The WT1 gene is a tumor-suppressor gene that was isolated as a gene responsible for Wilms' tumor, a childhood kidney neoplasm. We have previously reported that the WT1 gene is strongly expressed in leukemia cells with an increase in its expression levels at relapse and an inverse correlation between its expression levels and prognosis, thus making it a novel tumor marker for leukemic blast cells. Furthermore, WT1 antisense oligomers have been found to inhibit the growth of leukemic cells. These results strongly suggested the involvement of the WT1 gene in human leukemogenesis. The present study was performed to prove our hypothesis that the WT1 gene plays a key role in leukemogenesis and performs an oncogenic function in hematopoietic progenitor cells, rather than a tumor-suppressor gene function. 32D cl3, an interleukin-3–dependent myeloid progenitor cell line, differentiates into mature neutrophils in response to granulocyte colony-stimulating factor (G-CSF). However, when transfected wild-type WT1 gene was constitutively expressed in 32D cl3, the cells stopped differentiating and continued to proliferate in response to G-CSF. As for signal transduction mediated by G-CSF receptor (G-CSFR), Stat3α was constitutively activated in wild-type WT1-infected 32D cl3 in response to G-CSF, whereas, in WT1-uninfected 32D cl3, activation of Stat3α was only transient. However, most interesting was the fact that G-CSF stimulation resulted in constitutive activation of Stat3β only in wild-type WT1-infected 32D cl3, but not in WT1-uninfected 32D cl3. Thus, WT1 expression constitutively activated both Stat3α and Stat3β. A transient activation of Stat1 was detected in both wild-type WT1-infected and uninfected 32D cl3 after G-CSF stimulation, but no difference in its activation was found. No activation of MAP kinase was detected in both wild-type WT1-infected and uninfected 32D cl3 after G-CSF stimulation. These results demonstrated that WT1 expression competed with the differentiation-inducing signal mediated by G-CSFR and constitutively activated Stat3, resulting in the blocking of differentiation and subsequent proliferation. Therefore, the data presented here support our hypothesis that the WT1 gene plays an essential role in leukemogenesis and performs an oncogenic function in hematopoietic progenitor cells and represent the first demonstration of an important role of the WT1 gene in signal transduction in hematopoietic progenitor cells.
WILMS' TUMOR GENE (WT1) was identified as a tumor-suppressor gene responsible for Wilms tumor, a childhood kidney neoplasm.1,2 The WT1 gene encodes a zinc finger transcription factor and represses transcription of growth factor (PDGF-A chain,3 CSF-1,4 and IGF-II5) and growth factor receptor (IGF-IR6) genes, and the other genes (RAR-α,7 c-myc,8and bcl-28). We have previously reported high expression of wild-type WT1 in fresh leukemia cells regardless of the disease type, 9-11 an inverse correlation between WT1 expression levels and prognosis,9 increased expression of WT1 at relapse in acute leukemia,12 and growth inhibition of leukemic cells by WT1 antisense oligomers.13 These results suggested that WT1 plays an important role in leukemogenesis and may have an oncogenic function rather than a tumor-suppressor gene function in hematopoietic progenitor cells.
Acute myelocytic leukemia (AML) is an acute myeloproliferative disease characterized by maturation arrest within the myeloid lineage. The majority of AML cells, like their normal counterparts, proliferate dependently on growth factors,14 but are refractory to differentiation induction.15 Most responsive AML cells, for example, proliferate without differentiation in response to granulocyte colony-stimulating factor (G-CSF),16 suggesting the alteration in the G-CSF signaling pathway in AML cells.
32D cl3, an interleukin-3 (IL-3)–dependent myeloid progenitor cell line, differentiates into mature neutrophils in response to G-CSF like their normal myeloid cell counterparts.17 Thus, transfection of the WT1 gene into 32D cl3 provides us with an experimental system suitable for the elucidation of WT1 gene function in hematopoietic progenitor cells.
The present study was performed to prove our hypothesis that the WT1 gene plays a key role in leukemogenesis and performs an oncogenic function in hematopoietic progenitor cells. We describe here that 32D cl3 transfected with the WT1 gene proliferates without differentiation in response to G-CSF and that this proliferative response is associated with activation of both Stat3α and Stat3β.
MATERIALS AND METHODS
Construction of retroviral vectors.
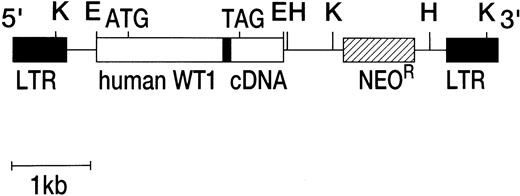
Murine retroviral vectors capable of expressing full-sized, nonspliced form [ie, 17 amino acids (+), KTS (+)] of human WT1 with or without the deletion of the third zinc finger domain18,19 was constructed with pM5Gneo retroviral vector, which was kindly provided by Dr Carol Stocking (Heinrich-Pette-Institut, Hamburg, Germany). This vector was derived from murine proliferative sarcoma virus and contained viral long terminal repeats (LTRs) and a neomycin resistance (NeoR) gene under the control of herpes virus thymidine kinase promoter. A full-sized, nonspliced type human WT1 cDNA (EcoRI-EcoRI fragment) with or without the deletion of the third zinc finger domain region18 was placed immediately downstream of the viral LTRs by digestion of the viral vector with EcoRI. The structure of retroviral vector is shown in Fig 1.
Structure of recombinant retroviral vectors. Human full-sized, nonspliced type WT1 cDNA with or without the deletion of the third domain of zinc finger region was inserted into a pM5GNeo retroviral vector. The solid bar within WT1 cDNA represents the deleted region of mutant WT1. K, Kpn I; E, EcoRI; H,HindIII.
Structure of recombinant retroviral vectors. Human full-sized, nonspliced type WT1 cDNA with or without the deletion of the third domain of zinc finger region was inserted into a pM5GNeo retroviral vector. The solid bar within WT1 cDNA represents the deleted region of mutant WT1. K, Kpn I; E, EcoRI; H,HindIII.
Cells and viruses.
The murine IL-3–dependent 32D cl3 cell line17 was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 50 U/mL recombinant murine IL-3 (rmIL-3). Recombinant retroviruses encoding WT1 were prepared by transfection of pM5Gneo/WT1 DNA into ϕ2 cells by the calcium phosphate method and selected for G418 resistance. The virus stock was prepared from supernatants of drug-resistant cell line. Viral titers were determined by infection of NIH3T3 cells and scored for G-418 resistant colonies. The expression of the human WT1 cDNA was confirmed by the detection of both human WT1 transcript and protein. Polymerase chain reaction–single-stranded conformation polymorphism (PCR-SSCP) analyses confirmed that the WT1 mRNA expressed by the retroviral vector contained neither point mutation nor deletion. 32D cl3 cells were infected with the recombinant retroviruses by coculturing the cells with virus-producing ϕ2 cells (virus titer, ∼105 cfu/mL) irradiated by x-rays for 48 hours and then grown in RPMI 1640 medium containing 10% FBS, 50 U/mL rmIL-3, and 500 μg/mL G418. G418-resistant clones were selected in 96-well microtiter plates. For induction of differentiation, exponentially growing cells were washed twice with serum-free RPMI 1640 medium and resuspended in RPMI 1640 medium containing 10% FBS and 20 ng/mL recombinant human G-CSF (rhG-CSF).
Reverse transcriptase-PCR (RT-PCR).
Extraction of total RNA and cDNA synthesis was performed as described previously.9 PCR for WT1 was performed as described previously.9 For detection of murine myeloperoxidase (MPO), lactoferrin (LF), and β-actin, specific primers were synthesized. The primer sequences are as follows: murine MPO: sense, 5′-ACAACATTGACATC-TGGATGG-3′, antisense, 5′-GGTCTCCTTCCAGGAAGTCA-3′; murine LF: sense, 5′-GACAAGGTGGAAGTCCTTCAG-3′, antisense, 5′-ACAACATTGACATCTGGATGG-3′; murine β-actin: sense, 5′-GTGGGCCGCCCTAGGCACCAG-3′, antisense, 5′-CTCTTTGATGTCACGCACGATTTC-3′. PCR were performed under the following conditions. For detection of MPO mRNA, denaturation at 94°C for 1 minute, primer annealing at 61°C for 1 minute, chain elongation at 72°C for 1.5 minutes, and 19 cycles of PCR. For detection of LF mRNA, denaturation at 94°C for 1 minute, primer annealing at 64°C for 1 minute, chain elongation at 72°C for 1.5 minutes, and 23 cycles of PCR. For detection of β-actin mRNA, denaturation at 94°C for 1 minute, primer annealing at 60°C for 1 minute, chain elongation at 72°C for 1.5 minutes, and 20 cycles of PCR.
Fluorescence-activated cell sorting (FACS) analysis.
Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MoAb) RB6-8C5 (anti-Gr-1) and FITC-conjugated MoAb M1/70.15 (anti-CD11b, Mac-1) were purchased from Pharmingen (San Diego, CA) and Caltag Laboratories (San Francisco, CA), respectively. Fluorescence intensity measurement was performed with a FACScan (Becton Dickinson, Mountain View, CA).
Western blot analysis.
Western blot analysis was performed as described previously.13 In brief, approximately 20 μg of cell lysates was transferred onto Immobilon polyvinylidene difluoride (PVDF) (Millipore, Bedford, MA), probed with anti-WT1 polyclonal antibodies,13 anti-Stat3 antibodies (Transduction Laboratories, Lexington, KY), anti-phosphorylated Stat3 antibodies (New England Biolabs, Beverly, MA), anti-Stat1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), or a mixture of anti-ERK1 and anti-ERK2 antibodies (Santa Cruz Biotechnology) and then with alkaline phosphatase- or peroxidase-conjugated anti-Ig antibodies. After washing, the filters were immersed in the buffer containing nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP; for WT1 protein) or enhanced chemiluminescence (ECL; for STAT and ERK proteins) for 10 to 60 minutes.
RESULTS
Introduction of the WT1 gene into 32D cells by retroviral infection.
32D cl3 (here designated 32D) cells were infected with recombinant retrovirus containing only a neomycin resistance gene, with a neomycin resistance gene plus a human full-sized, nonspliced type WT1 cDNA (wild-type WT1), or with a neomycin resistance gene plus a human full-sized, nonspliced type WT1 cDNA whose third zinc finger domain region was deleted (mutant WT1; Fig 1). G418-resistant clones were then isolated in medium containing IL-3 and G418 (V4, V5, and V6 were isolated as control clones expressing only the neomycin resistance gene; W2, W3, W4, W10, and W12were isolated as wild-type WT1-infected clones; and Z5, Z6, and Z7 were isolated as mutant WT1-infected clones).
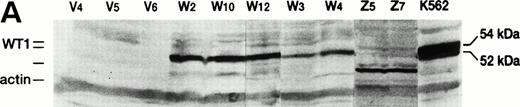
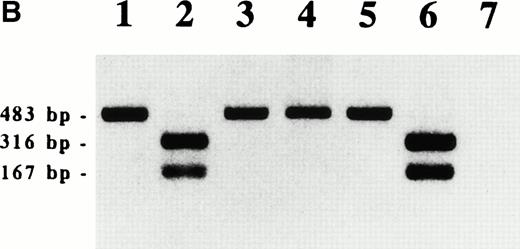
To confirm the production of WT1 protein from the infected WT1 gene, cell lysates were subjected to Western blot analysis using anti-WT1 antibodies. As shown in Fig 2A, wild-type WT1-infected clones produced various amounts of WT1 protein of 54 kD, whereas no WT1 protein was detected in 32D clones that were infected with the empty recombinant retrovirus containing only a neomycin resistance gene. Mutant WT1-infected 32D clones produced a short WT1 protein corresponding to the deletion of the third zinc finger domain. Because PCR primers used here amplified both human and mouse WT1 and the size of PCR products was the same, we could not discriminate between human and mouse WT1. However, because digestion of the PCR products with a restriction enzyme HaeIII occurred in human WT1, but not in mouse WT1, we could discriminate between the two PCR products. The results showed that the WT1-infected clones produced only human, but not mouse WT1 mRNA (Fig 2B). On the other hand, in both the parental 32D cells and the empty vector-infected clones (V4, V5, and V6) used as controls, neither human nor mouse WT1 mRNA was detected.
WT1 expression in WT1-infected 32D clones. (A) Western blot analysis of WT1 protein. V4, V5, and V6 , empty vector-infected-32D clones; W2 , W3 , W4 , W10, and W12, wild-type WT1-infected 32D clones; Z5 and Z7, mutant WT1-infected 32D clones. (B) RT-PCR analysis of WT1 transcript. RT-PCR was performed as described previously.9 WT1 PCR products were digested with a restriction enzyme HaeIII and electrophoresed on a 1.5% agarose gel. Lane 1, K562 (a human leukemia cell line); lane 2, digestion of 1; lane 3, mouse kidney cells; lane 4, digestion of 3; lane 5, a WT1-infected 32D clone (W2); lane 6, digestion of 5; lane 7, an empty vector-infected 32D clone (V4).
WT1 expression in WT1-infected 32D clones. (A) Western blot analysis of WT1 protein. V4, V5, and V6 , empty vector-infected-32D clones; W2 , W3 , W4 , W10, and W12, wild-type WT1-infected 32D clones; Z5 and Z7, mutant WT1-infected 32D clones. (B) RT-PCR analysis of WT1 transcript. RT-PCR was performed as described previously.9 WT1 PCR products were digested with a restriction enzyme HaeIII and electrophoresed on a 1.5% agarose gel. Lane 1, K562 (a human leukemia cell line); lane 2, digestion of 1; lane 3, mouse kidney cells; lane 4, digestion of 3; lane 5, a WT1-infected 32D clone (W2); lane 6, digestion of 5; lane 7, an empty vector-infected 32D clone (V4).
Proliferative response of wild-type WT1-infected 32D cells to G-CSF.
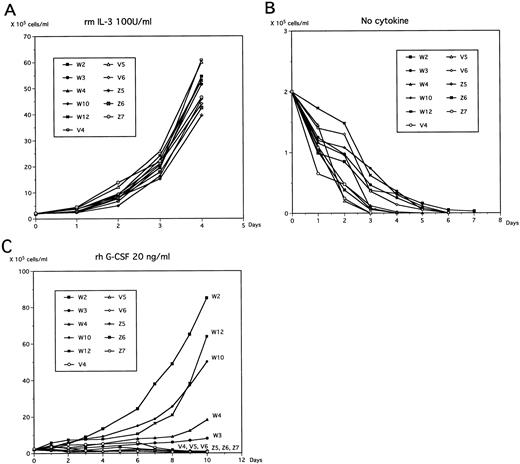
In the presence of IL-3, no significant difference in cell growth was found between controls and WT1-infected 32D clones (Fig 3A). It is well-known that deprivation of IL-3 from the medium induces apoptosis in 32D cells. In the current study, both control and WT1-infected 32D cells underwent apoptosis and died within 7 days after deprivation of IL-3 from the medium (Fig 3B).
Growth kinetics of WT1-infected 32D clones. Cells were cultured in 10% FBS-containing RPMI 1640 medium in the presence of IL-3 (50 U/mL; A), in complete deprivation of cytokines (B), or in the presence of G-CSF (rhG-CSF at 20 ng/mL; C), and viable cells were counted at the indicated time.
Growth kinetics of WT1-infected 32D clones. Cells were cultured in 10% FBS-containing RPMI 1640 medium in the presence of IL-3 (50 U/mL; A), in complete deprivation of cytokines (B), or in the presence of G-CSF (rhG-CSF at 20 ng/mL; C), and viable cells were counted at the indicated time.
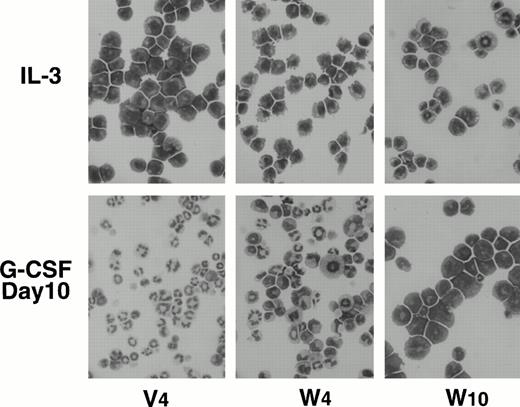
To determine the effects of WT1 expression on granulocytic differentiation pathways induced by G-CSF, control and WT1-infected 32D clones were removed from the IL-3–containing medium and cultured in G-CSF–containing medium. Control and mutant WT1-infected 32D clones completely differentiated into phenotypically matured neutrophils during 10 days of culture, whereas wild-type WT1-infected 32D clones stopped differentiating and began to proliferate (Figs 3C and4). An important finding was that the growth rate of the wild-type WT1-infected 32D clones in G-CSF–containing medium increased in parallel with the expression levels of the WT1 protein. In this context, a decrease in the expression levels of the WT1 protein in the wild-type WT1-infected 32D clones was accompanied by the appearance of phenotypically more differentiated cells during the culture in G-CSF–containing medium (Fig 4). Differentiated cells were frequently found in the W4 clone, which expressed low levels of WT1 protein.
Morphology of wild-type WT1-infected 32D clones cultured in IL-3– or G-CSF–containing medium. Cells maintained in rmIL-3 (50 U/mL)–containing medium (top) were washed for deprivation of IL-3, seeded in G-CSF (20 ng/mL)–containing medium, and cultured for 10 days (bottom). Cytospin cells were stained with May-Grünwald-Giemsa stain solution.
Morphology of wild-type WT1-infected 32D clones cultured in IL-3– or G-CSF–containing medium. Cells maintained in rmIL-3 (50 U/mL)–containing medium (top) were washed for deprivation of IL-3, seeded in G-CSF (20 ng/mL)–containing medium, and cultured for 10 days (bottom). Cytospin cells were stained with May-Grünwald-Giemsa stain solution.
Inhibition of G-CSF–induced differentiation of 32D cells by constitutive expression of the WT1 gene.
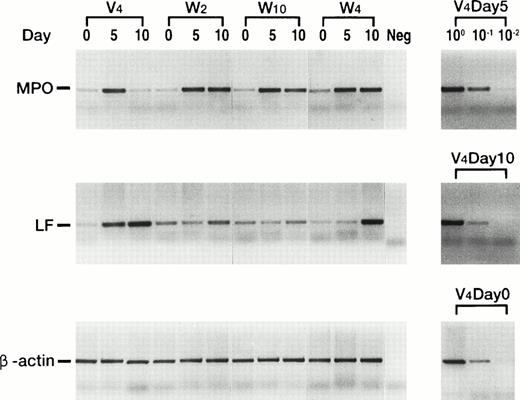
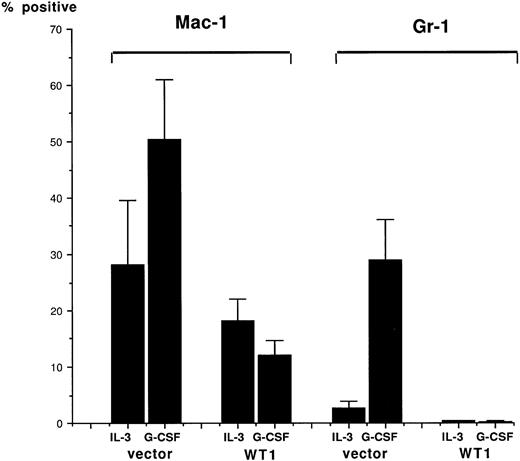
The mRNA expression levels of MPO20 and LF21genes, which are the respective differentiation markers for promyelocytes and metamyelocytes to segmented cells, were examined on the control and wild-type WT1-infected 32D clones cultured in G-CSF–containing medium (Fig 5). In the control clone V4, MPO mRNA expression increased, reached a peak 5 days after the treatment with G-CS F, and then decreased to the initial level 10 days after G-CSF treatment. In striking contrast, MPO mRNA expression increased in wild-type WT1-infected 32D clones and peaked at 5 days after G-CSF treatment, but then stayed at that level. In the control clone V4, LF mRNA expression increased during the 10 days of culture in G-CSF–containing medium, whereas in two wild-type WT1-infected 32D clones, W2 and W10, that expressed relatively high levels of WT1 protein, LF mRNA expression did not significantly change during the 10 days of culture in G-CSF–containing medium. In W4 that expressed low levels of WT1 protein, LF mRNA expression increased 10 days after G-CSF treatment, suggesting the differentiation of a part of the cells. In addition, the effects of WT1 expression on cell surface differentiation antigens were tested (Fig 6). In control clones (V4, V5, and V6) treated with G-CSF, expression of both Mac-1 and Gr-1 increased along with differentiation. In contrast, in wild-type WT1-infected clones (W2, W3, W4, and W10), both expressions remained unchanged during the 10-day culture period. These results showed that constitutive expression of the WT1 gene blocked the G-CSF–induced differentiation of the 32D cells. They also demonstrated that wild-type WT1-infected 32D cells can differentiate to the promyelocyte or near-promyelocyte stage, which is a little more differentiated than that of the parental 32D cells, but are blocked from differentiating further and are frozen at that stage, although WT1-low expressing 32D cells (W4) can differentiate in part into mature cells.
Quantitative RT-PCR analysis of MPO and LF transcripts after stimulation with G-CSF. Control and wild-type WT1-infected 32D clones were cultured in G-CSF–containing medium for the indicated days, and MPO and LF mRNAs were quantitated by RT-PCR under the optimized conditions.
Quantitative RT-PCR analysis of MPO and LF transcripts after stimulation with G-CSF. Control and wild-type WT1-infected 32D clones were cultured in G-CSF–containing medium for the indicated days, and MPO and LF mRNAs were quantitated by RT-PCR under the optimized conditions.
Inhibition of Mac-1 and Gr-1 differentiation antigen induction by WT1 expression. Control (V4, V5, and V6) and wild-type W T1-infected (W2, W4, W10, and W12) 32D clones were cultured in IL-3 (100 U/mL)–containing or G-CSF (20 ng/mL)–containing medium for 10 days. The cells were stained with FITC-conjugated M1/70.15 MoAb (CD 11b, Mac-1) or FITC-conjugated RB6-8C5 MoAb (Gr-1), and then FACS-analyzed. Means ± 2 SD of positive cells in three control clones and those in four WT1-infected clones are shown.
Inhibition of Mac-1 and Gr-1 differentiation antigen induction by WT1 expression. Control (V4, V5, and V6) and wild-type W T1-infected (W2, W4, W10, and W12) 32D clones were cultured in IL-3 (100 U/mL)–containing or G-CSF (20 ng/mL)–containing medium for 10 days. The cells were stained with FITC-conjugated M1/70.15 MoAb (CD 11b, Mac-1) or FITC-conjugated RB6-8C5 MoAb (Gr-1), and then FACS-analyzed. Means ± 2 SD of positive cells in three control clones and those in four WT1-infected clones are shown.
Constitutive activation of Stat3 by WT1 expression.
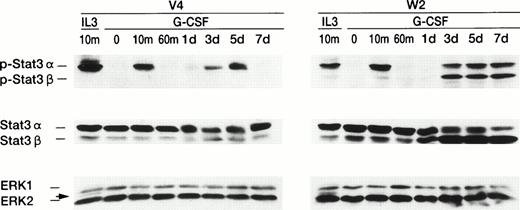
We examined the effects of WT1 expression on two major signals, STAT (signal transducers and activators of transcription)22,23and MAP kinase,24 mediated by the G-CSF receptor (G-CSFR; Fig 7). First, Western blot analysis using antibodies directed against phosphorylated (P-) Stat3 was performed. In the control clone V4 , only P-Stat3α was detected 10 minutes after G-CSF stimulation, after which it became undetectable. Three days after G-CSF stimulation, P-Stat3α became distinctly detectable again, peaked at 5 days after G-CSF stimulation, and once more became undetectable. In the wild-type WT1-infected clone W2, P-Stat3α was also detected 10 minutes after G-CSF stimulation, then became undetectable, and 3 days after G-CSF stimulation became detectable again. However, in contrast to in V4, P-Stat3α was detected continuously from days 3 through 7 after G-CSF stimulation. The most striking contrast was seen in P-Stat3β,25 which became detectable in W23 days after G-CSF stimulation and persisted from day 3 through 7 after G-CSF stimulation. Next, the amounts of Stat3α and Stat3β were examined with Western blot analysis using anti-Stat3 antibodies directed against the sequence common to Stat3α and Stat3β. In V4, the amounts of Stat3α and Stat3β did not significantly change at any time, and those of Stat3α were significantly greater than those of Stat3β. In W2, on the other hand, the quantity of Stat3α was significantly greater than that of Stat3β at 0, 10, and 60 minutes after G-CSF stimulation. One day after stimulation, the amounts of Stat3α had become equivalent to those of Stat3β, but then quantity of Stat3β exceeded that of Stat3α from days 3 through 7 after G-CSF stimulation, so that the ratio of Stat3α to Stat3β was reversed. As for Stat1, a transient activation was detected in both V4 and W2, but neither quantitative nor qualitative differences were found between V4 and W2 at any time after G-CSF stimulation (data not shown). Examination of the second signal, MAP kinase showed no significant difference in the amounts of MAP kinase between V4 and W2 after G-CSF stimulation, and we did not detect any active forms of MAP kinase in either V4 or W2 (Fig 7). Altogether, these data demonstrated that WT1 expression constitutively activated both Stat3α and Stat3β and that this activation might block differentiation and induce proliferation.
Constitutive activation of Stat3 in wild-type WT1-infected 32D cells. IL-3–deprived 32D cells were stimulated with IL-3 (100 U/mL) or G-CSF (20 ng/mL). Cell lysates were prepared at the indicated time and subjected to Western blot analysis, using antibodies directed against phosphorylated Stat3 protein (top panel) or Stat3 protein (middle panel), or using a mixture of antibodies directed against ERK1 or ERK2 (bottom panel). Arrows indicate activated ERK2.
Constitutive activation of Stat3 in wild-type WT1-infected 32D cells. IL-3–deprived 32D cells were stimulated with IL-3 (100 U/mL) or G-CSF (20 ng/mL). Cell lysates were prepared at the indicated time and subjected to Western blot analysis, using antibodies directed against phosphorylated Stat3 protein (top panel) or Stat3 protein (middle panel), or using a mixture of antibodies directed against ERK1 or ERK2 (bottom panel). Arrows indicate activated ERK2.
DISCUSSION
Mutational studies of G-CSFR have shown that the cytoplasmic domain of G-CSFR consists of two distinct functional domains. The membrane-proximal region, which contains two subdomains designated boxes 1 and 2, is responsible for the transduction of the proliferation signal and the C-terminal domain, called box 3, transduces the signal for cell differentiation.26-28 32D cells differentiate into neutrophils in G-CSF–containing medium. This indicates that the differentiation-inducing signal mediated by G-CSFR is transduced, whereas the proliferation signal transduction is inhibited. Our present study demonstrated that 32D cells stopped differentiating into neutrophils and continued to proliferate in G-CSF–containing medium in the presence of constitutive WT1 expression. This indicated that constitutive WT1 expression competed with the differentiation-inducing signal mediated by G-CSFR and blocked transduction of the differentiation-inducing signal and, instead, transduced only the proliferation signal mediated by G-CSFR. Thus, the results clarified that WT1 involves in the determination of whether differentiation-inducing signal mediated by G-CSFR can be transduced. Fresh leukemic cells from patients with AML usually show proliferation, rather than differentiation, in response to G-CSF.14-16This proliferative response of AML cells to G-CSF can now be explained by the high levels of WT1 expression in AML cells,9-11which allow for transduction of only the proliferation signal, but not of the differentiation-inducing signal mediated by G-CSFR.
The constitutive WT1 expression in 32D cells increased the amount of Stat3β protein, resulting in the reversion of ratio of Stat3α to Stat3β and activated both Stat3α and Stat3β. It is known that Stat3β results from a truncation of the C-terminal 55 amino acids of Stat3α and the addition of 7 new C-terminal amino acids encoded by 3′ sequence of Stat3α.25 Chakraborty et al29 investigated whether G-CSF signaling in immature normal and leukemic human myeloid cells diverges at the level of activation of STAT protein. In their experiments, the isoform composition of Stat3 proteins activated by G-CSF was examined on immature normal and leukemic human myeloid cells. In CD34+cells and in a leukemic myeloid cell line capable of differentiating in response to G-CSF, only Stat3β was activated, whereas in leukemic cells not capable of differentiating in response to G-CSF, both Stat3α and Stat3β were activated. In acute myeloid leukemia cell line AML-193, which proliferates, but does not differentiate in response to G-CSF, there was activation of both Stat3α and Stat3β. These results suggested that the balance of the two Stat3 isoforms in myeloid cells may influence the cellular pattern of gene activation and consequently the ability of these cells to differentiate in response to G-CSF, because the transcriptional activity of Stat3β is distinct from Stat3α.29 30 Therefore, wild-type WT1-infected 32D cells are leukemia cell-like in the sense that they are activated in both Stat3α and Stat3β and proliferate in response to G-CSF. Taken together, it may be assumed that blocking of differentiation and induction of proliferation in response to G-CSF in wild-type WT1-expressing 32D cells mimics in vivo leukemogenesis, in which a normal myeloid progenitor cell might be disturbed to differentiate and be turned to proliferation in response to physiological concentration of G-CSF when constitutive WT1 expression happened in the cell by as yet undetermined causes.
The hypothesis that the WT1 gene has basically two functional aspects, namely that of a tumor-suppressor gene and that of an oncogene, but in leukemic cells performs an oncogenic rather than a tumor-suppressor gene function, can be established on the basis of the following data: (1) high expression of wild-type WT1 in fresh leukemic cells,9-11,31-34 (2) an inverse correlation between WT1 expression levels and prognosis,9 (3) increased expression of WT1 at relapse as compared with that at diagnosis in acute leukemia,12 (4) growth inhibition of leukemic cells by WT1 antisense oligomers,13 and (5) our results presented here. Because it is known that WT1 protein interacts with P5335or par-436 protein, differences in the interaction of WT1 protein with other regulator proteins might determine whether the WT1 gene acts as a tumor-suppressor gene or performs an oncogenic function. Transfection of v-abl,37 v-src,38v-ras,39 v-myb,40 or c-myb41oncogenes blocked the G-CSF–induced granulocytic differentiation of 32D cells and caused them to proliferate in response to G-CSF. The v-abl– or v-src–infected 32D cells could proliferate independently from IL-3, whereas, like the WT1 gene, the v-ras, v-myb, or c-myb oncogene could not abrogate the dependency of the infected 32D cells on IL-3 for proliferation. The morphology of the rapidly proliferating cells of the wild-type WT1-infected 32D clones in G-CSF–containing medium was a little more differentiated than that of the parental 32D cells maintained in IL-3–containing medium (Fig 4). In addition, MPO expression levels increased after G-CSF stimulation (Fig 5). These results suggested that wild-type WT1-infected 32D cells can differentiate to promyelocyte or near-promyelocyte stage, which is a little more differentiated than that of the parental 32D cells, but are blocked from differentiating further and frozen at that stage, although WT1 expression higher than that in the wild-type WT1-infected 32D clones examined here might never allow differentiation. Transfection of v-ras or c-myb induced MPO in the 32D cells in response to G-CSF, whereas transfection of v-abl, v-src, or v-myb did not induce MPO. Thus, the WT1 gene might have an aspect similar to the oncogenic function of the v-ras or c-myb oncogene.
ACKNOWLEDGMENT
The authors thank Dr Hisamaru Hirai (Tokyo University, Tokyo, Japan) for providing us with 32D cl3. We also thank Tsuyomi Yajima for typing the manuscript and Machiko Mishima for her skillful technical asistance with the PCR.
Address reprint requests to Haruo Sugiyama, MD, Department of Clinical Laboratory Science, Osaka University Medical School, 1-7, Yamada-Oka, Suita City, Osaka 565, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal