Plasma of patients with thrombotic thrombocytopenic purpura (TTP) has been shown to contain unusually large von Willebrand factor (vWF) multimers that may cause platelet agglutination in vivo. Fresh frozen plasma infusions and plasma exchange represent the most efficient therapy of acute TTP. A specific protease responsible for cleavage of vWF multimers has been recently isolated from normal human plasma and was found to be deficient in four patients with chronic relapsing TTP. We examined the activity of the vWF-cleaving protease in plasma samples collected over a period of 400 days from a further patient with recurrent episodes of TTP who was treated by plasma exchange, plasma infusion, vincristine, corticosteroid therapy, and splenectomy. Complete deficiency of the vWF-cleaving protease was established during the first episode of TTP. The ensuing normalization of the platelet count was associated with the appearance of the protease activity. Three months after remission from the initial TTP event, the vWF-cleaving protease again disappeared and the platelet count gradually decreased. Relapses of severe thrombocytopenia occurred 7 and 11 months after the first acute episode of TTP. Deficient protease activity was associated with the presence in the patient plasma of an inhibitor that was found to be an IgG. Plasma exchange/infusion was followed by a temporary increase in the antibody titer, whereas treatment with vincristine led to a recovery of the platelet count without affecting the inhibitor concentration. Splenectomy and corticosteroid treatment resulted in disappearance of the autoantibody and normalization of the protease activity and of the platelet count. Our data suggest that the thrombocytopenia in this patient with TTP was associated with a lack of the vWF-cleaving protease activity depleted by an autoimmune mechanism. This case, together with our previously reported patients, leads us to conclude that acquired as well as constitutional deficiency of the vWF-cleaving protease may predispose to TTP.
THROMBOTIC thrombocytopenic purpura (TTP) is a disorder characterized by intravascular platelet clumping leading to thrombocytopenia, microangiopathic hemolytic anemia, renal dysfunction, central nervous system ischemia, and fever. The hypotheses concerning the etiology of TTP are controversial and suggest different pathogenetic mechanisms being responsible for development of the disorder.1 2 Endothelial injury is generally considered as the primary event. Besides familial forms of the disease, TTP has also been associated with infection, free radical formation, chemotherapy, pregnancy, HELLP syndrome, and abnormal processing of von Willebrand factor (vWF) multimers.
Unusually large vWF multimers have been observed in plasma samples from patients with chronic relapsing forms of TTP.3,4 vWF is a plasma glycoprotein composed of a variable number of 270-kD subunits linked by disulfide bonds. The binding affinity for collagen or other fibrillar components of the subendothelium and for platelets is strongly enhanced in the larger multimeric forms of vWF. Unusually large vWF multimeric forms, larger than those found in normal plasma, are present in endothelial cells and platelets.5,6 They are highly efficient in interacting with platelets at elevated levels of shear stress7 and may aggregate intact circulating platelets. Unusually large vWF multimers secreted from the endothelial cells into the plasma are thought to become proteolytically degraded.8-10 A specific protease cleaving purified vWF in vitro to the same fragments as those produced in vivo has been recently isolated from normal human plasma.11 12
Deficient activity of the vWF-cleaving protease has been recently established in four patients with chronic relapsing TTP.13In none of these patient plasmas was an inhibitor of or an antibody against the vWF-cleaving protease found. In the present study, we describe the occurrence of an autoantibody inhibiting the vWF-cleaving protease in another patient with TTP. Episodes with severe thrombocytopenia occurred in the absence of vWF-cleaving protease activity and in the presence of high levels of inhibitor. Clinical remission after splenectomy and under corticosteroid therapy was associated with disappearance of the autoantibody and reappearance of the protease activity.
MATERIALS AND METHODS
All samples for vWF analysis and protease assay were obtained from blood anticoagulated with 1/9 vol 0.106 mol/L Na3-citrate. Platelet-poor plasma was recentrifuged for 15 minutes at 3,000gat 25°C and stored at −20°C for subsequent testing. Platelet count, lactate dehydrogenase (LDH), hemoglobin (Hb), and bilirubin determinations were performed by conventional methods. vWF antigen (vWF:Ag) was determined by an enzyme-linked immunosorbent assay using a commercial rabbit antiserum against human vWF (Nordic, Tilburg, The Netherlands). Plasma thrombomodulin levels were quantified by an enzyme-linked immunosorbent technique with reagents (Asserachrom Thrombomodulin) kindly supplied by Diagnostica Stago (Asnières, France).
The multimeric composition of vWF was analyzed by sodium dodecyl sulfate (SDS) electrophoresis in 1% agarose gels [SeaKem HGT(P); FMC, Rockland, ME] according to Ruggeri and Zimmerman.14 Before electrophoresis, each sample containing 0.0025 U vWF:Ag was incubated for 20 minutes at 60°C with an equal volume of sample buffer containing SDS. Electrophoretically separated vWF multimers were electroblotted to nitrocellulose membranes and visualized using rabbit antiserum against human vWF (A0082; Dako, Glostrup, Denmark) and alkaline phosphatase-labeled goat antibodies against rabbit IgG (D0487; Dako).
Before the assay of vWF-cleaving protease activity, Pefabloc SC (Boehringer, Mannheim, Germany) was added at 10 mmol/L final concentration to citrated normal plasma or patient plasmas, and 1/20 dilutions of these plasmas were made with 0.15 mol/L NaCl-10 mmol/L Tris, pH 7.4 (TBS), containing 1 mmol/L Pefabloc. The protease was activated by 5 minutes of incubation at 37°C with 10 mmol/L BaCl2 as previously described.11 One hundred microliters of the incubation mixture was added immediately to 50 μL of protease-free vWF (adjusted to about 5 U vWF:Ag/mL) purified by gel filtration on Sepharose CL-2B (Pharmacia-LKB, Uppsala, Sweden) of a normal human plasma cryoprecipitate. Thus, the ratio of patient plasma vWF to purified normal vWF was about 1:50. The reaction mixture was dialyzed on the surface of a hydrophilic filter (VSWP; 25-mm diameter; Millipore, Bedford, MA) for 24 hours at 37°C against 1.5 mol/L urea/5 mmol/L Tris-HCl, pH 8.0. The reaction was stopped by the addition of 10 μL 0.2 mol/L EDTA, pH 7.4, and the extent of vWF degradation was assayed by multimer analysis using SDS-electrophoresis in 1.4% agarose gels. After electrophoresis, the proteins were electrotransferred to nitrocellulose, and vWF was visualized with peroxidase-conjugated rabbit antibodies against human vWF (P0226; Dako).11 A citrated normal human plasma pool (NHP), prepared from 42 healthy male subjects and stored at −70°C, was used for calibration of the protease assay in patient plasmas. Inhibitor of the vWF-cleaving protease was assayed by measuring the protease activity in mixtures of patient plasma and normal plasma at three different volume/volume (vol/vol) ratios, ie, 4:1, 1:1, and 1:5, after 10-minute preincubation at 37°C.
Affinity chromatography of the protease inhibitor was performed on a 4-mL column of protein A-Sepharose (Pharmacia-LKB). Two milliliters of patient plasma, collected on day 221, was applied onto the column equilibrated with TBS. Plasma proteins were eluted with TBS followed by 0.1 mol/L citrate, pH 4.0, and by 0.1 mol/L glycine-HCl, pH 3.0. The inhibitor of the vWF-cleaving protease was assayed in the breakthrough fraction of the patient plasma as well as in two IgG fractions eluted at pH 4.0 and 3.0, respectively. The same conditions were also used for affinity chromatography of the protease inhibitor on protein G-Sepharose (Pharmacia-LKB).
CASE REPORT
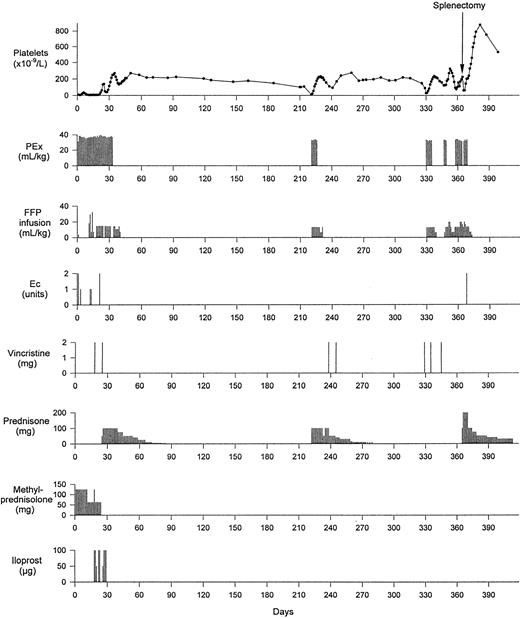
In January 1996, the 34-year-old previously healthy Turkish man developed flu-like symptoms with intermittent headaches and little productive cough. On January 30, he was admitted to a tertiary hospital because of fatigue, jaundice, anemia, and thrombocytopenia. Coombs-negative hemolytic anemia (Hb 9.3 g/dL and reticulocytes 10.1%) with schistocytes in the peripheral blood smear and thrombocytopenic purpura of all extremities (platelets, 17 × 109/L) were found. Leukocytes, including differential count, and prothrombin time were normal. Erythrocyte sedimentation rate was 62 mm/h and C-reactive protein was 16 mg/L (normal, <5.1 mg/L); there was no fever. Urinalysis showed 3 to 5 leukocytes and 20 to 30 erythrocytes per high power field and 0.68 g/L protein. Serum creatinine was repeatedly normal. Serologic examination was negative for human immunodeficiency virus, cytomegalovirus, Epstein-Barr virus, adenovirus, and mycoplasma pneumoniae. An intracutaneous Mantoux test was negative, as were immunologic tests (antinuclear antibodies, anti-DNA antibodies, anti-neutrophil cytoplasmic antibodies, and rheumatoid factor) for autoimmune disorders. Bone marrow aspiration showed an increased number of megakaryocytes but was otherwise normal. Ultrasonography of the abdomen indicated splenomegaly (15-cm longitudinal diameter), and computed tomography (CT) of the thorax showed a small subpleural density in the middle lung lobe. Echocardiography was unrevealing. One hundred milligrams of prednisone administered orally daily was initiated, but anemia deteriorated, and on February 6, after having suffered transient paresthesias on his left side, the patient became confused and agitated. He was then transferred to our hospital, where TTP was diagnosed and plasma exchange therapy using fresh frozen plasma (FFP) replacement was started immediately (Fig 1). Additional FFP was administered between plasma exchange sessions. Methylprednisolone and later prednisone were administered. Laboratory values are shown in Fig 2. CT scan of the brain performed on admission was normal. Ten days later, the patient again became confused and somnolent and developed homonymous hemianopsy to the left side. The CT scan now showed an ischemic zone of the right occipital lobe. Seven days later, the patient again suffered a transient ischemic attack with paresis of the left arm and dysarthry. Vincristine (2 mg intravenously [IV]) and iloprost (50 to 100 μg IV) were administered repeatedly, eventually allowing discharge from the hospital on day 46. Prednisone therapy was tapered and stopped on day 85. The patient was observed at regular intervals in our outpatient ward. Repeated thoracic CT scans showed unchanged subpleural lesions of 1-cm diameter. Except for morning stiffness of the fingers, the patient stayed well and had fully recovered without any residual neurological deficits. In September 1996 (day 221), the patient suffered a first relapse of TTP with hemolysis and thrombocytopenia, but without neurological signs. Plasma exchange and prednisone therapy were resumed successfully, but platelets decreased again after discharge from the hospital. When the platelet count decreased to 110 × 109/L, 2 mg vincristine IV was administered each on days 238 and 245, in addition to continued prednisone therapy. The platelet count increased and remained in the normal range for 11 weeks, when a second relapse of TTP occurred that was partially refractory to treatment with plasma exchange, additional FFP infusions, and vincristine. After vaccination against pneumococcal bacteriae and haemophilus influenzae and stabilization of the platelet count with intensified plasmatherapy, the patient underwent splenectomy on day 365, with continued postoperative plasma exchange, plasma infusion, and resumption of corticosteroid therapy. Platelets reached a maximum count of 876 × 109/L on day 382, and prednisone was tapered slowly and withdrawn on day 480. There was no relapse as of the last visit on September 18, 1997 (day 591), and the platelet count stabilized between 455 and 579 × 109/L during the last 7 months of observation.
Time course of the platelet count related to treatment with plasma exchange (PEx), plasma (FFP) infusion, packed erythrocytes (Ec), vincristine, prednisone, methylprednisolone, iloprost, and splenectomy.
Time course of the platelet count related to treatment with plasma exchange (PEx), plasma (FFP) infusion, packed erythrocytes (Ec), vincristine, prednisone, methylprednisolone, iloprost, and splenectomy.
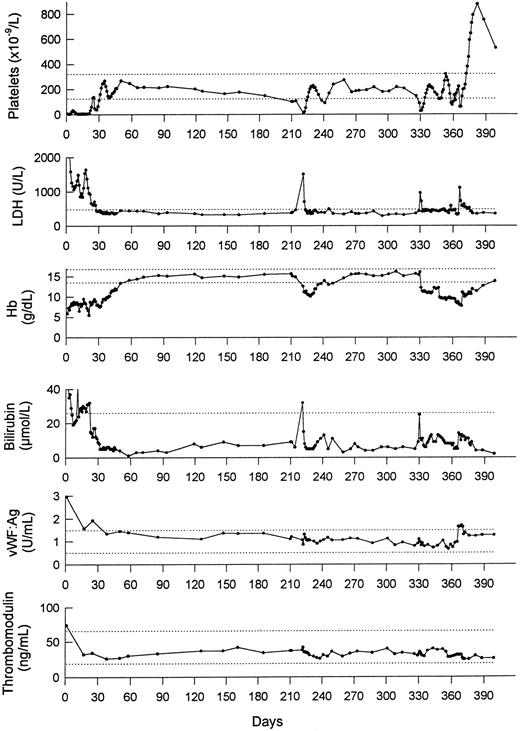
Laboratory parameters during 400 days after the first episode of TTP. LDH, lactate dehydrogenase; Hb, hemoglobin; vWF:Ag, vWF antigen. The dotted lines denote the limits of (upper) normal ranges.
Laboratory parameters during 400 days after the first episode of TTP. LDH, lactate dehydrogenase; Hb, hemoglobin; vWF:Ag, vWF antigen. The dotted lines denote the limits of (upper) normal ranges.
RESULTS
Upon admission to our hospital, TTP was diagnosed. Treatment and laboratory values during the course of the disease are presented in Figs 1 and 2. To examine whether acute episodes of TTP were associated with activation or damage of the endothelial cells, two endothelial cell markers, vWF:Ag and plasma thrombomodulin, were assayed during the course of the disease (Fig 2). Markedly elevated vWF:Ag (2.96 U/mL; normal range, 0.5 to 1.5 U/mL) was observed at the time of admission to our hospital. The level of vWF:Ag remained within normal limits before the relapses of thrombocytopenia and was only slightly increased immediately after splenectomy. The level of plasma thrombomodulin was moderately increased at presentation (74 ng/mL; normal range, 19 to 66 ng/mL), whereas normal values were observed during the subsequent course of the disease. These results suggest that the relapses of TTP were not associated with marked activation or damage of the endothelial cells. No bacterial or viral infection was noted immediately before or during the acute events. Normal levels of fibrinogen were found during the whole observation period (data not shown).
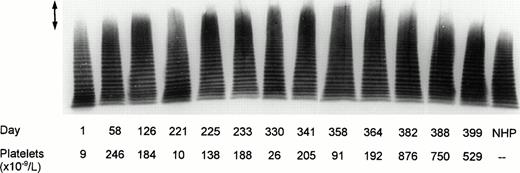
Analysis of vWF multimeric pattern in patient plasma is shown in Fig 3. Patient samples collected on days 126 and 225 to 364 contained considerable proportions of unusually large vWF multimers that disappeared after splenectomy. Two plasma samples, drawn at the onset of the first (day 1) and of the second (day 221) TTP episode comprised decreased amounts of large multimers and, in addition, contained increased amounts of low molecular weight forms of vWF suggesting that, during acute TTP, there occurred not only consumption of the unusually large vWF species but also enhanced degradation of the normal vWF multimers.
Multimeric analysis of plasma vWF in SDS-1% agarose gel. Equal amounts of vWF (0.0025 U vWF:Ag) from plasma samples of the patient and from NHP were applied on top of the gel. Days of plasma collection and the platelet counts on the respective days are shown beneath the immunoblot of the electrophoretic gel. The arrow (↕) denotes the unusually large vWF multimers.
Multimeric analysis of plasma vWF in SDS-1% agarose gel. Equal amounts of vWF (0.0025 U vWF:Ag) from plasma samples of the patient and from NHP were applied on top of the gel. Days of plasma collection and the platelet counts on the respective days are shown beneath the immunoblot of the electrophoretic gel. The arrow (↕) denotes the unusually large vWF multimers.
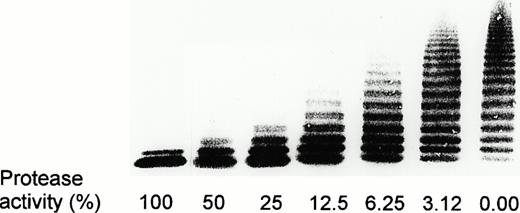
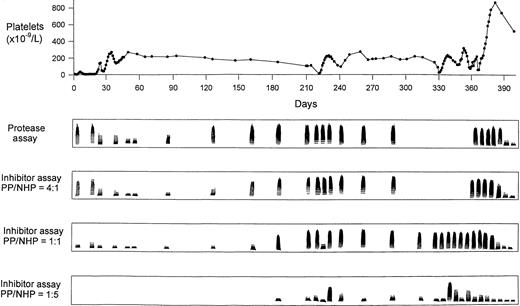
Calibration of the vWF-cleaving protease assay was performed with dilutions of NHP by defining the activity in a 1:20 dilution of NHP as 100% activity (Fig 4). Figure 5 shows activities of the vWF-cleaving protease in the patient plasma and the levels of an inhibitor of this protease in the course of the observation period. Deficient protease activity is represented by the presence of high molecular weight multimers remaining after incubation of purified vWF substrate with diluted patient plasma, whereas decreasing proportions of the large multimers reflect increasing protease activity. Screening for a protease inhibitor was performed by measuring the protease activity in mixtures of patient and normal plasma at three different ratios (4:1, 1:1, or 1:5 vol/vol) that had been preincubated for 10 minutes at 37°C. Results in Fig 5 indicate the absence of protease activity at the occasion of the first TTP episode. Combined treatment by several therapeutic regimens (Fig 1) led to appearance of protease activity that persisted for about 2 months after termination of plasma exchange/infusion. The disappearance of the protease was concomitant with a gradual decrease in the platelet count. No activity of vWF-cleaving protease was detectable from month 4 until after splenectomy. Patient plasma inhibited the vWF-cleaving protease activity of NHP in a 4:1 mixture, both at the time of the first TTP event as well as during months 5 through 12. Using 1:1 mixtures of patient plasma and NHP, the presence of the inhibitor was detectable after month 6. Its level was only transiently depressed during extensive plasma exchange at the occasion of the first relapse. However, the inhibitor concentration significantly increased a few days after cessation of plasma exchange, as shown by results of the inhibitor assay using 1:5 mixtures of patient plasma and NHP on days 233, 338, and 358 (Figs 1 and 5). It should be noted that the first relapse of TTP (days 221 to 230) was initially treated only with plasma exchange/infusion and prednisone. This treatment resulted in a temporary recovery of the platelet count. Subsequently, the plasmapheresis was discontinued and two injections of 2 mg vincristine each on days 238 and 245 were administered (Fig 1). The platelet count normalized within about 1 week after the first vincristine injection and remained in the normal range until the next relapse 3 months later, although the level of the protease inhibitor was not affected. Three weeks after splenectomy and under prednisone therapy, the protease inhibitor completely disappeared and full activity of the vWF-cleaving protease was observed as of the last visit on day 591, ie, 111 days after withdrawal of prednisone (not shown).
Calibration of the vWF-cleaving protease assay. The 1:20 dilution of NHP was denoted as 100% activity. TBS/1 mmol/L Pefabloc was used to prepare further dilutions (1:40 to 1:640). The lane denoted as 0.00 represents multimeric distribution of the vWF substrate that had been incubated with dilution buffer alone.
Calibration of the vWF-cleaving protease assay. The 1:20 dilution of NHP was denoted as 100% activity. TBS/1 mmol/L Pefabloc was used to prepare further dilutions (1:40 to 1:640). The lane denoted as 0.00 represents multimeric distribution of the vWF substrate that had been incubated with dilution buffer alone.
Activity of vWF-cleaving protease and the level of its inhibitor in comparison with the platelet count during 400 days of observation. The inhibitor assays were performed after 10 minutes of preincubation of patient plasma (PP) and NHP at three different ratios (4:1, 1:1, and 1:5 vol/vol).
Activity of vWF-cleaving protease and the level of its inhibitor in comparison with the platelet count during 400 days of observation. The inhibitor assays were performed after 10 minutes of preincubation of patient plasma (PP) and NHP at three different ratios (4:1, 1:1, and 1:5 vol/vol).
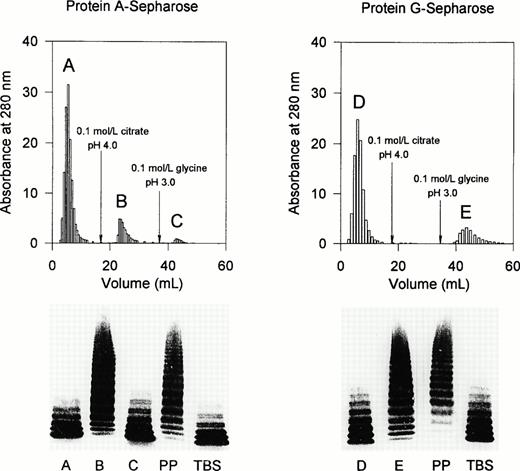
To examine the nature of the inhibitor, a plasma sample collected on day 221 was applied onto protein A-Sepharose (Fig 6). The breakthrough fraction denoted as peak A and the IgG fraction in peak C contained no protease inhibitor. The latter was present in the IgG fraction eluted as peak B by an acid citrate buffer (pH 4.0). Similar results were observed using affinity chromatography on protein G-Sepharose. The protease inhibitor was completely adsorbed to protein G-Sepharose and was subsequently eluted with glycine-HCl buffer at pH 3.0.
Affinity chromatography of the inhibitor of the vWF-cleaving protease on protein A-Sepharose and protein G-Sepharose. Two milliliters of patient plasma (PP) obtained on day 221 was applied onto a column of either protein A-Sepharose or protein G-Sepharose (4 mL bed volume each). Protease activity was assayed in mixtures of NHP (4 vol) and peak fractions eluted from the chromatographic columns (1 vol). A, B, C, D, and E refer to the chromatographic fractions eluted; TBS, Tris-buffered saline.
Affinity chromatography of the inhibitor of the vWF-cleaving protease on protein A-Sepharose and protein G-Sepharose. Two milliliters of patient plasma (PP) obtained on day 221 was applied onto a column of either protein A-Sepharose or protein G-Sepharose (4 mL bed volume each). Protease activity was assayed in mixtures of NHP (4 vol) and peak fractions eluted from the chromatographic columns (1 vol). A, B, C, D, and E refer to the chromatographic fractions eluted; TBS, Tris-buffered saline.
DISCUSSION
In patients with TTP, platelet aggregates obstruct the arterioles and capillaries of various organs. Brain ischemia and infarction can occur, along with thrombocytopenia, red blood cell fragmentation, and intravascular hemolysis.1,2,15 An abnormal interaction between the vascular endothelium and circulating platelets is suspected as the crucial defect in acute TTP. However, the primary event responsible for this abnormality has not yet been elucidated. Siddiqui and Lian16 isolated from plasma of patients with TTP a platelet agglutinating protein with a molecular weight of 37 kD. Other investigators17,18 established in plasma and serum of patients with TTP a calcium-dependent cysteine protease that may be responsible for platelet activation and aggregation. Abnormalities of vWF have been found in patients with TTP,3,4,19-21 but their interpretations widely differed. Thus, Moake et al3and Charba et al19 attributed the enhanced intra-vascular platelet clumping to the extremely large polymers of vWF. On the other hand, Moore et al21 suggested that increased platelet aggregation in TTP is due to the low molecular weight forms of vWF produced by platelet calpain. Furthermore, Mannucci et al4observed in TTP patients increased proteolytic degradation of vWF subunits despite the presence of unusually large vWF multimers in unreduced SDS-electrophoretic gels.
We have recently reported deficient activity of the vWF-cleaving protease11,12 in four patients, which included two brothers, with chronic relapsing TTP,13 supporting the hypothesis3 that the unusually large vWF multimers are responsible for formation of platelet aggregates in the circulating blood. In none of these patients was an inhibitor of or an antibody against the vWF-cleaving protease detected.13
The patient with relapsing TTP described in the present study showed a lack of the vWF-cleaving protease at the occasion of the first TTP event. This episode was associated with an initially increased concentration of vWF:Ag and plasma thrombomodulin, although no inciting agent that might be associated with a vascular lesion was established preceding the acute TTP. During the observation period of 400 days, the patient suffered two severe relapses of TTP, and in none of these bouts were increased levels of vWF:Ag or thrombomodulin, two markers of endothelial cell activation or damage, noted.
Unusually large vWF multimers were detected within the whole observation period before splenectomy, except in the two plasma samples collected at the time of the first (day 1) and of the second (day 221) acute TTP episode, with platelet counts of ≤10 × 109/L on both occasions. These results comply with findings of Moake et al,3 who reported that the unusually large vWF multimers disappeared from plasma of TTP patients during relapse and reappeared during remission, suggesting that they were consumed in the process of platelet aggregation. Moreover, both plasma samples collected from our patient during severe thrombocytopenia contained an abnormally high proportion of low molecular weight vWF, indicating enhanced proteolytic degradation of vWF. Because the specific vWF-cleaving protease in our patient was completely lacking at both occasions, it appears that vWF was degraded by another protease. A likely candidate responsible for increased vWF breakdown may be the calpain-like calcium-dependent cysteine protease that has been found in the sera of patients with TTP.17,18 The latter protease is apparently not involved in normal proteolytic processing of vWF, as shown by Moore et al,21 who demonstrated that the degradation products of vWF generated by calpain are strikingly different from those found in normal plasma. We have recently shown that the specific vWF-cleaving protease is distinct from calpain and that platelet calpain does not cleave vWF under conditions of our assay of the vWF-cleaving protease.11
The initial lack of the specific vWF-cleaving protease in our patient was caused by the presence of an inhibitor. Prolonged treatment by plasma exchange and plasma infusion, vincristine, and corticosteroid therapy resulted in depression of this inhibitor with transient appearance of the protease activity. In the further course of the disease, the platelet count gradually decreased with concomitant reappearance of the inhibitor and disappearance of the vWF-cleaving protease activity. The first relapse of acute TTP was initially treated only with plasma exchange, plasma infusion, and prednisone. As a result of plasma replacement, the platelet count was transiently normalized but decreased again along with a brief increase of the inhibitor level. Subsequent injections of two doses of vincristine led to normalization of the platelet count within the following week.
The patient plasma collected during the first relapse was submitted to affinity chromatography on protein A-Sepharose as well as on protein G-Sepharose. Binding of the inhibitor to insolubilized protein A and protein G suggests that the inhibitor is an Ig. Injection of vincristine resulted in improved platelet counts without affecting the levels of the protease activity or its inhibitor. Although vincristine has been reported to act as a potent immunosuppressive agent,22 its mode of action in our patient appears to be caused by a direct effect on the platelets. Vincristine has been shown to interfere with polymerization of microtubules associated with platelet membranes.23 Furthermore, Matera et al24 reported that low concentrations (0.1 μg/mL) of vincristine markedly inhibited the ADP- and collagen-induced platelet aggregation in vitro. Vincristine also inhibits generation of thromboxane A2 on platelet stimulation with collagen or thrombin, whereas it has no influence on the release of prostacyclin from rat aorta.25 The experience with vincristine in our patient is in agreement with that of other investigators who reported that treatment with vincristine resulted in complete remission in several patients with TTP refractory to plasma exchange.26-28
It has been suggested that in some patients TTP might be mediated by an autoimmune mechanism, because it could be controlled clinically with high doses of glucocorticoids.15 Complete remission of TTP in a patient treated by immunosuppressive therapy with prednisone and azathioprine29 led the investigators to hypothesize that the patient had developed an autoantibody directed against the depolymerase converting the unusually large vWF multimers to somewhat smaller forms normally found in the circulation. Our demonstration that the vWF-cleaving protease is inactivated by a circulating autoantibody confirms the above-noted hypothesis. Removal of IgG by 4–12 protein A immunoabsorption treatments led to durable complete remission of TTP in 7 of 10 patients that had previously failed to respond to therapeutic plasma exchanges.30
Infusion of FFP or its cryosupernatant fraction was found to be effective in preventing or reversing acute bouts of TTP31,32 by providing an activity required for degradation of unusually large vWF multimers. Only about 70% to 80% of patients respond to plasma exchange with FFP replacement,32 and the explanation for this variable response to therapy has thus far remained unclear. It appears that, in a subset of patients, the lack of vWF-cleaving protease activity is caused by an inhibitor against this protease. Our preliminary study on protease deficiency in TTP/HUS provides evidence for complete lack of protease activity and presence of protease inhibitor(s) in about two thirds of the patients, whereas no deficiency of vWF-cleaving protease was found in 120 normal subjects (unpublished data). Results of the present study suggest that the level of inhibitor may even become increased after plasma therapy. For patients who are refractory to treatment with plasma exchange, treatment with glucocorticoids, vincristine, and/or splenectomy seems to be a promising therapy.
In conclusion, our study indicates that an autoantibody inhibiting the vWF-cleaving protease activity may predispose to TTP similarly to constitutional deficiency of the vWF-cleaving protease.13We propose that the described assay of the vWF-cleaving protease activity and of its inhibitor may provide useful information regarding the prognosis and necessary treatment in patients with TTP.
Supported by grants from the Swiss National Science Foundation (Grant No. 32-47033.96); from the Central Laboratory, Blood Transfusion Service, Swiss Red Cross; from IMMUNO, Vienna, Austria; and from the Malcolm Hewitt Wiener Foundation (New York, NY).
Address reprint requests to Miha Furlan, PhD, Central Hematology Laboratory, University Hospital, Inselspital, CH-3010 Bern, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal