Expression of tissue factor (TF) by activated monocytes in several diseases leads to disseminated intravascular coagulation. Lipopolysaccharide (LPS)-induced monocyte TF expression is downregulated by the nuclear hormone all-trans retinoic acid (ATRA). In this study, we examined the mechanism by which ATRA inhibits monocyte TF expression. We show that ATRA selectively inhibited LPS induction of TF expression in human monocytes and monocytic THP-1 cells without affecting LPS induction of tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8). Inhibition of TF expression occurred at the level of transcription as determined by nuclear run-on. ATRA did not significantly alter the binding or functional activity of the transcription factors c-Fos/c-Jun and c-Rel/p65, which are required for LPS induction of the TF promoter in monocytic cells. In contrast to the ATRA inhibition of the endogenous TF gene, LPS induction of the cloned TF promoter was not inhibited by ATRA in transiently transfected THP-1 cells. Our results demonstrate that ATRA selectively inhibited LPS-induced TF gene transcription in human monocytic cells by a mechanism that does not involve repression of AP-1– or NF-κB–mediated transcription.
TISSUE FACTOR (TF) is the primary cellular initiator of the coagulation protease cascades.1,2TF-initiated thrombosis is associated with many disease states, including gram-negative sepsis, atherosclerosis, and cancer.3-6 TF expression by leukemic cells in patients with acute promyelocytic leukemia (APL) may initiate disseminated intravascular coagulation and lead to a consumptive coagulopathy.7-9 All-trans-retinoic acid (ATRA) therapy downregulates TF expression in these patients.8-10In addition, several studies have established that ATRA also inhibits TF expression in bacterial lipopolysaccharide (LPS)-stimulated peripheral blood monocytes, as well as in tumor necrosis factor-α (TNF-α)–stimulated human endothelial cells.7,11 12
ATRA is a member of the retinoid family of hormones, which are ligands for a nuclear hormone receptor family known as the retinoic acid receptors (RARs).13 Recent studies have shown that RARα and RARβ are required for retinoid-induced downregulation of monocyte TF expression.14 Retinoids are lipophilic molecules that can pass through plasma membranes and effectively enter the nucleus of cells to bind RARs. Binding of retinoids to RARs induces allosteric changes that allow RARs to bind to specific DNA recognition sites and regulate gene transcription.13 15 Liganded RARα has been shown to modulate gene transcription by directly interacting with other proteins, such as c-Jun.16
LPS stimulates monocytes to rapidly and transiently express several proinflammatory mediators, including TF, TNF-α, and interleukin-8 (IL-8).17 LPS induces the transcriptional activation of these genes, which require cooperative interaction between several transcription factors, including those belonging to the NF-κB/Rel and AP-1 families.18-21 We have established that binding of proteins to a 56-bp (−227 bp to −172 bp) region located in the human TF promoter mediates LPS induction of the TF gene in monocytes.22 This region contains two AP-1–binding sites and a nonconsensus κB-binding site, which bind c-Fos/c-Jun and c-Rel/p65 heterodimers, respectively.23,24 Functional interaction between these transcription factors is required for maximal LPS induction of TF gene transcription in monocytic cells.22 23
Our laboratory and others have shown that agents that block the nuclear translocation of c-Rel/p65 heterodimers by preventing IκBα phosphorylation or proteolytic degradation inhibit LPS-induced TF gene transcription in monocytic cells. These agents include protease inhibitors and salicylates.25-27 Additionally, agents that elevate intracellular cyclic adenosine monophosphate (cAMP) levels can inhibit LPS-induced TF gene transcription in monocytic cells and endothelial cells by affecting nuclear NF-κB activity.28Our recent studies indicate that cAMP inhibits NF-κB activity by activating the PKA signaling pathway, which leads to competition between p65 and CREB for limiting amounts of the coactivator CBP.29 Importantly, all of these NF-κB inhibitors also inhibit LPS induction of TNF-α gene transcription in human monocytic cells.25-28 30
Previous studies report that ATRA can inhibit both AP-1– and NF-κB–dependent transcription.31,32 RARα liganded with ATRA is a negative regulator of AP-1–dependent genes, such as the stromolysin and collagenase genes, which is proposed to be the result of direct interactions between RARs and c-Jun or binding of RARs to c-Jun sites.31,32 In addition, ATRA was recently shown to selectively inhibit TNF-α–induced vascular cell-adhesion molecule-1 (VCAM-1) expression in cultured dermal microvascular endothelial cells without affecting other NF-κB–regulated genes, such as E-selectin and intracellular adhesion molecule 1.33 The mechanism of this ATRA inhibition was proposed to be due to a specific reduction of NF-κB binding to κB sites in the VCAM-1 promoter without affecting binding to other κB sites.
In this study, we examined the mechanism by which ATRA inhibits LPS-induced TF gene expression in human monocytic cells.
MATERIALS AND METHODS
Materials.
LPS (Escherichia coli serotype O111:B4) was purchased from CalBiochem (San Diego, CA). ATRA was obtained from Sigma (St Louis, MO) and was dissolved in ethanol.
Monocyte isolation and cell culture.
Human peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation from heparinized blood drawn from volunteers. Cells were diluted 1:1 (vol:vol) in endotoxin-free RPMI 1640 medium (BioWhittaker, Walkersville, MD) and were overlaid onto Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). After centrifugation at 400g, the PBMCs were removed and washed twice with medium and resuspended in RPMI 1640 media containing 2% fetal calf serum (Gemini Bioproducts, Calabasas, CA). Monocytes were isolated from PBMCs using Sepracell-MN (Sepratech, Oklahoma City, OK). Monocytes were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. The purity of the monocyte preparations was typically 80% to 90% as determined by nonspecific esterase staining.34 Human monocytic leukemia THP-1 cells were obtained from the American Type Culture Collection (Rockville, MD) and were cultivated (nonsynchronized) at a density of 2 to 5 × 106 cells/mL as described.35 All reagents were tested by Limulus amebocyte lysate assay (BioWhittaker) and contained less than 0.005 ng/mL of endotoxin.
TF activity.
THP-1 cell pellets were solubilized at 37°C for 15 minutes using 15 mmol/L octyl β-d-glucopyranoside. TF activity in cell lysates was assayed using a one-stage clotting assay as described.36 Clotting times were converted to milliunits of TF activity by comparison with a standard curve established with purified human brain TF.36 For reference, a clotting time of 50 seconds corresponds to 1,000 mU of TF activity.
TF, TNF-α, and IL-8 enzyme-linked immunosorbent assays.
Monocytes (1 × 106 cells) or THP-1 cells (1 × 106 cells) were pretreated with various doses of ATRA for 30 minutes before LPS stimulation. Monocytes were stimulated with 100 ng/mL of LPS and THP-1 cells were stimulated with 10 μg/mL of LPS. Cells were incubated for 5 hours before measuring TF antigen levels in cell lysates and 24 hours before measuring TNF-α and IL-8 antigen levels in culture supernatants. The TF enzyme-linked immunosorbent assay (ELISA) kit was purchased from American Diagnostica (Greenwich, CT) and the TNF-α and IL-8 ELISA kits were purchased from R & D Systems (Minneapolis, MN). TF, TNF-α, and IL-8 protein concentrations from the ELISAs were determined using standard curves for each assay.
Analysis of TF, TNF-α, and IL-8 mRNA.
Total cellular RNA, purified using TRIZOL reagent (GIBCO-BRL, Gaithersburg, MD), was analyzed by Northern blotting as described.37 THP-1 cells were pretreated for 30 minutes with ATRA followed by a 2-hour induction with LPS (10 μg/mL). A 641-bp human TF cDNA fragment,38 an 800-bp human TNF-α cDNA fragment,39 and a 400-bp human IL-8 cDNA fragment (kindly provided by Dr R. Terkeltaub) were used as probes. cDNA fragments were labeled with [α-32P]deoxycytidine triphosphate (ICN, Costa Mesa, CA) using the Prime-It Kit (Stratagene, La Jolla, CA). Blots were rehybridized with the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (G3PDH; Clontech Laboratories, Palo Alto, CA). Autoradiography was performed at −70°C using Kodak Biomax film (Eastman-Kodak, Rochester, NY). Band intensities were quantified using a Personal Densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Nuclear run-on.
Nuclear run-on assays were performed as described previously.40 Cells were pretreated for 30 minutes with ATRA (1 × 10−5 mol/L) before a 1-hour stimulation with LPS. Cells (5 × 107) were lysed in a homogenizer and nuclei collected by centrifugation using a 2-mmol/L sucrose cushion. Nuclear RNA was labeled using [α-32P]uridine triphosphate (ICN). Samples were treated sequentially with DNase I (final concentration, 1 U/μL) for 10 minutes at 37°C and with proteinase K (final concentration, 200 μg/mL) for 45 minutes at 37°C. Nuclear RNA was extracted with phenol/chloroform, ethanol-precipitated, and purified using a G-50 sephadex column. TF, G3PDH, and TNF-α cDNAs present in the vectors pGEM3Z and pSP73 (Promega, Madison, WI) were used as targets in the nuclear run-on assays. Prehybridization and washing of the filters was performed as described.41 Radioactivity was quantified using a Phosphoimager and ImageQuant software (Molecular Dynamics).
Plasmids and transfections.
p(AP-1)4LUC and p(κB)4LUC contain four copies of the proximal TF AP-1 site and TF κB site, respectively, cloned upstream of the minimal simian virus 40 (SV40) promoter expressing the firefly luciferase (LUC) reporter gene in pGL2-Promoter (Promega).24 pTF(−2106)LUC has previously been described.22 Cells were transfected using diethyl aminoethyl (DEAE)-dextran22 and were cultivated for 46 hours before a 5-hour stimulation with LPS (10 μg/mL). Luciferase activity was determined using the Luciferase Assay System (Promega) and Monolight 2010 luminometer (Analytical Luminescence, San Diego, CA).
Electrophoretic mobility shift assay.
Nuclear extracts were prepared from 5 × 106 cells as described.42 Protein concentrations were 1 to 5 mg/mL, as determined by a bicinchoninic acid (BCA) protein assay (Price, Rockford, IL). An oligonucleotide (Operon Technologies, Alameda, CA) containing the TF κB site (underlined), 5′-GTCCCGGAGTTTCCTACC-3′, was annealed with a complementary primer and was radiolabeled using [α-32P]deoxycytidine triphosphate (ICN) as described.24 Similarly, binding of AP-1 was performed using a radiolabeled oligonucleotide containing the proximal TF AP-1 site (underlined), 5-CTGGGGTGAGTCATCCCTT-3′.23 An oligonucleotide containing an Sp1 site (underlined), 5′-ATTCGATCGGGGCGGGGCGAGC-3′, was purchased from Promega. Protein-DNA complexes were separated from free DNA probe by electrophoresis through 6% nondenaturing acrylamide gels (NOVEX, San Diego, CA) in 0.5× Tris borate-EDTA and bands visualized by autoradiography. Band intensities were quantified by densitometric analysis using a Personal Densitometer and ImageQuant software. The following antibodies from Santa Cruz Biotechnology were used for supershift studies: anti–p50 sc-114; anti–p65 sc-109; anti–c-Rel sc-070; anti-Sp1 (PEP2).
Statistical analysis.
Statistical significance was calculated using a paired Student'st test and was considered significant at P values ≤.05.
RESULTS
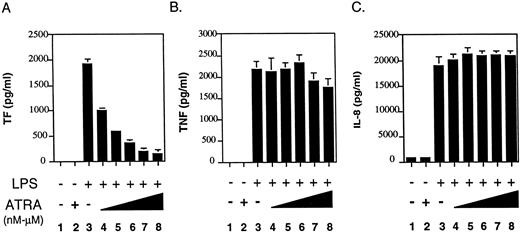
ATRA selectively inhibits LPS induction of TF expression in human monocytes and monocytic cells. ATRA has previously been shown to downregulate TF procoagulant activity in LPS-stimulated human monocytes.7 11 To determine if ATRA was a general inhibitor of LPS-induced genes in human monocytes, we analyzed the effect of ATRA on LPS induction of TF, TNF-α, and IL-8. Monocytes were incubated for 30 minutes with various doses of ATRA (1 × 10−9 mol/L to 1 × 10−5 mol/L) before LPS stimulation. ATRA inhibited LPS induction of TF expression in a dose-dependent manner (Fig 1A). Statistically significant inhibition (P = .005) was observed using 10−9 mol/L ATRA. In contrast, ATRA had no effect on LPS induction of TNF-α expression (Fig1B) or IL-8 expression (Fig 1C). These results indicate that ATRA selectively inhibits the LPS induction of TF expression in human monocytes without affecting induction of TNF-α and IL-8 expression.
Effect of ATRA on TF, TNF-α, and IL-8 protein expression in LPS-stimulated human monocytes. Freshly isolated human monocytes (1 × 106 cells) were incubated with LPS alone, ATRA alone (10−5 mol/L) or with various doses of ATRA (1 × 10−9 to 1 × 10−5 mol/L) for 30 minutes before LPS stimulation (100 ng/mL). (A) Monocytes were incubated for 5 hours and TF antigen levels were measured by ELISA. (B) Monocytes were incubated for 24 hours and TNF-α antigen level in culture supernatants were measured by ELISA. (C) Monocytes were incubated for 24 hours and IL-8 antigen levels in culture supernatants were measured by ELISA. Results from three independent experiments (mean ± SE) are shown.
Effect of ATRA on TF, TNF-α, and IL-8 protein expression in LPS-stimulated human monocytes. Freshly isolated human monocytes (1 × 106 cells) were incubated with LPS alone, ATRA alone (10−5 mol/L) or with various doses of ATRA (1 × 10−9 to 1 × 10−5 mol/L) for 30 minutes before LPS stimulation (100 ng/mL). (A) Monocytes were incubated for 5 hours and TF antigen levels were measured by ELISA. (B) Monocytes were incubated for 24 hours and TNF-α antigen level in culture supernatants were measured by ELISA. (C) Monocytes were incubated for 24 hours and IL-8 antigen levels in culture supernatants were measured by ELISA. Results from three independent experiments (mean ± SE) are shown.
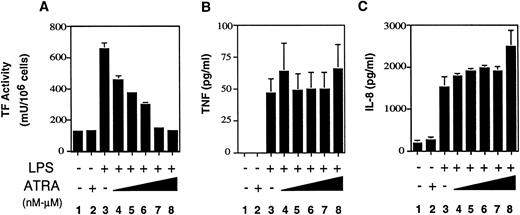
We next determined if ATRA selectively inhibited LPS induction of TF expression in human monocytic THP-1 cells, which we use as a model of human monocytes. THP-1 cells were incubated for 30 minutes with ATRA at various doses (1 × 10−9 to 1 × 10−5mol/L) before LPS stimulation. LPS induction of TF activity in THP-1 cells was inhibited by ATRA in a dose-dependent manner (Fig2A). Statistically significant inhibition (P = .01) was observed using 10−9 mol/L ATRA. Similar to the results observed with human monocytes, ATRA did not inhibit the LPS induction of TNF-α (Fig 2B) or IL-8 (Fig 2C). These results indicated that THP-1 cells were a valid model to examine the molecular mechanism by which ATRA selectively inhibits LPS-induced TF expression in human monocytic cells.
Effect of ATRA on TF, TNF-α, and IL-8 expression LPS-stimulated THP-1 cells. THP-1 cells (1 × 106 cells) were incubated LPS alone, ATRA (10−5 mol/L), or various doses of ATRA (1 × 10−9 to 1 × 10−5mol/L) before LPS stimulation (10 μg/mL). (A) THP-1 cells were exposed to LPS for 5 hours and TF activity was determined using a one-stage clotting assay. (B) THP-1 cells were exposed to LPS for 24 hours and TNF-α antigen levels in culture supernatants measured by ELISA. (C) THP-1 cells were exposed to LPS for 24 hours and IL-8 antigen levels in culture supernatants measured by ELISA. Results from three independent experiments (mean ± SE) are shown.
Effect of ATRA on TF, TNF-α, and IL-8 expression LPS-stimulated THP-1 cells. THP-1 cells (1 × 106 cells) were incubated LPS alone, ATRA (10−5 mol/L), or various doses of ATRA (1 × 10−9 to 1 × 10−5mol/L) before LPS stimulation (10 μg/mL). (A) THP-1 cells were exposed to LPS for 5 hours and TF activity was determined using a one-stage clotting assay. (B) THP-1 cells were exposed to LPS for 24 hours and TNF-α antigen levels in culture supernatants measured by ELISA. (C) THP-1 cells were exposed to LPS for 24 hours and IL-8 antigen levels in culture supernatants measured by ELISA. Results from three independent experiments (mean ± SE) are shown.
ATRA selectively inhibits LPS induction of TF mRNA expression.
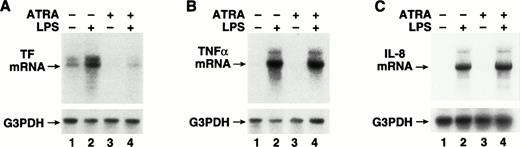
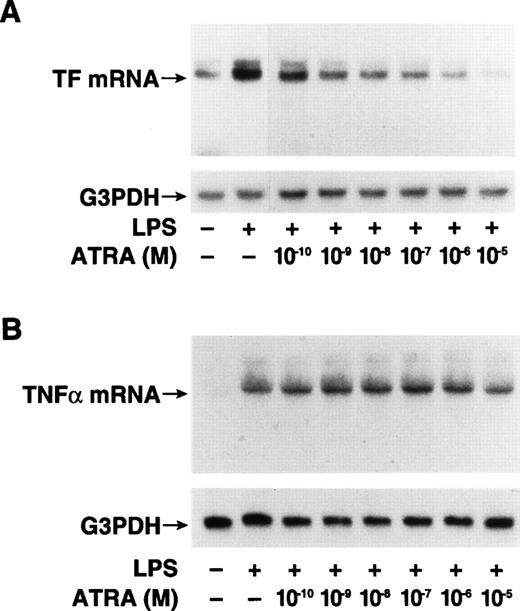
To determine if ATRA inhibited LPS-induced TF mRNA expression, we analyzed TF mRNA levels by Northern blot analysis in LPS-stimulated THP-1 cells with or without ATRA. THP-1 cells were incubated for 30 minutes with ATRA (10−5 mol/L) before LPS stimulation for 2 hours. The basal levels of TF mRNA observed in unstimulated cells were strongly induced by LPS (Fig 3A). ATRA reduced the basal levels of TF mRNA in unstimulated cells and abolished the LPS induction of TF mRNA (Fig 3A). ATRA did not inhibit LPS induction of TNF-α mRNA (Fig 3B) or IL-8 mRNA (Fig 3C). We also performed dose-titration experiments. ATRA inhibited LPS induction of TF mRNA expression in a dose-dependent manner (Fig4A), but did not inhibit LPS induction of TNF-α mRNA expression at any concentration (Fig 4B). These results indicate that ATRA selectively inhibits LPS induction of TF mRNA expression in human monocytic cells.
Effect of ATRA on TF, TNF-α, and IL-8 mRNA expression in LPS-stimulated THP-1 cells. Total RNA was extracted from THP-1 cells exposed to LPS (10 μg/mL) for 2 hours with or without a 30-minute pretreatment with ATRA (1 × 10−5 mol/L). Control samples were incubated with ATRA alone or without LPS or ATRA. Various mRNA levels were determined by Northern blot analysis using radiolabeled human cDNA probes. Membranes were reprobed with a G3PDH cDNA probe to assess RNA loading. The positions of TF (A), TNF-α (B), and IL-8 (C) mRNAs are indicated. Similar results were observed in one or more independent experiments.
Effect of ATRA on TF, TNF-α, and IL-8 mRNA expression in LPS-stimulated THP-1 cells. Total RNA was extracted from THP-1 cells exposed to LPS (10 μg/mL) for 2 hours with or without a 30-minute pretreatment with ATRA (1 × 10−5 mol/L). Control samples were incubated with ATRA alone or without LPS or ATRA. Various mRNA levels were determined by Northern blot analysis using radiolabeled human cDNA probes. Membranes were reprobed with a G3PDH cDNA probe to assess RNA loading. The positions of TF (A), TNF-α (B), and IL-8 (C) mRNAs are indicated. Similar results were observed in one or more independent experiments.
Dose-dependent ATRA inhibition of TF mRNA expression in LPS-stimulated THP-1 cells. Total RNA was extracted from THP-1 cells exposed to LPS (10 μg/mL) for 2 hours with or without a 30-minute pretreatment with ATRA at doses ranging from 1 × 10−10to 1 × 10−5 mol/L. The control sample was incubated without LPS or ATRA. TF and TNF-α mRNA levels were determined by Northern blot analysis using radiolabeled human cDNA probes. Blots were reprobed with a G3PDH cDNA probe to assess RNA loading. The positions of TF (A) and TNF-α (B) mRNAs are indicated.
Dose-dependent ATRA inhibition of TF mRNA expression in LPS-stimulated THP-1 cells. Total RNA was extracted from THP-1 cells exposed to LPS (10 μg/mL) for 2 hours with or without a 30-minute pretreatment with ATRA at doses ranging from 1 × 10−10to 1 × 10−5 mol/L. The control sample was incubated without LPS or ATRA. TF and TNF-α mRNA levels were determined by Northern blot analysis using radiolabeled human cDNA probes. Blots were reprobed with a G3PDH cDNA probe to assess RNA loading. The positions of TF (A) and TNF-α (B) mRNAs are indicated.
ATRA selectively inhibits LPS induction of TF gene transcription.
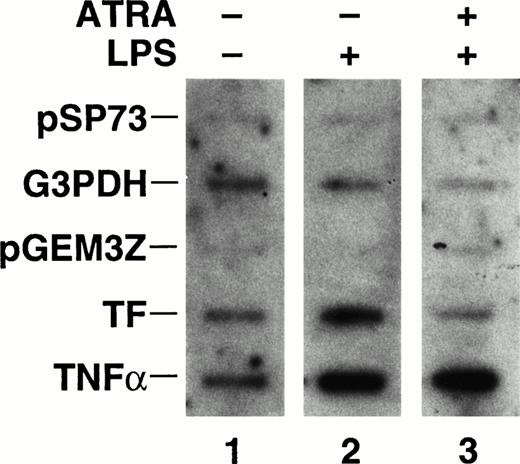
ATRA inhibition of TF mRNA expression could be due to changes in TF mRNA stability and/or an inhibition of TF gene transcription. To determine if ATRA inhibited LPS induction of TF gene transcription, nuclear run-on experiments were performed. THP-1 cells were incubated with ATRA (10−5 mol/L) for 30 minutes before a 1-hour stimulation with LPS. LPS stimulation of THP-1 cells increased the rate of TF gene transcription, which was abolished by ATRA (Fig 5). In contrast, ATRA did not affect the LPS induction of TNF-α gene transcription (Fig 5). The band intensity representing the rate of transcription of the housekeeping gene, G3PDH, was slightly reduced in cells incubated with ATRA and LPS in this experiment (Fig 5). However, this decrease was not observed in an independent experiment and is likely to be due to experimental variations in the assay. Importantly, ATRA and LPS did not decrease the levels of G3PDH mRNA in cells (Figs 3 and 4). These results demonstrate that ATRA selectively inhibits TF gene transcription in human monocytic cells.
Effect of ATRA on TF and TNF-α gene transcription in LPS stimulated THP-1 cells. Nuclei were isolated from unstimulated cells, cells stimulated with LPS (10 μg/mL) for 1 hour, and cells treated with ATRA (10−5 mol/L) for 30 minutes before LPS stimulation for 1 hour. The rate of transcription of the TF, TNF-α, and G3PDH gene was determined by nuclear run-on. Vector controls used in the experiment were pSP73 and pGEM3Z. The autoradiogram was exposed for 7 days at −80°C with intensifier screens. Similar results were observed in an independent experiment.
Effect of ATRA on TF and TNF-α gene transcription in LPS stimulated THP-1 cells. Nuclei were isolated from unstimulated cells, cells stimulated with LPS (10 μg/mL) for 1 hour, and cells treated with ATRA (10−5 mol/L) for 30 minutes before LPS stimulation for 1 hour. The rate of transcription of the TF, TNF-α, and G3PDH gene was determined by nuclear run-on. Vector controls used in the experiment were pSP73 and pGEM3Z. The autoradiogram was exposed for 7 days at −80°C with intensifier screens. Similar results were observed in an independent experiment.
ATRA does not affect DNA binding of c-Fos/c-Jun, c-Rel/p65, or Sp1 transcription factors.
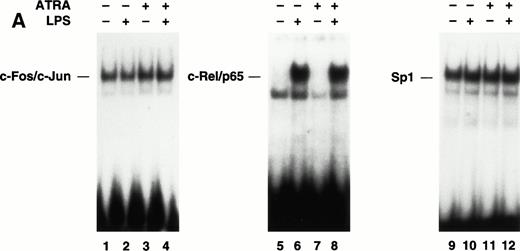
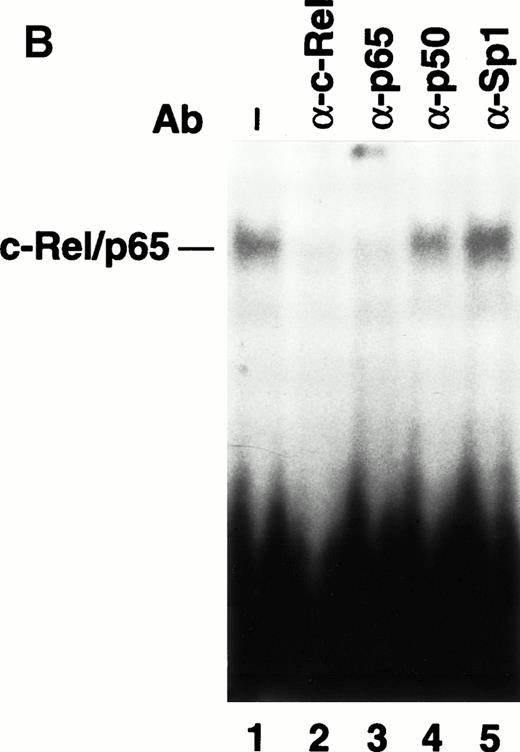
LPS induction of TF gene transcription in monocytic cells is primarily regulated by the cooperative interaction of c-Fos/c-Junand c-Rel/p65 heterodimers bound to a 56-bp LPS response element in the TF promoter.22,23 Previous electrophoretic mobility shift assays (EMSAs) and antibody supershift analysis of complexes have established that c-Fos/c-Jun and c-Rel/p65 heterodimers bind to the TF promoter. To examine if ATRA inhibited TF gene transcription by preventing binding of either c-Fos/c-Jun or c-Rel/p65 heterodimers, we performed EMSAs using oligonucleotides containing either the proximal AP-1 site or the κB site from the human TF promoter. THP-1 cells were incubated with ATRA for 30 minutes before a 2-hour stimulation with LPS. The binding of c-Fos/c-Jun heterodimers and Sp1 in unstimulated or LPS-stimulated THP-1 cells was not affected by ATRA (Fig 6A). We have shown that LPS stimulation of THP-1 cells induces nuclear translocation of c-Rel/p65 heterodimers and binding to the TF κB site.24 LPS-induced nuclear translocation and DNA binding of c-Rel/p65 heterodimers was also not inhibited by ATRA (Fig6A). Similar results were observed using a κB site that binds p50/p65 heterodimers (data not shown), which regulate TNF-α gene transcription.43 To exclude the possibility that ATRA induced binding of an inhibitory complex to the TF κB site, we performed supershift experiments using antibodies raised against NF-κB proteins. These studies demonstrated that ATRA did not change the composition of the c-Rel/p65 complex (Fig 6B). These results indicated that ATRA does not inhibit TF gene transcription by preventing the binding of Sp1, c-Fos/c-Jun, or c-Rel/p65 to the TF promoter.
The effect of ATRA on the binding of transcription factors to the TF promoter. (A) Effect of ATRA on the binding of c-Fos/c-Jun, Rel/p65, and Sp1. Nuclear extracts were isolated from THP-1 cells treated with or without ATRA (1 × 10−5 mol/L) for 30 minutes before the addition of LPS (10 μg/mL) for 2 hours. EMSAs were performed using AP-1, κB, and Sp1 sites. The position of c-Fos/c-Jun,c-Rel/p65, and Sp1 is indicated. Similar results were observed in an independent experiment. (B) Antibody supershift analysis of the protein-DNA complex formed using nuclear extracts from ATRA- and LPS-treated cells. Antibodies to various NF-κB proteins and Sp1 were incubated with nuclear extracts before the addition of a radiolabeled TF κB site.
The effect of ATRA on the binding of transcription factors to the TF promoter. (A) Effect of ATRA on the binding of c-Fos/c-Jun, Rel/p65, and Sp1. Nuclear extracts were isolated from THP-1 cells treated with or without ATRA (1 × 10−5 mol/L) for 30 minutes before the addition of LPS (10 μg/mL) for 2 hours. EMSAs were performed using AP-1, κB, and Sp1 sites. The position of c-Fos/c-Jun,c-Rel/p65, and Sp1 is indicated. Similar results were observed in an independent experiment. (B) Antibody supershift analysis of the protein-DNA complex formed using nuclear extracts from ATRA- and LPS-treated cells. Antibodies to various NF-κB proteins and Sp1 were incubated with nuclear extracts before the addition of a radiolabeled TF κB site.
ATRA does not inhibit LPS-induced AP-1– or NF-κB/Rel–dependent transcription in monocytic cells.
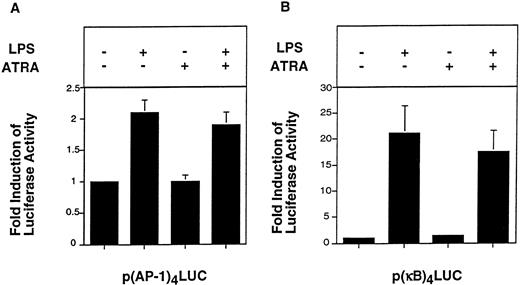
EMSAs analyze DNA binding of transcription factors, but do not measure the transcriptional activity of these proteins. For instance, phosphorylation of c-Jun increases the transcription activity of AP-1 without affecting DNA binding.44 Therefore, we performed functional studies to analyze the effect of ATRA on AP-1– and NF-κB/Rel–dependent transcription in THP-1 cells. Cells were transfected with either p(AP-1)4LUC, p(κB)4LUC, or the control plasmid pGL2-Promoter. LPS induced a twofold increase in luciferase activity expressed by p(AP-1)4LUC (Fig 7A). ATRA (10−5 mol/L) did not significantly reduce LPS induction of AP-1–dependent transcription (Fig 7A). LPS induced a 21-fold increase in luciferase activity expressed by p(κB)4LUC, which was slightly reduced (17%) by ATRA. LPS did not induce luciferase activity expressed by pGL2-Promoter, which contains only the minimal SV40 promoter (data not shown). These results indicated that ATRA did not significantly inhibit LPS-induced AP-1– or NF-κB–dependent transcription in THP-1 cells.
Effect of ATRA on LPS-induced AP-1– and NF-κB–dependent transcription in THP-1 cells. THP-1 cells were transfected with p(AP-1)4LUC or p(κB)4LUC (6 μg) using DEAE-dextran. After 46 hours, cells were divided into four equal portions and incubated with or without LPS (10 μg/mL) for 5 hours in the presence or absence of a 30-minute pretreatment with ATRA (1 × 10−5 mol/L). AP-1–mediated transcription (A) and c-Rel/p65–mediated transcription (B) are shown. Luciferase activity (light units) expressed by p(AP-1)4LUC and p(κB)4LUC was used to determine fold inductions (mean ± SE) in three independent experiments.
Effect of ATRA on LPS-induced AP-1– and NF-κB–dependent transcription in THP-1 cells. THP-1 cells were transfected with p(AP-1)4LUC or p(κB)4LUC (6 μg) using DEAE-dextran. After 46 hours, cells were divided into four equal portions and incubated with or without LPS (10 μg/mL) for 5 hours in the presence or absence of a 30-minute pretreatment with ATRA (1 × 10−5 mol/L). AP-1–mediated transcription (A) and c-Rel/p65–mediated transcription (B) are shown. Luciferase activity (light units) expressed by p(AP-1)4LUC and p(κB)4LUC was used to determine fold inductions (mean ± SE) in three independent experiments.
ATRA does not inhibit LPS induction of the cloned human TF promoter.
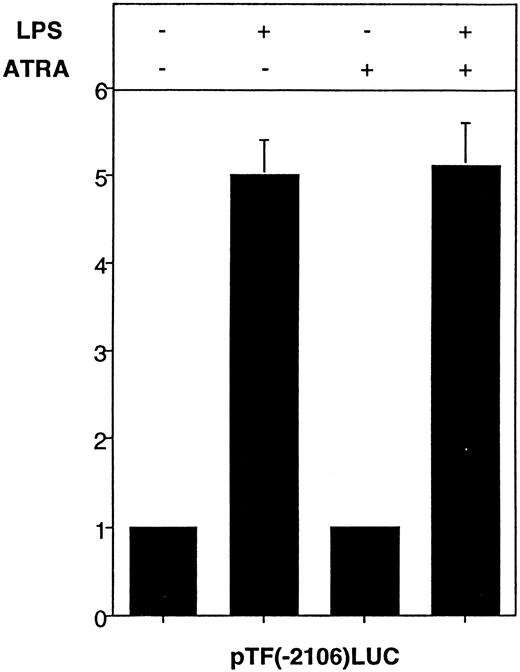
To determine if ATRA inhibited LPS induction of TF gene transcription by assembling an inhibitory complex at putative retinoic acid response elements at −1050 and −615 in the human TF promoter, we assessed the effect of ATRA on LPS induction of the cloned TF promoter in transiently transfected THP-1 cells. pTF(−2106)LUC contains the wild-type TF promoter (−2106 to +121 bp, relative to the start site of transcription) and is strongly induced by LPS.22 23 LPS induced a fivefold increase in luciferase activity expressed by pTF(−2106)LUC, which was not affected by ATRA (Fig8). These results indicated that ATRA does not inhibit the cloned TF promoter in transiently transfected THP-1 cells.
Effect of ATRA on LPS-induced TF promoter activity in THP-1 cells. pTF(−2106)LUC (6 μg) was transfected into THP-1 cells using DEAE-dextran. After 46 hours, cells were divided into four equal portions and were treated with LPS (10 μg/mL) for 5 hours with or without a 30-minute pretreatment of ATRA (1 × 10−5mol/L). The control sample was incubated without LPS or ATRA. Luciferase activity (light units) expressed by pTF(−2106)LUC was used to determine fold inductions (mean ± SE) in three independent experiments.
Effect of ATRA on LPS-induced TF promoter activity in THP-1 cells. pTF(−2106)LUC (6 μg) was transfected into THP-1 cells using DEAE-dextran. After 46 hours, cells were divided into four equal portions and were treated with LPS (10 μg/mL) for 5 hours with or without a 30-minute pretreatment of ATRA (1 × 10−5mol/L). The control sample was incubated without LPS or ATRA. Luciferase activity (light units) expressed by pTF(−2106)LUC was used to determine fold inductions (mean ± SE) in three independent experiments.
DISCUSSION
This study showed that ATRA inhibited LPS-induced TF expression in human monocytic THP-1 cells by preventing induction of TF gene transcription. ATRA inhibited monocyte TF expression at clinically relevant doses and required only a short preincubation period. A previous study showed that the inhibitory effect of ATRA was also observed when monocytes were exposed to retinoids for 10 minutes and washed before challenge with LPS, indicating that the inhibitory effect was rapid and irreversible.11 Short-term incubation with ATRA was not a general inhibitor of LPS signaling pathways in monocytes, because it had no effect on the LPS induction of TNF-α and IL-8. In contrast, long-term incubation of human monocytes (>72 hours) with ATRA inhibits LPS induction of cytokine expression, including IL-8,45 suggesting a different inhibitory mechanism.
The mechanism by which ATRA inhibits monocyte TF gene transcription is not known. Here, we showed that inhibition was not due to reduced DNA binding or reduced functional activity of c-Fos/c-Junor c-Rel/p65 heterodimers in monocytic cells. These results are consistent with the failure of ATRA to inhibit LPS induction of the TNF-α and IL-8 expression, both of which are regulated by AP-1 and NF-κB proteins.18,21,43,46 The small reduction in AP-1– and NF-κB–dependent transcription observed in transfected cells in the presence of ATRA may be due to competition for limiting amounts of the coactivator CBP. Recent studies have shown that CBP functions as a coactivator in transcription mediated by AP-1, NF-κB, and nuclear hormone receptors, such as RARα.29 47-50
Low-level (basal) expression of TF activity in unstimulated PBMCs is abolished by NF-κB inhibitors.25 Similarly, basal expression of TF mRNA expression in unstimulated THP-1 cells is abolished by NF-κB inhibitors (N. Mackman, unpublished data, July 1994), suggesting that it is mediated by c-Rel/p65. Thus, the same transcription factor complex appears to regulate both basal and LPS-induced TF expression in monocytic cells. We observed that ATRA inhibited both basal and LPS-induced TF mRNA expression in THP-1 cells (Fig 3), suggesting that it affected a common factor in the transcription complex that is required for monocyte TF expression. ATRA did not fully inhibit LPS-induced TF mRNA expression in all experiments, which is probably due to slight variations in the LPS and ATRA response of different cultures of THP-1 cells. Clinically, ATRA is used to treat APL patients suffering from coagulation disorders that often result in disseminated intravascular coagulation.10TF expression by the leukemic cells themselves, as well as monocytes, is thought to significantly contribute to this condition.9Our studies and others have shown that ATRA inhibits TF expression in both APL cells and monocytes.8,10 11 At present, there are no studies on how the TF gene is regulated in APL cells. However, the ability of ATRA to inhibit both constitutive TF expression in APL cells and LPS-induced TF expression in monocytes suggest that a similar regulatory mechanism may be employed in both cell types. This is currently being investigated.
ATRA inhibited LPS induction of the endogenous TF gene, but not the cloned TF promoter (−2106 to +121 bp) in transiently transfected THP-1 cells. The failure to inhibit the cloned TF promoter was not due to limiting amounts of RARα, because similar results were observed in cells cotransfected with a plasmid expressing RARα (data not shown). These results suggest that liganded RARα may assemble a repressor complex at a retinoic acid response element outside the cloned TF promoter region examined in this study. RARα has been shown to bind a transcriptional repressor called N-CoR.51,52 Recent studies have shown that unliganded RXR/TR heterodimers assemble a repressor complex that includes N-CoR.53,54 Importantly, in the presence of thyroid hormone this repressor complex is disrupted and the liganded thyroid receptor now recruits an activator complex. Our results suggest a novel inhibitory mechanism for ATRA because inhibition requires liganded RARα. Alternatively, inhibition may require assembly of the DNA into chromatin for recognition by ATRA. A recent study indicated that chromatin structure played a role in the regulation of gene expression by steroid hormone receptors.55 Finally, liganded RARα may compete with an as yet unidentified activator that induces the endogenous TF gene, but does not contribute to the regulation of the cloned TF promoter. ATRA inhibition of vitamin D3-stimulated transcription of the avian β3-integrin gene is mediated by competition for binding of VDR/RXR heterodimers and RAR/RXR heterodimers to a unique response element.56 Further studies are required to elucidate the molecular mechanism by which ATRA selectively inhibits TF gene expression in monocytes. This may require cloning of additional 5′ and 3′ flanking regions of the TF gene and/or the generation of stable THP-1 cell lines containing TF promoter-luciferase reporter genes.
ACKNOWLEDGMENT
We acknowledge H. McClary for technical assistance, G. Parry for helpful discussion, J. Erlich and M. O'Connell for critical reading of the manuscript, C. Glass for providing pRARα, and J. Robertson for preparing the manuscript.
Supported by Grant No. HL-48872 (N.M.) from the National Institutes of Health, and performed during the tenure of an Established Investigatorship from the American Heart Association (N.M.).
Address reprint requests to Nigel Mackman, PhD, The Scripps Research Institute, 10660 N Torrey Pines Rd, IMM-17, La Jolla, CA 92037.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal