Apoptosis mediated by the CD95 (Fas/Apo-1) molecule plays a crucial role in the regulation of the B-cell immune response. In this study, we examined the function of the CD95 antigen in B-cell–derived non-Hodgkin's lymphoma (NHL), a malignant disease of mature B cells. Membrane CD95 molecules were found to be constitutively expressed in a large number of NHL, including mantle cell (MCL, n = 10), lymphocytic (LCL, n = 10), follicular (FL, n = 11), and diffuse large cell lymphoma (DLCL, n = 9) with, however, different levels of intensity. Indeed, the levels of CD95 were low in MCL and LCL as compared with FL and DLCL. However, regardless of the intensity of expression, CD95 triggering with anti-CD95 monoclonal antibody (MoAb) did not induce apoptosis of lymphoma B cells, while these cells underwent apoptosis after irradiation or staurosporine treatment. Further experiments were then performed to address whether apoptosis could be restored by B-cell activation via CD40 cross-linking. We showed that CD40 engagement in the presence of interleukin (IL)-4 was more effective than CD40 engagement alone in upregulating the CD95 antigen and induced CD95-mediated cell death in nontumoral B cells. Concerning malignant B cells, CD40 ligation in the presence of IL-4 strongly increased CD95 expression, but did not markedly increase CD95-induced apoptosis. Furthermore, using cytotoxic T cells, we showed that CD95L was also ineffective in inducing apoptosis in lymphoma B cells, whereas these cells were killed by the perforin pathway. Our findings suggest that the CD95-mediated cell death pathway is altered in malignant cells from the NHL we tested. This could be a mechanism allowing lymphoma B cells to escape from immune regulation.
CD95 (FAS/APO-1) BELONGS to the tumor necrosis factor (TNF) receptor superfamily1,2 and mediates apoptosis after cross-linking with CD95 ligand (CD95L)3 or specific antibodies.4,5 The homeostasis of the immune response is highly regulated by such interactions. Indeed, CD95-mediated apoptosis plays a crucial role in the activation-induced cell death of T lymphocytes6,7 and in T-cell–mediated cytotoxicity.8,9 Concerning the human B-cell immune response, the role of CD95 is underlined by the development of an autoimmune lymphoproliferative syndrome in children who have defective CD95-mediated apoptosis.10,11 Moreover, recent work has demonstrated the key role of in vivo CD95 ligation in the expansion of antigen-reactive B cells and elimination of tolerant B cells.12 The dual reactivity following CD95 engagement is regulated by signals from both the B-cell antigen receptor (BCR) and CD40. Like CD95L, CD40 ligand (CD40L) is a member of the TNF superfamily and is expressed by activated CD4+ T cells.13,14 It promotes the growth of B cells by ligation with CD40. CD40 cross-linking also upregulates CD95 expression on B cells and induces susceptibility to CD95-mediated apoptosis of tolerant B cells12 or of B cells in the absence of BCR stimulation.15-17 In contrast, CD40-activated B cells become resistant to CD95-based apoptosis if the BCR is engaged18,19 and even proliferate in vivo.12
Malignant non-Hodgkin's lymphomas (NHL) are derived from a clonal expansion of B cells arrested at different stages of differentiation.20 Thus, lymphoma cells are the neoplastic counterparts of naive, activated, or memory normal B cells that each express a unique BCR. The nature of the antigen recognized by tumoral BCR is generally unknown or thought to be an autoantigen.21,22 Malignant B cells also share, with normal B cells, an antigen presenting function that allows them to generate antitumor cytotoxic lymphocytes.23 Moreover, CD40 is functional on tumor B cells because its ligation can induce resistance to spontaneous apoptosis.24 CD95 expression has been detected in NHL by immunohistochemical analysis,25-27however, its role in the induction of apoptosis or proliferation has not been fully investigated. In human lymphoma B-cell lines, some reports show that CD95-ligation induces apoptosis.4,28,29In chronic B-lymphocytic leukemia, malignant cells activated by Staphylococcus aureus Cowan I plus interleukin (IL)-2 undergo apoptosis after CD95 engagement except in one case, in which tumor cells proliferated.30
We investigated whether CD95 could be involved in NHL malignant B-cell development and in their susceptibility to T-cell–mediated cytotoxicity. We show here that all isolated tumor cells express CD95, but at various levels, and are resistant to apoptosis mediated by CD95 cross-linking either by specific monoclonal antibody (MoAb) or by CD95L expressed on cytotoxic T cells. CD40 activation upregulates CD95 expression on malignant B cells, but poorly restores responsiveness to CD95-mediated apoptosis.
MATERIALS AND METHODS
Cell lines, medium, and cytokines.
The mouse fibroblastic L cells stably transfected with the human CD40 Ligand (CD40Lig-L cells)15 was kindly provived by Dr J. Banchereau (Schering-Plough, Dardilly, France). CD40Lig-L cells, Jurkat T cells, and Epstein-Barr virus (EBV)-immortalized B-lymphoblastoid cell lines (BLCL) were grown at 37°C in 5% CO2 in air, in RPMI 1640 containing 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and nonessential amino acids (complete medium) supplemented with 10% fetal calf serum.
Purified human rIL-4 was purchased from R&D Systems (Oxon, UK) and human rIL-2 was provided by Roussel Uclaf (Romainville, France).
Preparation and CD40-activation of lymphoma and nontumoral B cells.
Lymphoma B cells were obtained from lymph nodes or spleens from 40 NHL patients, including 10 lymphocytic (LCL), 10 mantle cell (MCL), 11 follicular (FL), and nine diffuse large cell (DLCL) NHL according to the Revised European-American Lymphoma (REAL) classification.20 Nontumoral cells were obtained from lymph nodes from patients with benign hyperplasia or from spleens from cadavers. Biopsies were gently dissociated with a scalpel in RPMI 1640 and filtered through a 100-μm cell strainer to remove aggregates. The lymphoma samples were included in this study when the malignant clone represented more than 98%, evaluated by the positivity with anti-κ or -λ chain MoAb, of all B cells present. The malignant or nontumoral B lymphocytes were separated using a standard rosetting technique using 2-aminoethyl-isothiouronium bromide (AET)-sensitized sheep red blood cells. A flow cytometric analysis with CD3/CD19 antibodies was performed to evaluate the purity of the suspension, which was at least 97%. CD19+ B cells were then cryopreserved until phenotypic or functional assays were performed. The viability of cells used in all experiments was at least 90%.
CD40-activation of nontumoral and lymphoma B cells was performed using CD40Lig-L cells. B cells were seeded in 24-well flat-bottom plates at 5 × 105 per well in the presence of 5 × 104 irradiated (7,500 rad) CD40Lig-L cells in complete medium supplemented with 15% human A serum in the presence or absence of 10 ng/mL IL-4 for 3 days at 37°C in 5% CO2 in air.
CD95 expression analysis.
CD95 expression on cells was determined by indirect fluorescence. Briefly, 5 × 105 cells were incubated for 20 minutes at 4°C with anti-CD95 MoAb CH-11 (Immunotech, Marseille, France) and washed twice with Hanks' Balanced Salt Solution (HBSS) supplemented with 2% newborn calf serum. They were then incubated with 0.2 mL phycoerythrin-conjugated goat antimouse IgM (Immunotech) for 20 minutes at 4°C and again washed twice.
Immunostained cells were analyzed by flow cytometry on a FACScan (Becton Dickinson, Pont de Claix, France). Nonspecific staining was determined using an IgM control MoAb. Both percentages of positive cells and mean fluorescence intensities (MFI) were recorded.
Measurement of apoptosis by 51Cr-release assays.
Cells (1 × 106 ) were labeled with 100 μCi51Cr-sodium chromate for 1 hour at 37°C in 5% CO2 in air, then washed three times and resuspended at final concentration 1 × 105 cells/mL in complete medium supplemented with 15% serum A. Each V-shaped well of 96-well microtiter plates received 100 μL of cells (104) and 100 μL of anti-CD95 MoAb CH-11 or isotype control IgM MoAb at final concentration indicated within the text. After 4 hours or 18 hours at 37°C, microplates were centrifuged, and 100 μL aliquots of supernatants were assayed for radioactivity. The percentage of apoptotic cells, evaluated by the percentage of51Cr-release, was calculated according to the following formula: % 51Cr-release = 100 × (ER − SR)/(MR − SR), where ER, SR, MR represent experimental, spontaneous, and maximum 51Cr-release, respectively. All expressed values are derived from averaged quadruplicate determinations.
Measurement of apoptosis by annexin V/propidium iodide (PI) double staining.
Annexin V binds to phosphatidylserine and allows the detection of the loss of cell phospholipid asymmetry, an event that appears during the early phases of apoptosis. The Apoptest containing annexin V-fluorescein isothiocyanate (FITC) was purchased from Nexins Research B.V. (Maastricht, The Netherlands). As described,31 cells (5 × 105) were washed with ice-cold phosphate-buffered saline (PBS) and were incubated for 10 minutes in the dark in 500 μL of binding buffer containing annexin V-FITC solution and 10 μg/mL propidium iodide (PI). Without washing, cells were then analyzed on a FACScan. Early apoptotic cells were only stained by annexin V-FITC, whereas late apoptotic or necrotic cells were double-stained by annexin V-FITC and PI. The specific apoptosis, consisting of the percentage of viable cells undergoing apoptosis by the effect of the inducer, was calculated according to the following formula: 100 × ([D assay − D control]/100 − D control) where D represents the percentage of cells dying during the culture through apoptosis or necrosis. For induction of apoptosis, cells were incubated in complete medium supplemented with 15% human serum in the presence of 1 μg of anti-CD95 MoAb CH-11 or isotype control IgM MoAb. Irradiation exposure (3,000 rad) and staurosporin treatment was also performed. Apoptosis was evaluated after 4 hours, 18 hours, or both at 37°C.
Cytotoxicity assay.
The cytotoxicity of allogeneic T cells against tumor targets was measured in standard 4-hour 51Cr-release assays as previously described.23 Briefly, 104 51Cr-labeled target cells were mixed with the effector cells at different E/T ratios (25/1 to 0.01/1). After a 4-hour incubation at 37°C in 5% CO2 in air, the radioactivity in the supernatants was counted. The percentage of specific lysis was calculated according to the following formula: % lysis = 100 × (ER − SR)/(MR − SR), where ER, SR, MR represent experimental, spontaneous, and maximum 51Cr-release, respectively. All expressed values are derived from averaged quadruplicate determinations.
Effector allogeneic T cells were obtained from unidirectional mixed lymphocyte reaction (MLR) performed by mixing HLA mismatched purified CD3+ T lymphocytes and irradiated (3,000 rad) lymphoma B cells, restimulated once with lymphoma cells and IL-2. The effector cells were CD3+/T-cell receptor (TCR)αβ+ (99%), CD4+ and CD8+. Before cytotoxicity assays, effector cells were preincubated for 2 hours with a mixture of phorbol myristy acetate (PMA; Sigma, St Louis, MO; final concentration, 5 ng/mL) and the Ca2+ ionophore ionomycin (Sigma; final concentration, 1.5 μg/mL). The cytotoxicity tests were performed with Jurkat cells or lymphoma B cells as target cells in complete medium supplemented with 15% human A serum in the presence or absence of 3 mmol/L EGTA/4 mmol/L Mg2+.
RESULTS
CD95 expression on lymphoma B cells is heterogenous.
Membrane CD95 expression was analyzed by flow cytometry on purified malignant B cells from 40 NHL patients, including 10 LCL, 10 MCL, 11 FL, and nine DLCL NHL. Nontumoral B cells (n = 9), EBV-immortalized (n = 5), and Jurkat T cells were also included in this study as controls. We used the anti-CD95 MoAb CH-11 for these assays because it allows a better detection of CD95 molecule than other clones (data not shown). However, because MoAb CH-11 can induce cell death when used in a soluble form,5 all experiments were performed on ice. The absence of induction of apoptosis in this protocol was verified on Jurkat T cells.
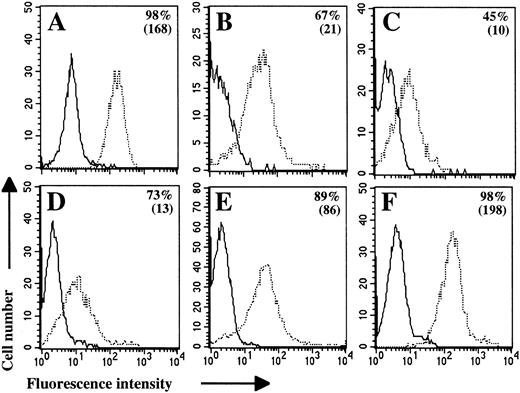
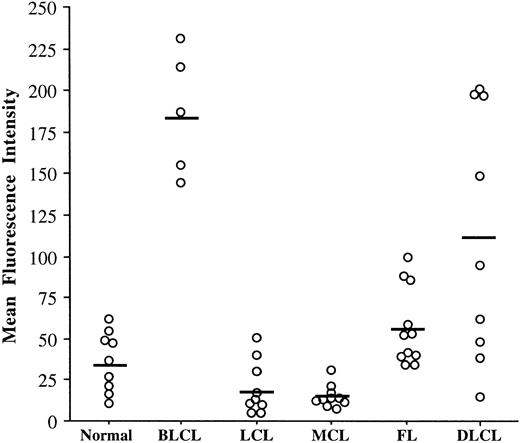
All the studied cells expressed CD95 and this expression was unimodal (Fig 1). However, because the intensity of this expression was very heterogeneous, the MFI was a more discriminating parameter between NHL than the percentages of positive cells (Fig 2). Our results indicate clearly that the levels of the CD95 molecule were relatively homogeneous on nontumoral B cells and within each group of the REAL classification except DLCL. Lymphoma cells from LCL and MCL expressed low levels of the CD95 molecule (mean MFI, 18 and 15, respectively), as compared with FL (mean MFI, 57). The level of CD95 on nontumoral B cells was relatively low (mean MFI, 36) whereas in BLCL, it was higher (mean MFI, 186).
CD95 molecule is expressed on freshly isolated lymphoma B cells. Flow cytometric analyses were performed on Jurkat cells (A), nontumoral B cells (B; Donor NT-1), and lymphoma B cells (C through F). Lymphoma B cells were obtained from LCL-1 (C), MCL-5 (D), FL-6 (E), and DLCL-4 (F) patients. Cells were stained with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Percentages of positive cells and MFI values (in brackets) are indicated for each cell population.
CD95 molecule is expressed on freshly isolated lymphoma B cells. Flow cytometric analyses were performed on Jurkat cells (A), nontumoral B cells (B; Donor NT-1), and lymphoma B cells (C through F). Lymphoma B cells were obtained from LCL-1 (C), MCL-5 (D), FL-6 (E), and DLCL-4 (F) patients. Cells were stained with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Percentages of positive cells and MFI values (in brackets) are indicated for each cell population.
The levels of CD95 expression vary according to NHL types. CD95 expression has been analyzed by flow cytometry on nontumoral B cells (n = 9), BLCL (n = 5), LCL (n = 10), MCL (n = 10), FL (n = 11), and DLCL (n = 9). Cells were stained with anti-CD95 MoAb CH-11 or with IgM control MoAb followed by PE-conjugated antimouse IgM. The MFI value was calculated by subtracting the IgM control MFI value from the anti-CD95 MoAb MFI value. Bars indicate the mean of MFI values within each entity.
The levels of CD95 expression vary according to NHL types. CD95 expression has been analyzed by flow cytometry on nontumoral B cells (n = 9), BLCL (n = 5), LCL (n = 10), MCL (n = 10), FL (n = 11), and DLCL (n = 9). Cells were stained with anti-CD95 MoAb CH-11 or with IgM control MoAb followed by PE-conjugated antimouse IgM. The MFI value was calculated by subtracting the IgM control MFI value from the anti-CD95 MoAb MFI value. Bars indicate the mean of MFI values within each entity.
Resistance of lymphoma B cells to CD95-based apoptosis mediated by anti-CD95 MoAb CH-11.
CD95 engagement induces apoptosis of activated, but not resting, normal B cells.15-17 To determine the function of CD95 molecule on B cells from NHL, we first studied the effect of anti-CD95 MoAb CH-11 on cell viability using a 4-hour 51Cr-release assay. This method allows the detection of late stages of apoptosis characterized by loss of membrane integrity, as already shown in other studies on CD95-based lymphocyte-mediated cytotoxicity.7 9 As shown in Fig 3, a 4-hour CD95 ligation by MoAb induces apoptosis of Jurkat T cells in a dose-dependent manner. Likewise, EBV-immortalized B lymphoblastoid cell lines were also susceptible to the CD95-mediated death signal (data not shown). However, CD95-ligation failed to induce apoptosis in three lymphoma cell samples (Fig 3). All of the malignant B-cell suspensions, which have been tested (two LCL, four MCL, two FL, and two DLCL), and four nontumoral B-cell populations did not undergo apoptosis when the membrane CD95 molecules were cross-linked by CH-11 MoAb, even at increased incubation times (18 hours).
Lymphoma B cells are resistant to CD95-mediated apoptosis in an assay detecting late stages of apoptosis. Jurkat cells (□) and three lymphoma cell populations isolated from LCL-4 (▴), MCL-6 (⧫), and DLCL-1 (•) patients were labeled with [51Cr]-sodium chromate and then incubated with increased concentrations of anti-CD95 MoAb CH-11 for 4 hours. The apoptosis intensity was evaluated by the percentage of specific 51Cr-release.
Lymphoma B cells are resistant to CD95-mediated apoptosis in an assay detecting late stages of apoptosis. Jurkat cells (□) and three lymphoma cell populations isolated from LCL-4 (▴), MCL-6 (⧫), and DLCL-1 (•) patients were labeled with [51Cr]-sodium chromate and then incubated with increased concentrations of anti-CD95 MoAb CH-11 for 4 hours. The apoptosis intensity was evaluated by the percentage of specific 51Cr-release.
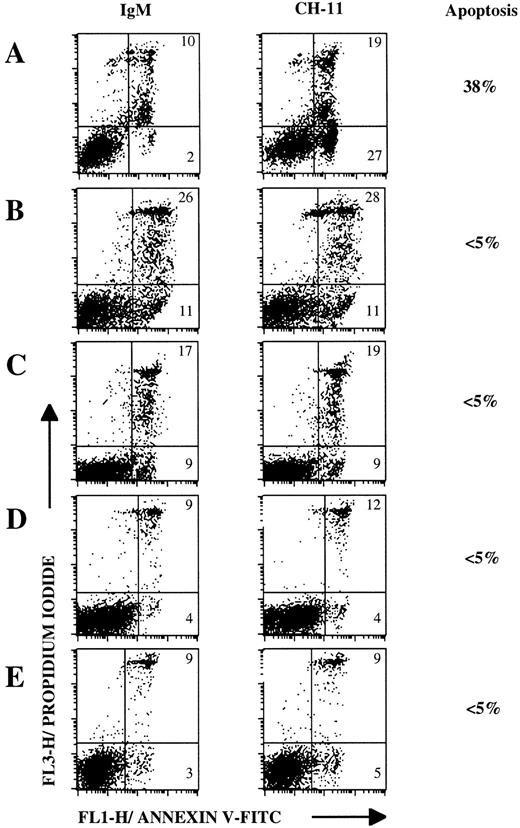
We next used annexin V staining, as it is more suitable than the 4-hour51Cr-release assay for detection of early stages of apoptosis.31 Annexin V binding is combined with PI staining to distinguish between early and late apoptotic cells. This test has been used in several reports to measure apoptosis, in particular in populations of germinal center B-lymphocytes.32 As a control, Jurkat T cells that expressed high levels of CD95 molecule (Fig 1A) were treated with anti-CD95 MoAb CH-11 or IgM control MoAb for 4 hours. As shown in Fig 4A, 38% of Jurkat cells underwent apoptosis and were mostly in early apoptosis, as indicated by annexin V staining. Representative dot-plots of annexin V versus PI fluorescence obtained with lymphoma B cells from MCL (Fig4C), FL (Fig 4D), DLCL (Fig 4E), and nontumoral (Fig 4B) B cells show that no CD95-based apoptosis was detected after an 18-hour incubation period. CD95 ligation never induced apoptosis either in malignant cell populations tested (four LCL, six MCL, five FL, and four DLCL) or in three nontumoral B-cell populations (Table1).
Lymphoma B cells are resistant to CD95-mediated apoptosis in an assay detecting early stages of apoptosis. Flow cytometric analysis of Jurkat cells (A), nontumoral B cells (B; Donor NT-1), and lymphoma B cells (C through E). Lymphoma B cells were obtained from MCL-4 (C), FL-3 (D), DLCL-3 (E). Cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 4 hours (A) or 18 hours (B through E) and then double-stained wirth annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). The specific apoptosis, that is the percentage of viable cells undergoing apoptosis with the inducing agent, was calculated according to the following formula: ((DCH-11 − DIgM)/100 − DIgM) ×100, where D represents the percentage of cells dying during the culture by apoptosis or necrosis.
Lymphoma B cells are resistant to CD95-mediated apoptosis in an assay detecting early stages of apoptosis. Flow cytometric analysis of Jurkat cells (A), nontumoral B cells (B; Donor NT-1), and lymphoma B cells (C through E). Lymphoma B cells were obtained from MCL-4 (C), FL-3 (D), DLCL-3 (E). Cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 4 hours (A) or 18 hours (B through E) and then double-stained wirth annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). The specific apoptosis, that is the percentage of viable cells undergoing apoptosis with the inducing agent, was calculated according to the following formula: ((DCH-11 − DIgM)/100 − DIgM) ×100, where D represents the percentage of cells dying during the culture by apoptosis or necrosis.
Percentage of Apoptosis Induced by CD95 Cross-Linking, Irradiation, and Straurosporine Treatment of Lymphoma and Nontumoral B Cells
| Case . | CD95 Expression . | CD95 MoAb . | Irradiation . | Staurosporine . | |||
|---|---|---|---|---|---|---|---|
| 4 h . | 18 h . | 4 h . | 18 h . | 4 h . | 18 h . | ||
| Jurkat | 168 | 38 | 86 | 35 | 83 | 65 | 96 |
| NT-1 | 21 | ≤5 | ≤5 | 18 | 59 | 71 | 88 |
| NT-2 | 47 | ≤5 | ≤5 | 11 | 58 | 66 | 84 |
| NT-3 | 62 | ≤5 | ≤5 | 27 | 86 | 81 | 97 |
| LCL-1 | 9 | ≤5 | ≤5 | ≤5 | 52 | 76 | 95 |
| LCL-2 | 5 | ≤5 | ≤5 | ≤5 | 38 | 29 | 92 |
| LCL-3 | 30 | ≤5 | ≤5 | ND | ND | ND | ND |
| LCL-4 | 40 | ≤5 | ≤5 | ≤5 | 42 | 61 | 91 |
| MCL-1 | 14 | ≤5 | ≤5 | ND | ND | ND | ND |
| MCL-2 | 14 | ≤5 | ≤5 | ND | ND | ND | ND |
| MCL-3 | 9 | ≤5 | ≤5 | ≤5 | 36 | 61 | NC-150 |
| MCL-4 | 31 | ≤5 | ≤5 | 8 | 55 | 60 | NC |
| MCL-5 | 13 | ≤5 | ≤5 | 9 | 69 | 51 | NC |
| MCL-6 | 17 | ≤5 | ≤5 | ≤5 | 33 | 88 | 98 |
| FL-1 | 34 | ≤5 | 8 | ≤5 | 58 | 35 | 97 |
| FL-2 | 34 | ≤5 | ≤5 | ≤5 | 59 | 30 | 82 |
| FL-3 | 59 | ≤5 | ≤5 | ≤5 | 20 | 24 | 87 |
| FL-4 | 42 | ≤5 | ≤5 | 7 | 44 | 10 | 69 |
| FL-5 | 88 | ≤5 | 7 | ≤5 | 20 | 40 | 68 |
| FL-6 | 86 | ND | ND | ≤5 | 35 | 16 | 84 |
| DLCL-1 | 48 | ≤5 | ≤5 | ND | ND | ND | ND |
| DLCL-2 | 15 | ≤5 | ≤5 | ≤5 | 22 | 39 | 84 |
| DLCL-3 | 38 | ≤5 | ≤5 | ≤5 | 13 | ≤5 | 24 |
| DLCL-4 | 198 | ≤5 | ≤5 | ≤5 | 28 | 8 | 81 |
| Case . | CD95 Expression . | CD95 MoAb . | Irradiation . | Staurosporine . | |||
|---|---|---|---|---|---|---|---|
| 4 h . | 18 h . | 4 h . | 18 h . | 4 h . | 18 h . | ||
| Jurkat | 168 | 38 | 86 | 35 | 83 | 65 | 96 |
| NT-1 | 21 | ≤5 | ≤5 | 18 | 59 | 71 | 88 |
| NT-2 | 47 | ≤5 | ≤5 | 11 | 58 | 66 | 84 |
| NT-3 | 62 | ≤5 | ≤5 | 27 | 86 | 81 | 97 |
| LCL-1 | 9 | ≤5 | ≤5 | ≤5 | 52 | 76 | 95 |
| LCL-2 | 5 | ≤5 | ≤5 | ≤5 | 38 | 29 | 92 |
| LCL-3 | 30 | ≤5 | ≤5 | ND | ND | ND | ND |
| LCL-4 | 40 | ≤5 | ≤5 | ≤5 | 42 | 61 | 91 |
| MCL-1 | 14 | ≤5 | ≤5 | ND | ND | ND | ND |
| MCL-2 | 14 | ≤5 | ≤5 | ND | ND | ND | ND |
| MCL-3 | 9 | ≤5 | ≤5 | ≤5 | 36 | 61 | NC-150 |
| MCL-4 | 31 | ≤5 | ≤5 | 8 | 55 | 60 | NC |
| MCL-5 | 13 | ≤5 | ≤5 | 9 | 69 | 51 | NC |
| MCL-6 | 17 | ≤5 | ≤5 | ≤5 | 33 | 88 | 98 |
| FL-1 | 34 | ≤5 | 8 | ≤5 | 58 | 35 | 97 |
| FL-2 | 34 | ≤5 | ≤5 | ≤5 | 59 | 30 | 82 |
| FL-3 | 59 | ≤5 | ≤5 | ≤5 | 20 | 24 | 87 |
| FL-4 | 42 | ≤5 | ≤5 | 7 | 44 | 10 | 69 |
| FL-5 | 88 | ≤5 | 7 | ≤5 | 20 | 40 | 68 |
| FL-6 | 86 | ND | ND | ≤5 | 35 | 16 | 84 |
| DLCL-1 | 48 | ≤5 | ≤5 | ND | ND | ND | ND |
| DLCL-2 | 15 | ≤5 | ≤5 | ≤5 | 22 | 39 | 84 |
| DLCL-3 | 38 | ≤5 | ≤5 | ≤5 | 13 | ≤5 | 24 |
| DLCL-4 | 198 | ≤5 | ≤5 | ≤5 | 28 | 8 | 81 |
CD95 expression is expressed in MFI values.
Abbreviations: ND, not done; NC, not calculated.
Percentage of apoptotic cells was not calculated when the total number of dead cells in control was greater than 60%.
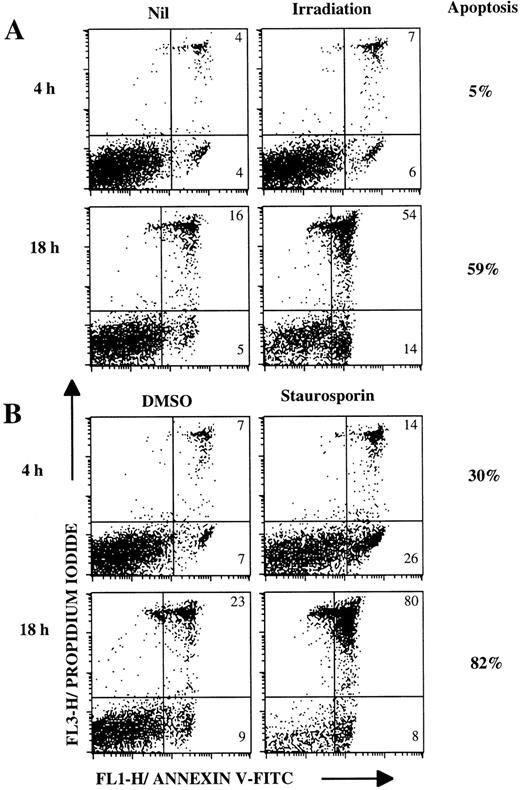
To verify that lymphoma cells did not present major abnormalities in intracellular mechanisms of apoptosis, we treated these malignant cells with other apopotic inducers (Fig 5, Table1). Apoptosis of lymphoma B cells can be induced in 4 hours and is greater after 18 hours of staurosporine treatment (Fig 5B). Irradiation exposure also induced apoptosis, but this was less marked (Fig 5A). It is noticable that lymphoma cells become necrotic following apoptosis during 18 hours of culture (Fig 5A and B). Most of the lymphoma cells tested (16 patients) underwent apoptosis after irradiation exposure or with staurosporine treatment (Table 1).
Lymphoma B cells undergo apoptosis after irradiation or staurosporine treatment. Lymphoma cells (FL-2 patient) were cultured for 4 hours or 18 hours (A) without treatment or after irradiation (3,000 rads), (B) with 5 μmol/L staurosporine or dimethyl sulphoxide (DMSO) as a control. Cells were stained with annexin V-FITC/PI and then analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
Lymphoma B cells undergo apoptosis after irradiation or staurosporine treatment. Lymphoma cells (FL-2 patient) were cultured for 4 hours or 18 hours (A) without treatment or after irradiation (3,000 rads), (B) with 5 μmol/L staurosporine or dimethyl sulphoxide (DMSO) as a control. Cells were stained with annexin V-FITC/PI and then analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
CD40 engagement greatly increases CD95 expression on lymphoma cells, but poorly induces sensitivity to CD95-based apoptosis.
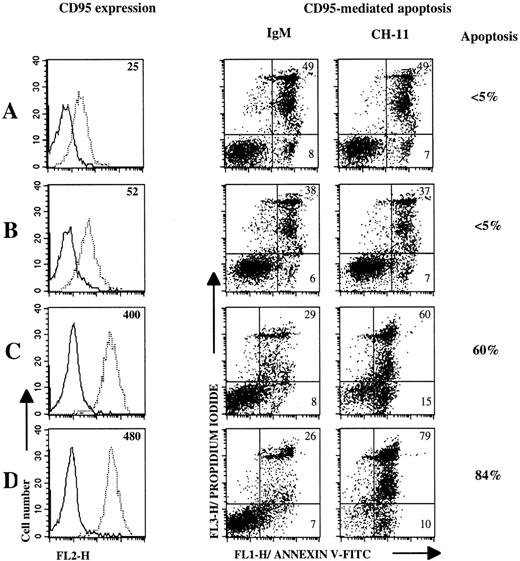
Recent reports have demonstrated that CD40 triggering on resting normal B cells upregulates CD95 expression and induces their susceptibility to CD95-mediated apoptosis.15-17 We first determined optimal activation conditions to increase CD95 expression and to induce CD95-based apoptosis responsiveness by performing 3-day cultures with nontumoral B cells (Donor NT-1) in the presence or absence of irradiated CD40Lig-L cells, IL-4, or both. To induce CD95-mediated death, anti-CD95 MoAb was added on day 3 and apoptosis was evaluated 18 hours later. Results in Fig 6 represent one of two separate experiments (Table 2). We showed that CD40 activation upregulated CD95 expression on B cells (Fig6C) and this phenomenon was stronger in the presence of IL-4 (Fig 6D), whereas IL-4 alone had little effect (Fig 6B) compared with cells cultured with medium (Fig 6A). Furthermore, CD40 cross-linking in the presence of IL-4 restored CD95-based apoptosis more efficiently than culture with CD40Lig-L cells alone (Fig 6C and D). Cells cultured with medium or IL-4 did not undergo apoptosis (Fig 6A and B).
CD40 ligation and CD40 ligation plus IL-4 upregulate CD95 expression on nontumoral B cells and induce CD95-based apoptosis. CD95 expression and apoptosis induction were measured on 3-day cultured B cells (Donor NT-1) in mediun alone (A), or with IL-4 (B), CD40Lig-L cells (C) and IL-4 plus CD40Lig-L cells. CD95 expression was analyzed by flow cytometry after staining with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Numbers within histogram plots represent MFI values. For CD95-mediated apoptosis experiments, cultured cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 18 hours and then double-stained with annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
CD40 ligation and CD40 ligation plus IL-4 upregulate CD95 expression on nontumoral B cells and induce CD95-based apoptosis. CD95 expression and apoptosis induction were measured on 3-day cultured B cells (Donor NT-1) in mediun alone (A), or with IL-4 (B), CD40Lig-L cells (C) and IL-4 plus CD40Lig-L cells. CD95 expression was analyzed by flow cytometry after staining with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Numbers within histogram plots represent MFI values. For CD95-mediated apoptosis experiments, cultured cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 18 hours and then double-stained with annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
Modulation of CD95 Expression and Sensitivity to CD95-Mediated Apoptosis of Lymphoma and Nontumoral B Cells by CD40L and IL-4
| Case . | CD95 Expression . | CD95-Mediated Apoptosis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium . | IL-4 . | CD40Lig-L Cells . | CD40Lig-L Cells + IL-4 . | Medium . | IL-4 . | CD40Lig-L Cells . | CD40Lig-L Cells + IL-4 . | |
| NT-1 | 25 | 52 | 400 | 480 | ≤5 | ≤5 | 60 | 84 |
| NT-2 | 20 | 30 | 407 | 434 | NC* | ≤5 | 55 | 65 |
| LCL-3 | 17 | ND | ND | 240 | NC | ND | ND | 14 |
| MCL-3 | 3 | ND | ND | 160 | ≤5 | ≤5 | ≤5 | ≤5 |
| MCL-4 | 3 | ND | ND | 134 | NC | ≤5 | ≤5 | ≤5 |
| MCL-6 | 12 | ND | ND | 213 | NC | ND | ND | 37 |
| FL-1 | 88 | ND | ND | 237 | ≤5 | ≤5 | 15 | NC |
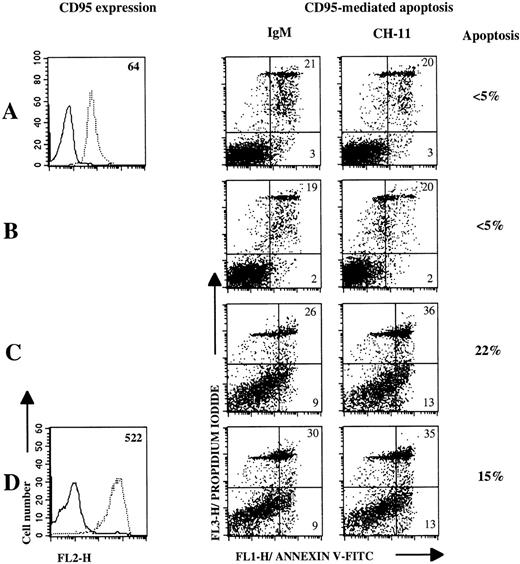
| FL-3 | 64 | ND | ND | 522 | ≤5 | ≤5 | 22 | 15 |
| DLCL-2 | ND | ND | ND | ND | NC | 9 | ≤5 | ≤5 |
| DLCL-3 | 39 | ND | ND | 347 | ≤5 | ≤5 | 7 | 6 |
| Case . | CD95 Expression . | CD95-Mediated Apoptosis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Medium . | IL-4 . | CD40Lig-L Cells . | CD40Lig-L Cells + IL-4 . | Medium . | IL-4 . | CD40Lig-L Cells . | CD40Lig-L Cells + IL-4 . | |
| NT-1 | 25 | 52 | 400 | 480 | ≤5 | ≤5 | 60 | 84 |
| NT-2 | 20 | 30 | 407 | 434 | NC* | ≤5 | 55 | 65 |
| LCL-3 | 17 | ND | ND | 240 | NC | ND | ND | 14 |
| MCL-3 | 3 | ND | ND | 160 | ≤5 | ≤5 | ≤5 | ≤5 |
| MCL-4 | 3 | ND | ND | 134 | NC | ≤5 | ≤5 | ≤5 |
| MCL-6 | 12 | ND | ND | 213 | NC | ND | ND | 37 |
| FL-1 | 88 | ND | ND | 237 | ≤5 | ≤5 | 15 | NC |
| FL-3 | 64 | ND | ND | 522 | ≤5 | ≤5 | 22 | 15 |
| DLCL-2 | ND | ND | ND | ND | NC | 9 | ≤5 | ≤5 |
| DLCL-3 | 39 | ND | ND | 347 | ≤5 | ≤5 | 7 | 6 |
CD95 expression was measured after a 3-day culture in the indicated conditions and is expressed in MFI values. CD95-mediated apoptosis was measured after an 18-hour incubation with CD95 MoAb or IgM MoAb as control after a 3-day culture in the indicated conditions. Apoptosis is expressed as a percentage of specific apoptosis as described in Materials and Methods.
Abbreviations: ND, not done; NC, not calculated.
Percentage of apoptotic cells was not calculated when the total number of dead cells in control was greater than 60%.
We then examined the effect of CD40Lig-L cells/IL-4 culture on the modulation of CD95 expression and function on lymphoma B cells. As indicated in Table 2, CD40 ligation and IL-4 activation significantly upregulated CD95 expression on lymphoma B cells. However, little apoptosis occured after incubation with anti-CD95 MoAb for the last 18 hours of culture (Fig 7 and Table 2), except for one case (MCL-6) where the specific apoptosis reached 37%. The intensity of CD95-mediated apoptosis was not significantly different when lymphoma cells were cultured with CD40Lig-L cells or IL-4 alone, as compared with CD40Lig-L cells plus IL-4.
CD40 ligation and CD40 ligation plus IL-4 poorly induce sensitivity to CD95-based apoptosis. CD95 expression and apoptosis induction were measured on 3-day cultured B cells (Patient FL-3) in mediun alone (A), or with IL-4 (B), CD40Lig-L cells (C), and IL-4 plus CD40Lig-L cells. CD95 expression was analyzed by flow cytometry after staining with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Numbers within histogram plots represent MFI values. For CD95-mediated apoptosis experiments, cultured cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 18 hours and then double-stained with annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
CD40 ligation and CD40 ligation plus IL-4 poorly induce sensitivity to CD95-based apoptosis. CD95 expression and apoptosis induction were measured on 3-day cultured B cells (Patient FL-3) in mediun alone (A), or with IL-4 (B), CD40Lig-L cells (C), and IL-4 plus CD40Lig-L cells. CD95 expression was analyzed by flow cytometry after staining with anti-CD95 MoAb CH-11 (dashed line) or with IgM control MoAb (solid line) followed by PE-conjugated antimouse IgM. Numbers within histogram plots represent MFI values. For CD95-mediated apoptosis experiments, cultured cells were incubated with 1 μg/mL anti-CD95 MoAb CH-11 or with IgM control MoAb for 18 hours and then double-stained with annexin V-FITC/PI and analyzed by flow cytometry. Numbers within dot plots represent the percentages of cells in early apoptosis (lower right, annexin V+/PI-) and in late apoptosis or in necrosis (upper right, annexin V±/PI+). Percentages next to the dot plots indicate the specific apoptosis.
Resistance of lymphoma cells to CD95-induced lysis, but not perforin-mediated cytotoxic T-cell killing.
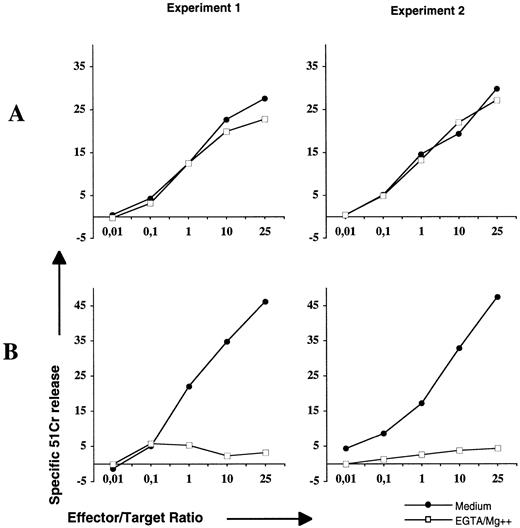
CD95/CD95L interactions and perforin/granzyme B pathways are the two main lytic mechanisms used by cytotoxic T cells.8,9 Thus, we next addressed the question of the ability of the physiologic ligand of CD95 (CD95L) expressed by cytolytic T cells to induce apoptosis of lymphoma B cells. For this purpose, we performed cytotoxic assays with activated T cells using a standard 4-hour 51Cr-release test in the presence or absence of EGTA that blocks the perforin pathway (Fig 8). Allogeneic cytolytic T cells were obtained after mixed lymphocyte reaction culture using lymphoma B cells as stimulators and then activated by PMA/ionomycin to upregulate CD95L expression as described elsewhere.33 Activated cells were tested with Jurkat cells to control functional expression of CD95L. As shown in Fig 8A, the lysis of Jurkat cells is only CD95-based, as it was not significantly changed in the presence of EGTA. When lymphoma cells were used as targets (Fig 8B), lysis that exceeded 45% in the absence of EGTA was completly abrogated in its presence. These results indicate that CD95 ligation by functional CD95L did not induce apoptosis of lymphoma B cells, and that the perforin pathway was the main mechanism by which malignant B cells were killed by cytotoxic T cells.
Resistance of lymphoma cells to CD95, but not perforin, lytic mechanisms of cytolytic T cells. Allogeneic antilymphoma T cells generated by MLR were preincubated for 2 hours with 5 ng/mL PMA and 1.5 μg/mL ionomycin. The subsequent 4-hour cytotoxicity assay was performed with Jurkat (A) or lymphoma B cells (B) in the presence of medium (•) or EGTA-Mg2+ (□). The cytotoxicity intensity was evaluated by the percentage of 51Cr-release. Experiments 1 and 2 were performed with lymphoma cells isolated from LCL-3 and MCL-1 patients, respectively.
Resistance of lymphoma cells to CD95, but not perforin, lytic mechanisms of cytolytic T cells. Allogeneic antilymphoma T cells generated by MLR were preincubated for 2 hours with 5 ng/mL PMA and 1.5 μg/mL ionomycin. The subsequent 4-hour cytotoxicity assay was performed with Jurkat (A) or lymphoma B cells (B) in the presence of medium (•) or EGTA-Mg2+ (□). The cytotoxicity intensity was evaluated by the percentage of 51Cr-release. Experiments 1 and 2 were performed with lymphoma cells isolated from LCL-3 and MCL-1 patients, respectively.
DISCUSSION
B-cell NHL is a particular neoplastic disease because the malignant clone develops essentially from the immune lymphoid system. In vivo, the development of normal B-cell responses appears to be under the control of both specific antigen recognition via the B-cell receptor (BCR) and CD4+ T lymphocytes via CD40L and CD95L molecules.12 Without adequate BCR engagement, CD40-activated B cells undergo apoptosis by CD95 ligation.12 15-19 This mechanism seems to allow the expansion of antigen-reactive B cells and elimination of tolerant B cells.
In NHL, an alteration of the control of B-cell growth or survival leads to cellular accumulation and tumor development. Thus, the overexpression or altered expression of proteins involved in apoptosis or the cell cycle are often found in NHL: Bcl-2 in follicular lymphoma,34 Bcl-6 in diffuse large cell lymphoma,35 cyclin D1 in mantle cell lymphoma,36 c-myc in Burkitt's lymphoma,37 and p53 in several types of lymphoma.38,39 However, a single abnormality is not sufficient to explain the development of lymphoma.40 Moreover, the observation that isolated lymphoma cells died rapidly in culture suggested that interaction with other cells in the tumoral environment is needed for their survival. Interestingly, one report has described that specific CD4+T-cell clones induced the proliferation of follicular lymphoma cells in vitro.41 Furthermore, lymphoma B cells can proliferate after CD40 cross-linking.24 The fact that both CD40L and CD95L are expressed on activated CD4+ T cells led us to postulate that lymphoma cells are responsive to CD40 activation and resistant to CD95-mediated apoptosis, independently of BCR triggering. Such a resistance to CD95-based apoptosis could be one mechanism involved in the development of the malignant process in NHL, allowing the escape of malignant B cells from immune regulation. Our observations provide evidence that such a mechanism probably exists. The present study describes the expression and the functionality of the CD95 molecule in a large number of tumor cells and addresses the question of the involvement of CD95 in NHL oncogenesis and tumor progression.
We first showed that all tumor cells were positive for CD95 expression with, however, a magnitude of positivity that varies according to NHL groups. In accordance with immunohistologic analysis,25-27the highest levels of CD95 expression were encountered in FL and DLCL, and the lowest in LCL and MCL. These observations were in agreement with studies reporting CD95 expression on normal B cells at different maturation stages.19 25 Indeed, germinal center B cells from which FL and most DLCL are probably derived, express high levels of the CD95 molecule. In contrast, naive B cells, which are thought to be the normal counterparts of MCL and most LCL, express low levels.
The susceptibility of normal B cells to CD95-mediated apoptosis depends on their activation state. Interestingly, we showed that CD95-ligation did not induce apoptosis of tumor cells isolated from from MCL and LCL NHL that are considered to be resting cells and expressed few CD95 molecules.
Conversely, FL and DLCL cells, which are considered to be activated, particularly DLCL, which are cycling, were also not responsive to CD95-based apoptosis. Such an absence of concordance between CD95 expression and CD95-mediated cell death has already been described in cases of normal B cells. Indeed, normal B lymphocytes were rendered sensitive to CD95-mediated death after mitogenic activation by CD40-cross-linking12,15,17 or pokeweed mitogen (PWM).30 Our results showed that CD40 engagement plus IL-4 greatly upregulated the expression of membrane CD95 molecules on lymphoma, as well as on nontumoral B lymphocytes. In these conditions, CD95 ligation induced a strong apoptosis in nontumoral activated B cells, whereas little apoptosis was detected in activated lymphoma cells. Concerning apoptosis induction in nontumoral human B cells, our results conflict with those published in the mouse system in which IL-4 induces CD95 resistance in CD40-stimulated primary B cells.42 Our results show that CD95 cross-linking induced more apoptosis in nontumoral B cells activated by CD40 plus IL-4 than by CD40 alone. The resistance of CD40-activated lymphoma cells to apoptosis was not due to IL-4, as similar results were obtained without IL-4. Discrepancies in experimental procedures could explain these differences.
The data presented in this study strongly suggest that the CD95 death pathway is partially blocked in human lymphoma cells and that activation by CD40 in the presence of IL-4 is not sufficient to restore it. This blockade was not related to late abnormalities in intracellular mechanisms of apoptosis because tumor B cells undergo apoptosis in response to other inducers such as irradiation or staurosporine treatment.
Several mechanisms could interact with the CD95 signaling pathway to inhibit apoptosis. Overexpression of Bcl-2 in follicular lymphoma cells and also in other tumors has been proposed as a mechanism blocking the apoptotic process induced by the CD95 molecule.26,43However, some studies have shown that CD95-mediated apoptosis is only partialy inhibited by Bcl-2.44,45 Other molecules could also interfere with CD95-mediated apoptosis, such as Bcl-xL, another Bcl-2–related protein that also prevents apoptosis and seems to be commonly expressed in lymphoma cells.46 Interestingly, Bcl-x expression is strongly enhanced by CD40 cross-linking in murine B cells and human tonsillar B-cell centroblasts.47 48 It would be of interest to measure Bcl-2 and Bcl-x expression in lymphoma cells after CD40 engagement in the presence of IL-4.
CD95-induced apoptosis could also be altered by mutations in genes coding for proteins of the CD95 death-inducing signaling complex as described in lymphoma T cells.49 At present, only one study has reported CD95 gene alterations in B-cell NHL, but at a relatively low frequency.50 Interestingly, novel proteins, one called sentrin and the others FLIPs (FLICE-inhibitory proteins), have been recently described that protect against CD95-induced cell death.51 52 An overexpression of such proteins in lymphoma cells could participate in their resistance to CD95-mediated apoptosis.
One alternative mechanism of resistance to CD95 apoptosis in lymphoma cells could be related to BCR signaling. Antigen receptor engagement has been shown to protect normal B cells from CD95-mediated apoptosis.12,18,19 In NHL, the existence of an antigen recognized by tumoral BCR has rarely been described. However, the demonstration of somatic hypermutations with intraclonal diversity in follicular lymphoma suggests that antigen stimulation and selection is involved in the evolution of the malignant clone.21 22Little is known about the intracellular signaling of BCR in lymphoma B cells. It can also be supposed that abnormalites driving constitutive signaling by the BCR could protect activated lymphoma B cells from CD95-induced death. Further investigations will be neccessary to explore the precise role of tumoral BCR in the resistance of lymphoma cells to CD95-mediated cell death.
The resistance of lymphoma cells to CD95-mediated apoptosis that we have demonstrated could signify that, in vivo, tumor cell could escape from the regulatory control of CD4+ lymphocytes and partly from the antitumor cytotoxic CD8+ T cells. Indeed, the CD95-death pathway is the major mechanism for CD4+ T cells to mediate apoptosis and one of the two killing pathways used by CD8+ T lymphocytes.8,9 We have previously described that allogeneic T lymphocytes were able to lyse tumor cells.23 Thus, we tested whether CD95 cross-linking by CD95 ligand, rather than by MoAbs, could drive apoptosis in lymphoma cells. We have shown that neither CD4+ nor CD8+effector cells were able to induce the death of tumor cells via a CD95-based mechanism. Indeed, the majority of the cytotoxicity observed against tumor cells (>45%) was strictly dependent on the perforin pathway. These results also support the notion that CD8+lymphocytes represent good, and perhaps the best, effectors to kill tumor cells.
We show in this report that a large number of B cells from distinct NHL groups were intrinsically resistant to CD95-mediated apoptosis, including DLCL or FL, which are considered to arise from activated cells. Interestingly, CD40 engagement poorly induced CD95-mediated cell death. So, particularly in NHL, the resistance to CD95-induced cell death is probably an important step in tumor development or progression, as this property allows lymphoma cells to escape from CD4+ T-cell–mediated apoptosis and partially from CD8+ T-cell–mediated cytotoxicity. However, the blockade in the CD95 signaling pathway cannot be considered as the sole mechanism involved in the tumoral development because children who have defective CD95-mediated apoptosis develop an autoimmune syndrome as their primary disease rather than a cancer.10,11Furthermore, recent work53 has shown that CD95-deficient mice (lpr) lack malignant tumors, but develop lethal B-cell lymphoma when they are deficient in T cells, suggesting a CD95-independent role for T cells in lymphoma development. Thus, in NHL, resistance to CD95-based apoptosis and functional abnormalities of T lymphocytes, such as anergy,54 could be of prime importance in facilitating lymphoma development or progression.
ACKNOWLEDGMENT
We are grateful to the staff of the Blood Center Immunological Department, the Grenoble CHU and Annecy CHR hematological departments, and to E. Keddari for their collaboration. We also thank Pierre Garrone for his comments and helpful suggestions.
Supported by Grant No. 1241 from the “Association pour la Recherche sur le Cancer.”
Address reprint requests to Joël Plumas, PhD, Laboratoire d'Immunologie, Etablissement de Transfusion Sanguine de l'Isère et de la Savoie, BP 35, F-38701 La Tronche Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Lymphoma B cells are resistant to CD95-mediated apoptosis in an assay detecting late stages of apoptosis. Jurkat cells (□) and three lymphoma cell populations isolated from LCL-4 (▴), MCL-6 (⧫), and DLCL-1 (•) patients were labeled with [51Cr]-sodium chromate and then incubated with increased concentrations of anti-CD95 MoAb CH-11 for 4 hours. The apoptosis intensity was evaluated by the percentage of specific 51Cr-release.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/8/10.1182_blood.v91.8.2875.2875_2875_2885/5/m_blod4081403.jpeg?Expires=1769303397&Signature=URGMHJAjo25WKuz6ldQ8RqopvX0Tvi8xXMt4Xk2T-iqHTeSAWsc9dUGjfGqy-j4GJw-8kJxDN2e6SEuHwJWPx1emfiDwXD7k2fn6bHt9wsSMDGuZ5Hv78idjMRz~AEyEfgQrdl5bpWqxvRK3XazbmmByQH-GXn8dfi2wSd7emCujFP~4RbTgBKmuMtLlJMSePTkDP1f9m2vlJmbVfMIgpil1pR8RwOFSxhbbPYcj7PrTt6DpXvglPYM4-v0cKGoZUbQg5V30Fp84RVLGHSoNch89GwM1Bid6OpqBKJdVatF0DaCG-lRC1rBgqkjoMGBwFd5xyTRuhoJ3pcX6w9F5qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal