation, Cys at position 442 in the Marburg fibrinogen Aα-chain loses its disulfide partner, Aα Cys 472, and part of the Marburg Aα-chain forms a disulfide bridge with albumin.8This dysfibrinogen was found in a 20-year-old woman suffering from recurrent thromboembolic diseases in addition to a severe uterine bleeding after delivery of her first child by caesarian section.9 In this study, we attempted to estimate the amount of albumin disulfide linked to the Marburg fibrinogen and observed the behavior of the albumin molecule on formation of factor XIIIa–cross-linked fibrin. We also studied fibrin facilitation of plasmin formation catalyzed by tissue-type plasminogen activator (t-PA) and degradation of cross-linked fibrin by plasmin in relation to the postoperative recurrent thromboembolic diseases observed in the patient.
MATERIALS AND METHODS
Purification of fibrinogen.
Fibrinogen was purified from the patient's and normal plasma by immunoaffinity chromatography using a monoclonal antibody, IF-1, which recognizes the calcium-dependent conformation of the D domain of human fibrinogen, essentially as described previously.10 By sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting under nonreducing conditions, the purified Marburg fibrinogen was confirmed to consist of at least five species, native and partly degraded fibrinogen species free of albumin, and those linked with one or two molecules of albumin (profiles not shown). As anticipated from the data presented by Koopman et al8 on this abnormal fibrinogen, the Aα-chain was found to migrate close to the γ-chain on SDS-PAGE under reducing conditions, and a faint protein band noted at the position for the normal Aα-chain was confirmed to be albumin by immunoblotting (profiles not shown).
Aggregation studies of preformed, acid-solubilized fibrin monomer and enhancement of t-PA–catalyzed activation of plasminogen by the polymerizing fibrin monomer.
Fibrinogen Marburg (0.5 mg/mL) was clotted with human α-thrombin (5 NIH U/mL) prepared from prothrombin as described elsewhere11 at 37°C for 30 minutes and allowed to form gels for 40 minutes at 37°C and for successive 18 hours at 4°C. The Marburg fibrin clots appeared to be translucent and fragile as compared with turbid and solid normal fibrin clots. The fibrin clots were washed three times with tris-buffered saline (TBS) and solubilized with 20 mmol/L acetic acid and subjected to aggregation studies essentially as described elsewhere.5 Enhancement of t-PA–catalyzed plasminogen activation by polymerizing fibrin monomer was measured in 200 μL of the reaction mixture composed of acid-solubilized fibrin monomer (0.2 μmol/L), two-chain t-PA (4 U/mL; Sumitomo Pharmaceutical Co,Osaka, Japan), plasminogen (1.0 μmol/L), and a chromogenic substrate, S-2251 (H-D-valine-leucine-lysine-p-nitroanilide; 0.3 mmol/L). Plasmin generation was monitored by measurement of A405 at 2-minute intervals.
Factor XIIIa–catalyzed cross-linking of fibrin.
The normal and Marburg fibrinogen (0.5 mg/mL) were clotted at 25°C with α-thrombin (2.5 NIH U/mL) and factor XIII (1.25 U/mL, prepared from pooled plasma essentially described elsewhere,12 and the activity of factor XIIIa was expressed as amine-incorporating units as described by Lorand et al13) in the presence or absence of α2-plasmin inhibitor (α2-PI, 10 μg/mL; Calbiochem-Novabiochem, La Jolla, CA) in 32 μL of TBS containing 5 mmol/L CaCl2. At timed intervals, the reaction was stopped by addition of ethylenediaminetetraacetate-Na2 (EDTA, 2 mmol/L), and the clots were dissolved in the reducing SDS-PAGE solution (1.5 mol/L Tris-HCl, pH 8.8 containing 3% SDS, 8 mmol/L dithiothreitol, 2 mmol/L EDTA, and 8 mol/L urea). One microgram of proteins per lane was loaded to SDS-PAGE gels. For immunoblot analyses, antibodies against the Aα (148-160) residue segment (a kind gift from Dr Willem Nieuwenhuizen, Gaubius Laboratory, Leiden, The Netherlands) and against the γ (89-273) residue segment, JIF25,5 were used to specify the subunit polypeptides. Incorporation of α2-PI into cross-linked fibrin clots was monitored by autoradiography by using 37 ng of 125I–α2-PI (5 × 109 cpm/mg) in the cross-linking reaction mixture. For this experiment, α2-PI was labeled with125I by the Iodobeads method using Na125I essentially according to the manufacturer's instruction (Pierce, Rockford, IL). After extensive washing with Tris-HCl, pH 7.4 containing 0.5 mol/L NaCl and 0.1 % Tween 20, the radiolabeled clots were subjected to SDS-PAGE followed by autoradiography.
Plasmic degradation of factor XIIIa–cross-linked fibrin.
Normal and the Marburg fibrinogen (0.5 mg/mL) were clotted with 5 NIH U/mL α-thrombin in the presence of 2 U/mL factor XIII, 4 U/mL t-PA, 10 nmol/L plasminogen, and 2 mmol/L CaCl2 at 37°C. At timed intervals, the fibrin clots were dissolved in the reducing SDS-PAGE solution, and 2 μg of proteins per lane was subjected to SDS-PAGE.
Characterization of factor XIIIa–cross-linked fibrin formed in the presence of α2-PI.
To characterize the Marburg fibrin in relation to clinical thromboembolic diseases, we attempted to analyze the subunit compositions of cross-linked Marburg fibrin formed in the presence of α2-PI. In this experiment, 3 μg of α2-PI was added to 90 μg of fibrinogen, 0.75 NIH U of α-thrombin, and 0.1 U of factor XIII in 300 μL of TBS containing 5 mmol/L CaCl2, and the mixture was allowed to clot for 30 minutes at 37°C. After treatment with 2 mmol/L EDTA, the clots were precipitated by centrifugation for 30 minutes at 12,000 rpm and solubilized with 150 μL of the reducing SDS-PAGE solution. To get precise band separation in SDS-PAGE gels and efficient recovery of the resolved peptides therefrom, as much as 90 μg of proteins was divided in two parts and loaded separately onto 7.5% to 12.5% polyacrylamide gradient gels (2-mm thick and 15-cm long separation gels). The resolved polypeptides were blotted onto Problot membrane (PE-Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. After protein staining with Coomassie Brilliant Blue (CBB), amino acid sequence analysis of polypeptide bands was conducted with a protein sequencer, model 476 A (PE; Applied Biosystems). The recovery of individual polypeptides was estimated from the recovery of phenylthiohydantoin (PTH) amino acids in the first five cycles.
RESULTS
Amount of albumin disulfide linked to the Marburg fibrinogen.
Based on the recoveries of the first six amino acid residues assigned to the subunit polypeptides of fibrinogen and albumin, we estimated the amount of albumin linked to fibrinogen in duplicate runs (Table 1). At each cycle, recoveries of only representative amino acid residues for individual polypeptides were used for estimation as indicated by bold letters. The amount of albumin per mole of fibrinogen was calculated to be 0.34 mol for the first run and 0.32 mol for the second run from the ratio of albumin versus 2γ-chains (2γ) representing a dimeric molecule of fibrinogen. Thus, approximately 0.33 mol of albumin was found to be linked to the Marburg fibrinogen by a disulfide bond. Because no free sulfhydryl (SH) groups in the Marburg fibrinogen were present, the remainder of unpaired Aα Cys442 was expected to be linked with other substances such as a Cys molecule as reported for fibrinogen Osaka II.5
Albumin Content Calculated on the Basis of Amino Acid Sequence Analysis Data
| Sequence Identified . | Polypeptide Assigned . | Polypeptide Recovered (pmol) . | |
|---|---|---|---|
| 1st Run . | 2nd Run . | ||
| YVATRD | γ | 88 | 150 |
| ADSGEG | Aα | 65 | 120 |
| DSGEGD | AYα | 10 | 26 |
| DAHKSEV | Albumin | 30 | 48 |
| Sequence Identified . | Polypeptide Assigned . | Polypeptide Recovered (pmol) . | |
|---|---|---|---|
| 1st Run . | 2nd Run . | ||
| YVATRD | γ | 88 | 150 |
| ADSGEG | Aα | 65 | 120 |
| DSGEGD | AYα | 10 | 26 |
| DAHKSEV | Albumin | 30 | 48 |
Polypeptide recovery was calculated from the PTH-amino acid as indicated by bold letters. Albumin content was estimated from the polypeptide recovery ratio of albumin/2γ.
Aggregation of acid-solubilized fibrin monomer and facilitation by the polymerizing fibrin monomer of t-PA–catalyzed plasminogen activation.
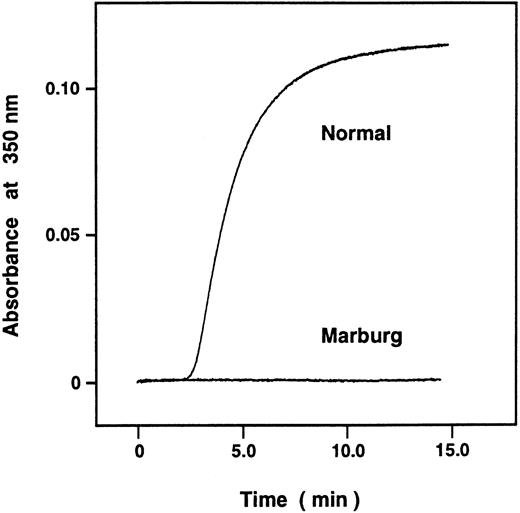
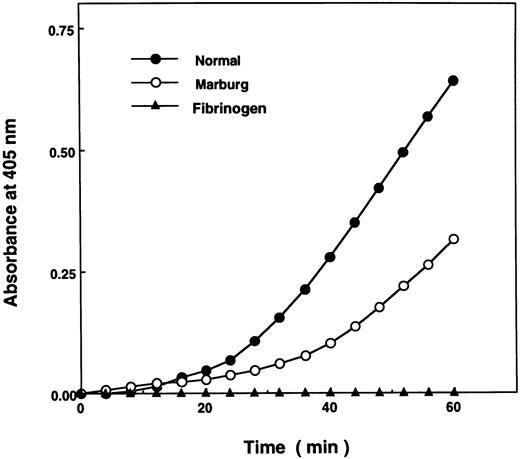
Although the turbidity of the acid solubilized fibrin monomer failed to increase when monitored by A350 (Fig 1), t-PA–catalyzed plasminogen activation was considerably enhanced in the presence of the polymerizing Marburg fibrin monomer at pH 7.4, though not as avidly as in the control (Fig 2). The result indicated that the Marburg fibrin monomer molecules were able to form double-stranded protofibrils to a certain extent and that failure to form fibrin gels could largely be attributed to impairment of lateral association of the double-stranded protofibrils, normally mediated by untethered carboxy-terminal regions of the α-chains (αC domains).14
Polymerization of acid-solubilized fibrin monomer was measured by monitoring A350 nm. The normal and Marburg acid-solubilized des-AB fibrin monomers were prepared as described in Materials and Methods. The reaction was started by dilution of 18.4 μL of fibrin monomer (20 μg) with 500 μL of 25 mmol/L imidazole-buffered saline, pH 7.4, and aggregation was monitored by A350.
Polymerization of acid-solubilized fibrin monomer was measured by monitoring A350 nm. The normal and Marburg acid-solubilized des-AB fibrin monomers were prepared as described in Materials and Methods. The reaction was started by dilution of 18.4 μL of fibrin monomer (20 μg) with 500 μL of 25 mmol/L imidazole-buffered saline, pH 7.4, and aggregation was monitored by A350.
Facilitation of t-PA–catalyzed activation of plasminogen by polymerizing fibrin monomer. Enhancement of t-PA–catalyzed plasminogen activation by fibrin monomer was measured in 180 μL of the reaction mixture composed of acid-solubilized fibrin monomer (0.2 mmol/L), plasminogen (1.0 μmol/L), t-PA (4 U/mL), and S-2251 (0.3 μmol/L) as described in Materials and Methods. Plasmin generation was measured by monitoring A405 nm at 2-minute intervals.
Facilitation of t-PA–catalyzed activation of plasminogen by polymerizing fibrin monomer. Enhancement of t-PA–catalyzed plasminogen activation by fibrin monomer was measured in 180 μL of the reaction mixture composed of acid-solubilized fibrin monomer (0.2 mmol/L), plasminogen (1.0 μmol/L), t-PA (4 U/mL), and S-2251 (0.3 μmol/L) as described in Materials and Methods. Plasmin generation was measured by monitoring A405 nm at 2-minute intervals.
Factor XIIIa–catalyzed cross-linking of the fibrin γ- and α-chains.
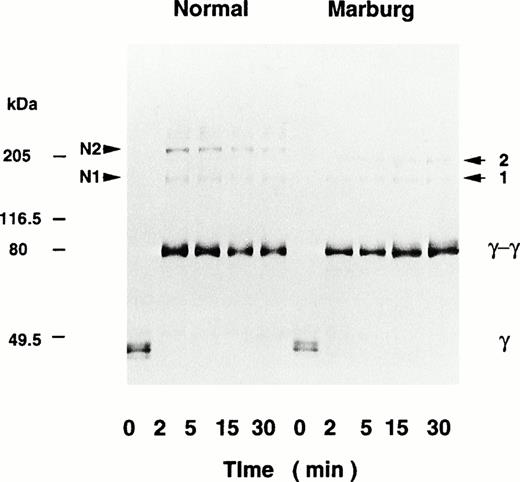
Despite severely altered fibrin monomer aggregation, factor XIIIa–catalyzed cross-linking of the Marburg fibrin γ-chains took place in a normal fashion (Fig 3). The result indicated that the initial double-stranded oligomers had been constructed by the A polymerization site in the E domain and its complementary a site in the D domain.15-17 Besides bands for the γ-dimer, at least two higher-molecular-weight bands containing the γ-chain were present as indicated by numbers 1 and 2 in the patient's sample. There were also bands denoted by N1 and N2 in the normal sample, apparently corresponding to the two bands for the Marburg fibrin, suggesting that they are heteromultimers composed of the α- and γ-chains. Indeed, this was confirmed by immunoblotting with an antibody recognizing the fibrin α-chain (148-160) segment. Furthermore, formation of high-molecular-weight α-polymers was almost missing in the patient's cross-linked fibrin, indicating that intramolecular α-chain cross-linking was severely disturbed by the lack of putative amine donor lysine residues at positions 508 and 556 or 562.18
Factor XIIIa–catalyzed cross-linking of the fibrin γ-chain analyzed by immunoblotting. Fibrinogen was clotted with thrombin and factor XIII in the presence of CaCl2. At timed intervals, the clots were subjected to SDS-PAGE followed by immunoblotting using an anti–γ-chain antibody. Besides the γ-dimer, high-molecular-weight polypeptides 1 and 2 were formed in the Marburg fibrin, and the corresponding peptides, N1 and N2, were formed in the normal fibrin. The molecular mass and the positions of the marker proteins are indicated in the left margin.
Factor XIIIa–catalyzed cross-linking of the fibrin γ-chain analyzed by immunoblotting. Fibrinogen was clotted with thrombin and factor XIII in the presence of CaCl2. At timed intervals, the clots were subjected to SDS-PAGE followed by immunoblotting using an anti–γ-chain antibody. Besides the γ-dimer, high-molecular-weight polypeptides 1 and 2 were formed in the Marburg fibrin, and the corresponding peptides, N1 and N2, were formed in the normal fibrin. The molecular mass and the positions of the marker proteins are indicated in the left margin.
Plasmic degradation of factor XIIIa–cross-linked fibrin.
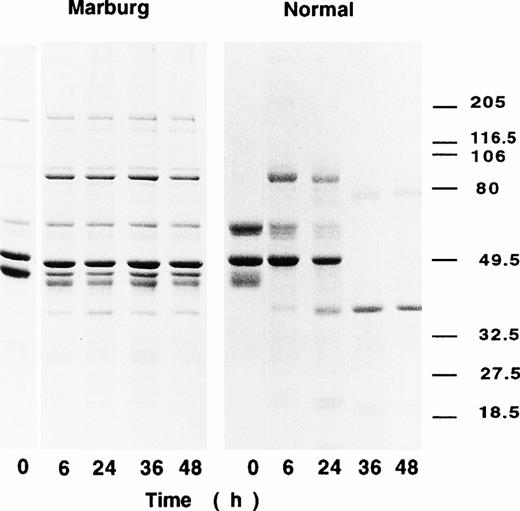
The Marburg fibrinogen was hardly clotted by an ordinary amount of thrombin used for the clotting assay, but addition of large amounts of thrombin together with factor XIII and Ca2+ resulted in formation of solid but fragile gels. Although the fibrin gels thus formed appeared to be fragile and translucent, they were found to be highly resistant against plasmin. In the degradation experiments of t-PA and plasminogen-enriched cross-linked fibrin, the Marburg fibrin clots remained as solid gels for more than 72 hours at 37°C. In fact, their subunit polypeptides were found to be largely preserved even at 48 hours as compared with those of normal control, which had been digested into much smaller segments at 36 hours of incubation as observed by SDS-PAGE (Fig 4).
Fibrin degradation by t-PA–catalyzed plasmin digestion. Fibrinogen was clotted with thrombin and factor XIII in the presence of plasminogen (1.0 nmol/L), t-PA (4 U/mL), and CaCl2. At 6 hours, 24 hours, 36 hours, and 48 hours, the reaction mixture was subjected to SDS-PAGE under reducing conditions.
Fibrin degradation by t-PA–catalyzed plasmin digestion. Fibrinogen was clotted with thrombin and factor XIII in the presence of plasminogen (1.0 nmol/L), t-PA (4 U/mL), and CaCl2. At 6 hours, 24 hours, 36 hours, and 48 hours, the reaction mixture was subjected to SDS-PAGE under reducing conditions.
Factor XIIIa–catalyzed cross-linking of α2-PI to the Marburg fibrin.
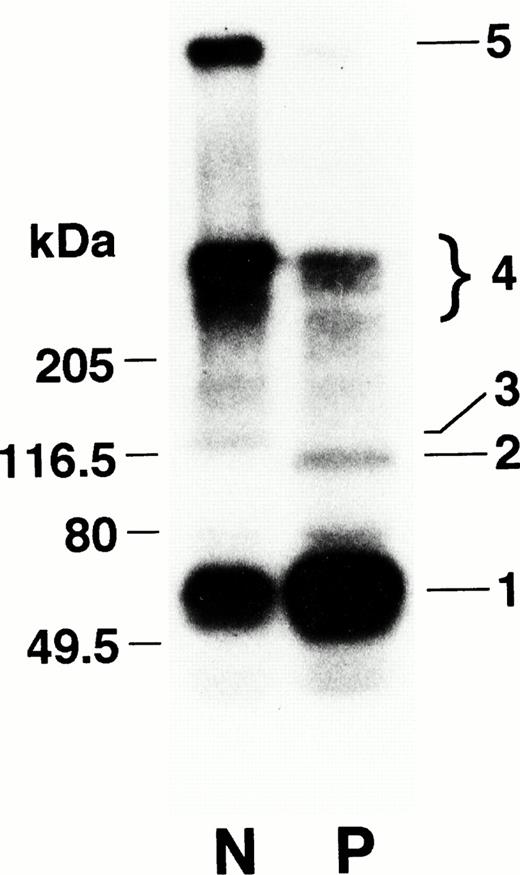
α2-PI is known to be cross-linked by factor XIIIa to the fibrin α-chain,19 where Gln2 of α2-PI serves as amine acceptor and Aα Lys303 of fibrin as amine donor.20 When the cross-linking profile of α2-PI to the Marburg fibrin was studied by autoradiography, the radiolabel was distributed to a 115-kD band corresponding to a complex of the Marburg α-chain and α2-PI (band 2) and to higher-molecular-weight proteins (band 4, Mr > 205 × 103) representing the polymerized Marburg α-chains (Fig 5). The extremely high-molecular-weight protein complex containing α2-PI in the normal sample (band 5) was, however, missing in the patient's sample. These results were in agreement with the observation as reported by Sobel et al21 on an in vitro plasma clotting system using the patient's plasma.
Factor XIIIa–catalyzed cross-linking of α2-PI to the Marburg fibrin analyzed by autoradiography. Fibrinogen was clotted with thrombin and factor XIII in the presence of125I–α2-PI and CaCl2 at 37°C for 30 minutes. After extensive washing, the radiolabeled clots were subjected to SDS-PAGE followed by autoradiography. Band 1 represents α2-PI and bands 2 and 3 represent a 115-kD (Marburg α-chain–α2-PI) and a 135-kD (normal α-chain–α2-PI) complex, respectively. Bands 4 and 5 represent high-molecular-weight complexes.
Factor XIIIa–catalyzed cross-linking of α2-PI to the Marburg fibrin analyzed by autoradiography. Fibrinogen was clotted with thrombin and factor XIII in the presence of125I–α2-PI and CaCl2 at 37°C for 30 minutes. After extensive washing, the radiolabeled clots were subjected to SDS-PAGE followed by autoradiography. Band 1 represents α2-PI and bands 2 and 3 represent a 115-kD (Marburg α-chain–α2-PI) and a 135-kD (normal α-chain–α2-PI) complex, respectively. Bands 4 and 5 represent high-molecular-weight complexes.
Behavior of the α-chain–linked albumin on factor XIIIa cross-linking of the Marburg fibrin.
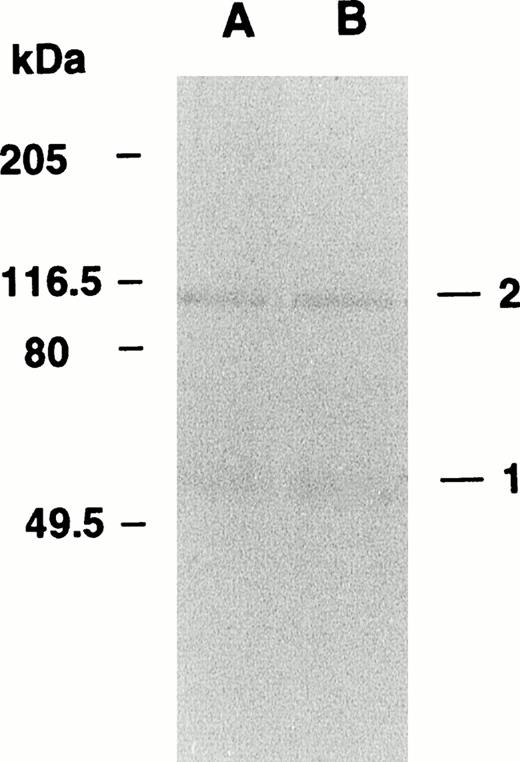
When the Marburg fibrinogen was clotted with thrombin, factor XIIIa, and Ca2+ and analyzed by immunoblotting using an antialbumin antibody, albumin was found to be distributed to a 115-kD protein complex (Fig 6, band 2 in lane A) as well as a 66-kD protein (Fig 6, band 1 in lane A), suggesting that some of the α-chain–linked albumin molecules had been cross-linked to an about 48-kD polypeptide subunit, ie, either the γ-chain or the truncated α-chain, by serving as substrate for factor XIIIa. The factor XIIIa–catalyzed cross-linking was also observed regardless of the presence or absence of α2-PI (band 2 in lane B), indicating that the fibrinogen-linked albumin did not share the cross-linking site of Aα Lys303 with α2-PI and that the albumin was most likely cross-linked to the γ-chain but not to the α-chain.
Behavior of fibrinogen-associated albumin in the cross-linked Marburg fibrin as analyzed by immunoblotting. Fibrinogen Marburg was clotted with thrombin and factor XIII in the presence (A) or absence (B) of α2-PI and then subjected to immunoblotting using an anti-human albumin antibody. Band 1 represents noncross-linked albumin, and band 2 represents the cross-linked albumin with a fibrin-derived subunit.
Behavior of fibrinogen-associated albumin in the cross-linked Marburg fibrin as analyzed by immunoblotting. Fibrinogen Marburg was clotted with thrombin and factor XIII in the presence (A) or absence (B) of α2-PI and then subjected to immunoblotting using an anti-human albumin antibody. Band 1 represents noncross-linked albumin, and band 2 represents the cross-linked albumin with a fibrin-derived subunit.
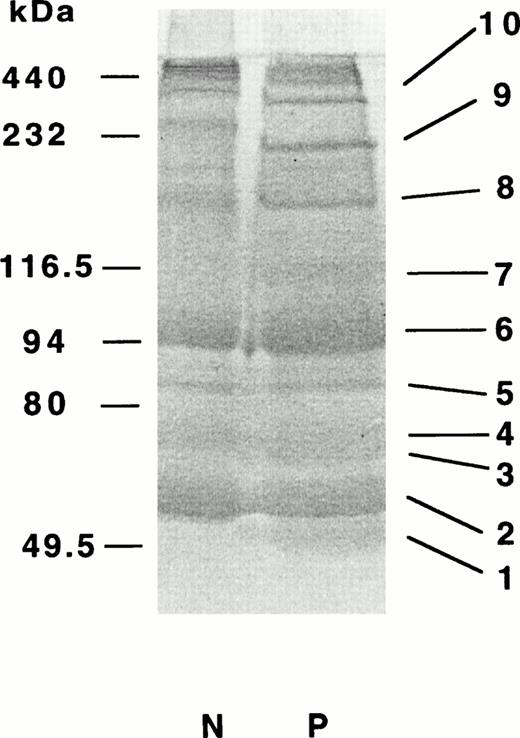
To further characterize the factor XIIIa–catalyzed cross-linking of the fibrinogen-linked albumin in the presence of α2-PI, we conducted amino acid analysis of the cross-linked polypeptide complexes resolved by SDS-PAGE under reducing conditions. To obtain enough amounts of polypeptides for sequence analysis of the 115-kD band, an unusually large amount, as much as 90 μg, of the Marburg fibrinogen was used for this study (for details, see Materials and Methods). In SDS-PAGE gels, at least 10 major polypeptide bands were noted (Fig 7, bands 1 to 10 in lane P). In a 115-kD polypeptide band (band 7), we were able to assign albumin and α2-PI in addition to the α- and γ-chains of fibrin at an approximate molar ratio of 5:3:1:1, based on PTH–amino acids recovered in the first five cycles (Table2). In other polypeptide bands we could assign the monomeric fibrin subunits, α2-PI and albumin (bands 1 to 4), the γ-dimer (band 6), or heteromultimers consisting of the α- and γ-chains (bands 8 to 10). Together with approximate molecular sizes of these polypeptide bands, we assigned most probable heteromultimers for the polypeptide complexes (Table 3).
Polypeptide compositions of proteins derived from α2-PI–incorporated cross-linked fibrin. Fibrinogen (90 μg) was clotted with thrombin and factor XIII for 30 minutes in the presence of CaCl2 and α2-PI. The clots were solubilized and subjected to PAGE in two lanes under reducing conditions followed by blotting onto PVDF-membranes as described in Materials and Methods. The protein bands were stained with CBB. Major bands 1-9 in the patient's sample were separetely subjected to amino-terminal sequence analysis.
Polypeptide compositions of proteins derived from α2-PI–incorporated cross-linked fibrin. Fibrinogen (90 μg) was clotted with thrombin and factor XIII for 30 minutes in the presence of CaCl2 and α2-PI. The clots were solubilized and subjected to PAGE in two lanes under reducing conditions followed by blotting onto PVDF-membranes as described in Materials and Methods. The protein bands were stained with CBB. Major bands 1-9 in the patient's sample were separetely subjected to amino-terminal sequence analysis.
Amino Acid Sequence Analysis of the 115-kD Band
| Proteins Assigned . | Amino Acid Recovered at Each Cycle (pmoles of PTH-Amino Acid) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| γ-chain | Y | V | A | T | R |
| (3.5) | (3.8) | (4.2) | t* | (3.9) | |
| α-chain | G | P | R | V | V |
| (6.4) | (6.1) | (3.8) | (4.2) | (7.4) | |
| Albumin | D | A | H | K | S |
| (0.9) | (1.2) | (0.9) | (1.2) | t* | |
| α2-PI | N | Q | E | Q | V |
| (1.2) | nd | (1.5) | (1.2) | (7.4) | |
| Proteins Assigned . | Amino Acid Recovered at Each Cycle (pmoles of PTH-Amino Acid) . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| γ-chain | Y | V | A | T | R |
| (3.5) | (3.8) | (4.2) | t* | (3.9) | |
| α-chain | G | P | R | V | V |
| (6.4) | (6.1) | (3.8) | (4.2) | (7.4) | |
| Albumin | D | A | H | K | S |
| (0.9) | (1.2) | (0.9) | (1.2) | t* | |
| α2-PI | N | Q | E | Q | V |
| (1.2) | nd | (1.5) | (1.2) | (7.4) | |
*Trace amounts were detected.
Abbreviation: nd, not detected.
Polypeptide Complexes in the Reduced Factor XIIIa–cross-linked Fibrin
| Band No. . | Mr (×10−3) . | Ratio of Polypeptides . | Assigned Polypeptide Complex . | ||||
|---|---|---|---|---|---|---|---|
| α . | β . | γ . | Alb* . | α2-PI . | |||
| 1 | 49.5 | 1 | α | ||||
| 2 | 52 | 1 | β | ||||
| 3 | 66 | 1 | Alb | ||||
| 4 | 68 | 1 | α2-PI | ||||
| 5 | 84 | 1 | 1 | α · γ | |||
| 6 | 95 | 1 | γ2 | ||||
| 7 | 115 | 5 | 3 | 1 | 1 | α · α2-PI, γ · Alb, α2 · γ† | |
| 8 | 235 | 1 | 4 | αγ4 | |||
| 9 | 282 | 1 | 2 | α2 · γ4 | |||
| 10 | >440 | m‡ | m‡ | αmγm | |||
| Band No. . | Mr (×10−3) . | Ratio of Polypeptides . | Assigned Polypeptide Complex . | ||||
|---|---|---|---|---|---|---|---|
| α . | β . | γ . | Alb* . | α2-PI . | |||
| 1 | 49.5 | 1 | α | ||||
| 2 | 52 | 1 | β | ||||
| 3 | 66 | 1 | Alb | ||||
| 4 | 68 | 1 | α2-PI | ||||
| 5 | 84 | 1 | 1 | α · γ | |||
| 6 | 95 | 1 | γ2 | ||||
| 7 | 115 | 5 | 3 | 1 | 1 | α · α2-PI, γ · Alb, α2 · γ† | |
| 8 | 235 | 1 | 4 | αγ4 | |||
| 9 | 282 | 1 | 2 | α2 · γ4 | |||
| 10 | >440 | m‡ | m‡ | αmγm | |||
Abbreviation: Mr, relative molecular mass.
Albumin.
Ratio of polypeptide complexes; α · α2-PI: γ · Alb:α2 · γ; 1:1:2.
m; positive integral number, m ≥ 4.
DISCUSSION
Fibrinogen Marburg is a unique dysfibrinogen, in that (1) the carboxy-terminal 150 residues are missing due to premature appearance of a stop codon TAA for AAA coding for Aα Lys461; (2) because of truncation of the Aα-chain, Aα Cys442 loses its disulfide bond partner Aα Cys472, and as a consequence some Aα Cys442 residues are linked with albumin by a disulfide bond. Indeed, the amount of disulfide-linked albumin to the Marburg fibrinogen was calculated to be 0.33 mol per mol of fibrinogen. In other words, one in every three Marburg fibrinogen molecules, or one in every six Marburg Aα-chains is linked with albumin near its carboxyl-terminus; and (3) the abnormality is associated with concomitant severe bleeding and thromboembolic diseases.8 9
In normal fibrinogen, the two carboxy-terminal Aα-chain segments (αC domains) interact with each other and associate with the central E domain. On thrombin-cleavage of fibrinopeptides A and B, the αC domains are loosened from the E domain and then untethered, thereby becoming available for association with other αC domains in promoting lateral association of fibrin protofibrils.12 In the Marburg fibrinogen, cleavage of fibrinopeptides A and B proceeded in a normal fashion (profiles not shown), and subsequent fibrin monomer assembly to form double-stranded protofibrils may also have progressed nearly normally, as evidenced by normal factor XIIIa–catalyzed cross-linking of the fibrin γ-chain and substantially enhanced t-PA–catalyzed plasminogen activation in the presence of polymerizing Marburg fibrin monomer (Fig 2). The failure for the Marburg fibrin monomer to increase the turbidity as monitored by A350 (Fig 1) could be accounted for by impaired lateral association of fibrin protofibrils. Based on our observation, we presume that the Marburg fibrin monomer molecules are able to bind with one another via the set of A-a polymerization sites and to form double-stranded fibrin protofibrils. These protofibrils are, however, unable to associate laterally in a normal fashion because of the truncated α-chain lacking the interaction sites assigned to the αC domain15 and the presence of the albumin molecule between the strands. In addition, factor XIIIa–catalyzed intermolecular ligation of the αC domains may not occur because the putative amine donor Lys residues at positions 508 and 556 or 562 are all missing in the Marburg α-chain. This interpretation may well agree with the delayed α-polymer formation and lack of the extremely high-molecular-weight α-polymer complex in the patient's fibrin in SDS-PAGE gels (data not shown). When the Marburg fibrinogen was clotted with thrombin in the presence of factor XIII, α2-PI, and Ca2+, a variety of heteromultimers composed of α- and γ-chains, such as αγ, α2γ, αγ4, α2γ4, and αmγm (m = 4) were identified (Fig 7 and Table 3). Moreover, some of the α-chain–linked albumin molecules were found to be cross-linked to an about 48-kD polypeptide, most probably γ-chain, forming a 115-kD polypeptide complex (Fig 6), which was noticeable only when factor XIII was present in the reaction mixture. This complex was not present in the normal sample either. Interestingly, formation of the 115-kD complex was not inhibited by α2-PI (Fig 6A), indicating that the albumin did not share the amine donor Aα Lys303 with Gln2 of α2-PI. If the albumin is ligated to the α-chain at another sites, a potential (αα2-PIγ) trimer can be formed, but this sort of trimolecular complex was not identified in this experiment.
We attempted to calculate the amount of cross-linked albumin on the basis of the recovery of amino acids assigned to albumin in the 115-kD polypeptide (Fig 7 and Table 2). Assuming that the γ-chain–ligated albumin molecules had been transferred at 100% to the membrane and that they had been totally recovered in the sequence analysis, the ligated albumin was estimated to be only 0.2% of the total Aα-chain–linked albumin. However, an extraordinarily large amount (as much as 90 μg) of the Marburg fibrin had been applied to SDS-PAGE gels in this experiment; therefore, the efficiency of protein transfer to the membrane may have been extremely low. Indeed, a large amount of proteins was retained in the SDS-PAGE gels even after electroblotting. Furthermore, the recovery of amino acids in our sequence study is estimated to be 30% to 40%. If the transfer efficiency was around 30%, the amount of albumin ligated to the γ-chain could be expected to be about 2% of the total albumin molecules.
On formation of factor XIIIa–cross-linked double-stranded protofibrils, the albumin-linked αC domain may be aligned closely to the carboxy-terminal segment of the γ-chain of an adjacent fibrin molecule in another strand of the protofibril, and a factor XIIIa–mediated cross-link is introduced between the albumin and either Gln398 (or 399) or Lys406 of the γ-chain. Consequently, extraordinary α-chain–albumin–γ-chain bridges are formed in the Marburg fibrin. At this stage of the investigation, we have no evidence for the amine donor-acceptor relationship in this cross-linking. In addition to cross-linking of the albumin to the γ-chain, the α-chain–linked albumin not involved in the cross-linking with the γ-chain must have been integrated into the cross-linked Marburg fibrin clots an affected their tertiary structure. Taking this sort of disoriented cross-linking into consideration, the Marburg cross-linked fibrin must have a distorted tertiary structure, manifesting unusual properties and behaviors. In fact, the cross-linked Marburg fibrin clots appeared to be fragile and less turbid, but they were extremely resistant against plasmin, remaining as solid gels for more than 72 hours and considerably retaining the subunit polypeptides even at 48 hours of incubation at 37°C (Fig 4). In view of the fact that fibrin clots containing thin fibers are digested more slowly by plasmin than intact normal fibrin clots,22 the Marburg fibrin may also be composed of thin fibers.
Recently, cross-linked and noncross-linked fibrin gels, derived from the two types of congenitally abnormal fibrinogens, were characterized by electron microscopic analyses.23-25 The Caracas II fibrin, which has a mutation of Aα Ser434 to Asn linked with an extra oligosaccharide,26 was shown to have thinner fibers in diameter and large pore or open areas bounded by local fiber networks.23 The presence of such irregular large pores allows fluids to get through the fibrin gels without any disturbance as evidenced by high permeation rates.23 This finding seems to account for the absence of thrombosis in the proband.27 On the other hand, the Dusart fibrin with an Aα Arg554 to Gly substitution partly linked with albumin2 was shown to have a less ordered structure than normal fibrin, but with many branch points resulting in greatly diminished pore size and high stiffness.24,25 In this fibrin, the α-chain–linked albumin may have distorted the fibrin structure and may have also contributed to the resistance against plasmin noted in the Dusart fibrin28 and in the Chapel Hill III fibrin29with the same type of mutation.3 The presence of albumin near the carboxy-terminal part of the fibrin α-chain, defective lateral association of protofibrils, and extremely high resistance of fibrin against plasmin relevant to thromboembolic diseases are shared by the Marburg fibrin.
At this stage, our study is still incomplete, but pieces of information provided in this study may partly account for the unique Marburg fibrin clot structure and behaviors relevant to concomitant bleeding and thromboembolic diseases observed in this patient.
ACKNOWLEDGMENT
We thank Michiko Takano for her expert secretarial assistance.
Supported in part by Grants-in-Aid for Scientific Research 06404043, 07680696, and 08407034; and for International Scientific Research Program, Joint Research Grants 06044196 and 09044329 from the Ministry of Education, Science and Culture of the Government of Japan.
Address reprint requests to Teruko Sugo, PhD, Division of Hemostasis and Thrombosis Research, Institute of Hematology, Jichi Medical School, Yakushiji 3311-1, Minamikawachi, Tochigi, 329-0498, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal