Platelet factor 4 (PF4) has been recognized as an inhibitor of myeloid progenitors. However, the mechanism of action of this chemokine remains poorly understood. The present study was designed to determine its structure/function relationship. A series of peptides overlapping the C-terminal and central regions of PF4 were analyzed in vitro for their action on murine hematopoietic progenitor growth to assess the minimal sequence length required for activity. The peptides p17-58 and p34-58 possessed an increased hematopoietic inhibitory activity when compared with PF4, whereas the shorter peptides p47-58 and p47-70 were equivalent to the native molecule and the peptide p58-70 was inactive. The PF4 functional motif DLQ located in 54-56 was required for the activity of these peptides. The peptide p34-58 impaired to a similar extent the growth of colony-forming unit-megakaryocyte (CFU-MK) as well as burst-forming unit-erythroid (BFU-E) and colony-forming unit–granulocyte-macrophage (CFU-GM), whereas PF4 was more active on CFU-MK. In the experiments using purified murine CD34+ marrow cells, statistically significant inhibition induced by p34-58 was shown at concentrations of 2.2 nmol/L or greater for progenitors of the three lineages, whereas that induced by PF4 was seen at 130 nmol/L for CFU-MK and 650 nmol/L for CFU-GM and BFU-E, indicating that the p34-58 acts directly on hematopoietic progenitors and its activity is approximately 60- to 300-fold higher than PF4. The p34-58, unlike PF4, lacked affinity for heparin and its inhibitory activity could not be abrogated by the addition of heparin. In addition, an antibody recognizing p34-58 neutralized the activity of p34-58 but not whole PF4 molecule. These results demonstrate that PF4 contains a functional domain in its central region, which is independent of the heparin binding properties, and provide evidence for a model of heparin-dependent and independent pathways of PF4 in inhibiting hematopoiesis.
PLATELET FACTOR 4 (PF4) is a 7.8-kD protein that is synthesized by megakaryocytes, stored in α-granules as a noncovalent tetramer and released from activated platelets. Each monomer has a conformational flexible N-terminal region that is anchored by two disulfide bridges to the protein core, which consists of three antiparallel β-strands and a carboxyl-terminal α-helix.1-5 PF4 shares 30% to 40% amino acid homology and general structural identity with the members of CXC chemokines family, including β-thromboglobulin (βTG) and its N-terminal cleavage product neutrophil-activating protein-2 (NAP2), interleukin-8 (IL-8), human proto-oncogene Gro/melanocyte growth-stimulating activity (Gro/MCSA), and interferon-inducible protein 10 (IP-10). In addition, these molecules have overlapping and some additive biologic activities in modulating inflammation, hemostasis, hematopoiesis, cell proliferation, angiogenesis, and glycosaminoglycan activity.6-10
PF4 has been recognized as inhibitor of hematopoiesis and angiogenesis.11-15 However, the mechanism of action of PF4 remains poorly understood, because no cell surface receptor has been yet identified. The basic nature of the C-terminus of PF4, which confers to the molecule a high affinity for heparin and other sulfated glycans, has been considered to be responsible for most of the activities of PF4.16 In human erythroleukemia (HEL) cells, PF4 directly inhibits the growth of HEL cells by fixation on heparan sulfate proteoglycans on the cell surface. The binding to cells and the inhibitory effect of PF4 can be inhibited by addition of exogenous heparin or other glycosaminoglycans (GAG), by treatment with heparinase and heparitinase as well as the inhibitors of proteoglycan synthesis.17,18 However, PF4 sequence p58-70, which contains the main heparin-binding domain, does not interfere with megakaryocytopoiesis in vitro and in vivo in mice.19Furthermore, a PF4 analogue lacking affinity for heparin has been found to retain the ability of PF4 to suppress angiogenesis in vivo.20 Recent studies on structure-function relationships of chemokines have shown that the two DLQ motives of PF4 located in positions 7-9 and 54-56 are necessary for this protein to inhibit myeloid progenitor proliferation.21 All these observations suggest that functional determinants other than the heparin binding sequence may also be involved in the mechanism of action of PF4. To address this issue, a series of peptides overlapping the C-terminal and central domains of PF4 were tested for inhibitory activity towards hematopoietic progenitor cells. We provide evidence here for a new pathway of hematopoietic inhibitory action of PF4 through its central functional domain independent of the heparin binding properties of PF4.
MATERIALS AND METHODS
Reagents and Growth Factors
Unfragmented heparin was purchased from Serbio Laboratories (France). Recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) was provided by Beite Kaito (Paris, France) and recombinant murine stem cell factor (rmSCF) was provided by R & D (Oxford, UK). Both growth factors were diluted in phosphate-buffered saline (PBS) + 0.01% bovine serum albumin (BSA) and stored at −20°C.
PF4 Purification
Highly purified human PF4 extracted from platelet concentrates and synthetic peptides related to PF4 were provided by Serbio (Gennevilliers, France). Briefly, human PF4 was prepared from fresh human platelet concentrates, washed, and disrupted by three repetitive deep-freezing (−80°C) and thawing (30 minutes at 37°C) cycles. Platelet debris were removed after centrifugation at 5,000g and all the platelet released proteins were recovered in the supernatant. This supernatant was used for the preparation of PF4 as described by Handin and Cohen,22 with the exception that a final gel filtration step was performed on Superdex 75, in 0.05 mol/L Tris, 0.5 mol/L NaCl buffer at pH 7.5. This preparation was highly purified (single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) and contained no fibronectin, fibrinogen, thrombospondin, or von Willebrand factor. Both Western blot and direct enzyme-linked immunosorbent assay (ELISA) analysis showed no contamination by human transforming growth factor β (TGFβ).12 13 PF4 was originally stored in a glycine buffer saline at a concentration of 100 μg/mL and then lyophilized and reconstituted with 1 mL distilled water.
Peptide Synthesis
Peptides were synthesized using standard solid-phase methodology and purified by high-performance liquid chromatography (HPLC) using a C18 column and a 0% to 80% linear acetonitrile gradient in 0.1% trifluoroacetic acid. Amino acid sequences of the peptides are shown in Fig 1.
Amino acid sequences of human PF4 and peptides tested for inhibitory activity on the formation of CFU-MK, CFU-GM, and BFU-E from total cells of bone marrow and purified CD34+ bone marrow cells.
Amino acid sequences of human PF4 and peptides tested for inhibitory activity on the formation of CFU-MK, CFU-GM, and BFU-E from total cells of bone marrow and purified CD34+ bone marrow cells.
Antibodies
Rabbit anti-PF4 polyclonal antibodies were immunopurified using a PF4 column and then digested with pepsin to obtain the anti-PF4 F(ab′)2 fragments that were further purified by gel filtration using a Sephazyl S100 column. This fraction was subsequently applied onto a p34-58 affinity column. Although still exhibiting high anti-PF4 reactivity, the F(ab′)2 contained in the filtrate did not recognize the PF4 sequence 34-58, and the fraction was thus named anti-PF4(p34-58)−. The F(ab′)2 fragments bound onto p34-58 column were separately eluted from the affinity column and designated as the anti-p34-58 fraction.
Heparin Binding Properties of the Peptides
Various 125I-PF4 and 125I-p34-58 aliquots were incubated overnight with heparin cross-linked to agarose (Bio-Rad, Ivry sur Seine, France). One milliliter of NaCl at increasing concentrations (from 0 to 2.0 mol/L) was added to each sample. After 20 minutes of incubation at room temperature, the heparin-associated PF4 and p34-58 were spun down and radioactivity in the supernatants was quantified by γ-counting to determine the nonbound PF4 and p34-58 fractions.
Colony Assays of Hematopoietic Progenitors
Cell preparation.
Total and CD34+ bone marrow cells obtained from Balb/c mice were used in the present study. The mice (6- to 8-week-old male mice) were purchased from IFFA CREDO Laboratories (L'Arbresle, France) and maintained under standard housing conditions with water and commercial rodent chow. After mice were killed by cervical dislocation, the femurs were removed and the total bone marrow was expelled with 5 mL α medium (Eurobio, Paris, France).
For the purification of CD34+ cells, bone marrow cells were incubated with biotinylated rat anti-CD34 MoAb (RAM34; Pharmingen, San Diego, CA) for 30 minutes on ice, washed twice in PBS-BSA, and stained with fluorescein isothiocyanate (FITC)-conjugated streptavidin (Caltag, San Francisco, CA) for 30 minutes. Control cells were stained with biotinylated rat-IgG2a (clone R35-95; Pharmingen) and streptavidin-FITC. Viable cells were defined by forward (FSC) and side (SSC) light-scattering properties and exclusion of propidium iodide on dual-laser FACS Vantage cell sorter (Becton Dickinson Immunocytometry Systems, San Jose, CA). The visible laser output was set at 150 mW with emission at 488 nm and the emitted light was collected with 530/30 bandpass filter. Cells were collected in a tube with α medium containing 10% aplastic anemia serum (AAS) obtained by blood collection from pigs 5 days after 8 Gy total body irradiation and were counted and reanalyzed for purity.
Colony-forming unit-megakaryocyte (CFU-MK) assay.
Megakaryocytes and their progenitor cells were studied using a plasma clot system.23 24 Briefly, 2 × 105nucleated marrow cells or 24,000 CD34+ marrow cells were cultured in at least triplicate in Petri dishes (35 mm) in a total volume of 1 mL with 1% BSA (Sigma Chemical Co, St Louis MO), 10% bovine citrated plasma (GIBCO, Cergy-Pontoise, France), 1 × 10−4 mol/L 2-mercaptoethanol (Sigma Chemical), 0.34 mg CaCl2 (Prolabo, Paris, France), 15 U penicillin plus 15 μg streptomycin, and 10% AAS. PF4, various synthetic peptides, and antibodies were added exogenously just before the plasma-clot assay. Heparin (5 IU/dish) was added after the clot formation. The cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. After 7 days of culture, the dishes were fixed with 1% paraformaldehyde and stained for acetylcholinesterase to determine the number of colonies derived from CFU-MK.
Identification of colonies was performed as previously described.23 A CFU-MK–derived colony was defined as a cluster of three or more cells. Megakaryocytes and CFU-MK were counted using a computerized automatic image analysis. Briefly, this analysis system was based on acetylcholinesterase staining, a specific stain for murine bone marrow megakaryocytes, and an image capturing instrument with a computer program (see addendum).
Burst-forming unit-erythroid (BFU-E) and colony-forming unit–granulocyte-macrophage (CFU-GM) assays.
BFU-E and CFU-GM were assayed using a methylcellulose system as previously described.24 A total of 1 × 105 marrow nucleated cells/mL or 16,000 CD34+marrow cells/mL were plated in semisolid medium containing 0.8% methylcellulose, 10% AAS, 1 × 10−4 mol/L 2-mercaptoethanol (Sigma Chemical), 10 ng/mL rmSCF, and 10 ng/mL rmGM-CSF for BFU-E and CFU-GM assays. Quadruplicate cultures for each assay were incubated at 37°C in a humidified atmosphere of 5% CO2. A BFU-E colony (≥3 clusters of 20 cells) and CFU-GM colonies (≥50 cells) were scored under an inverted microscope at 5 days of culture.
Statistical Analysis
Results were expressed as the mean ± SEM for data from 3 or more separate experiments. The significance of the difference between groups was determined by the Student's t-test. * and # indicateP < .05 and P < .01, respectively.
RESULTS
Effects of PF4-Related Peptides on Megakaryocyte Colony Formation From Bone Marrow Cells
In an attempt to detect new functional domains of PF4, various peptides were synthesized (Fig 1). The longer peptide p17-58 contains two cysteines and one of the sequences DLQ, a functional motif located in position 54-56 of PF4. The peptide p34-58 corresponds to the C-terminal part of p17-58, and thus it still includes the two cysteines and the DLQ motif. Other peptides synthesized included four peptides of C-terminal region of PF4, the p47-70, p47-58, p47-55, and p58-70, which have been previously characterized by us.19
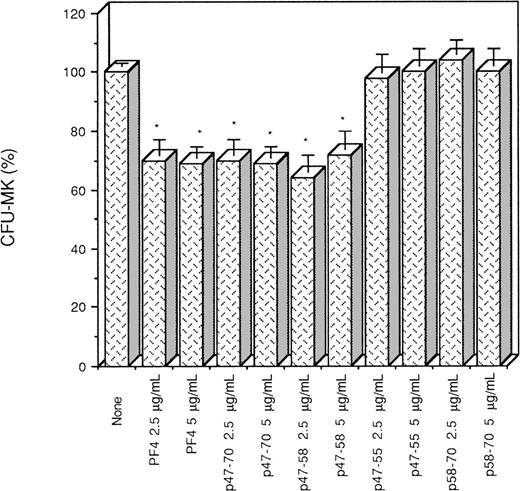
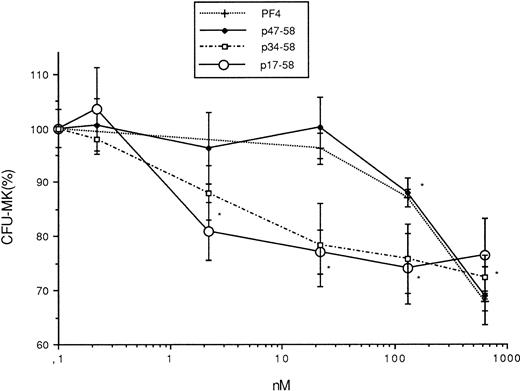
The capacity of these peptides to interfere with megakaryocytopoiesis was examined in vitro in comparison with PF4. It was found that p47-58 and p47-70, like PF4, significantly inhibited megakaryocyte colony formation from bone marrow cells to 30%, whereas p58-70 did not, suggesting that p47-58 and p47-70 retain the inhibitory activity of PF4 but p58-70 does not possess such an activity (Fig 2). Figure3 shows the results obtained from the experiments using a range of concentrations of p17-58 and p34-58 compared with PF4 and p47-58. As seen in Fig 3, a dose of at least 130 nmol/L (1 μg/mL) was required for PF4 to exert a significant inhibitory effect on the growth of CFU-MK. In contrast, 60-fold lower concentrations (2.2 nmol/L) of peptides p17-58 and p34-58 were sufficient to induce a similar effect on megakaryocytopoiesis. Further shortening of the peptide resulted in a loss of activity, because p47-58 inhibited the growth of CFU-MK at the same molar concentrations as PF4, suggesting that the central region of PF4 contains a functional domain responsible for a strong growth inhibitory activity. Because no statistical significant difference in the inhibitory activity was detected between peptides p17-58 and p34-58, further characterization of the central domain of PF4 was performed only using the shorter active peptide p34-58.
Effect of PF4 and various peptides (p47-55, p47-58, p47-70, and p58-70) of PF4 on the formation CFU-MK. Values are expressed as mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (75 colonies/mL) in control cultures.
Effect of PF4 and various peptides (p47-55, p47-58, p47-70, and p58-70) of PF4 on the formation CFU-MK. Values are expressed as mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (75 colonies/mL) in control cultures.
Effect of PF4 and various peptides (p17-58, p34-58, and p47-58) of PF4 (0, 0.2, 2.2, 22, 76.5, 130, and 630 nmol/L) on the formation CFU-MK. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (70 colonies/mL) in control cultures.
Effect of PF4 and various peptides (p17-58, p34-58, and p47-58) of PF4 (0, 0.2, 2.2, 22, 76.5, 130, and 630 nmol/L) on the formation CFU-MK. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (70 colonies/mL) in control cultures.
Structural Determinants for the Activity of p34-58
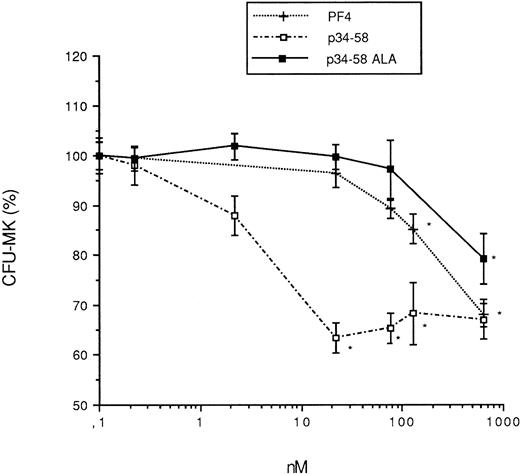
The motif DLQ is implicated in the inhibitory action of PF4. Thus, we synthesized a new peptide in which the DLQ in position 54-56 was mutated to ALA (Fig 1). In comparison to the native peptide, this peptide has lost most of the inhibitory activity on MK colony formation, suggesting that this motif was essential for the effect of the peptide (Fig 4). This is consistent with our previous observation on the peptide 47-58, in which the partial deletion of the DLQ sequence also resulted in the inactivation of peptide 47-55 (Fig 2).
Effect of PF4 and various peptides (p34-58 and p34-58ALA) on the formation CFU-MK. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (52 colonies/mL) in control cultures.
Effect of PF4 and various peptides (p34-58 and p34-58ALA) on the formation CFU-MK. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of megakaryocyte colonies (52 colonies/mL) in control cultures.
Because p34-58 contains two Cys (36 and 52), we could not discard the formation of dimeric or cyclic peptides with different inhibitory activities. To test this possibility, other peptides were synthesized in which the Cys36 or both Cys were replaced by Ser. As shown in Table 1, there was no difference in the effective inhibitory concentrations of p34-58S36 and p34-58S36S52 when compared with the native peptide. To examine the formation or intramolecular and intermolecular disulfide bridges, the native p34-58 peptide and its two mutated forms (p34-58S36 and p34-58S36S52) were analyzed by fast protein liquid chromatography (FPLC) using a TSK 3000 column. All peptides eluted at a molecular weight of about 2,500 Daltons, corresponding to the monomeric form. In addition, mass spectrometry analysis demonstrated for all peptides a molecular weight of 2,600 to 2,700 Daltons, confirming the monomeric presentation. This analysis also demonstrated that Cys residues, when present, are in the reduced form. Lastly, reduction studies, followed by a hydrophobic analysis, failed to show any modification of the molecular presentations of these peptides. All these data suggest that these peptides are in the monomeric form and that the Cys residues, when present, are not oxidized.
Effect of Native and Mutated p34-58 on CFU-MK, CFU-GM, and BFU-E Growth
| . | CFU-MK . | CFU-GM . | BFU-E . |
|---|---|---|---|
| Control | 100 ± 2.81 | 100 ± 3.55 | 100 ± 4.42 |
| p34-58 (22 nmol/L) | 63.4 ± 3.1-150 | 64.1 ± 2.9-150 | 65 ± 5.9-150 |
| p34-58S36 (22 nmol/L) | 70.3 ± 2.2-150 | 59.3 ± 4.1-150 | 62 ± 3.7-150 |
| p34-58S36S52 (22 nmol/L) | 68.0 ± 3.67-150 | 64.5 ± 5-150 | 68.3 ± 3.6-150 |
| . | CFU-MK . | CFU-GM . | BFU-E . |
|---|---|---|---|
| Control | 100 ± 2.81 | 100 ± 3.55 | 100 ± 4.42 |
| p34-58 (22 nmol/L) | 63.4 ± 3.1-150 | 64.1 ± 2.9-150 | 65 ± 5.9-150 |
| p34-58S36 (22 nmol/L) | 70.3 ± 2.2-150 | 59.3 ± 4.1-150 | 62 ± 3.7-150 |
| p34-58S36S52 (22 nmol/L) | 68.0 ± 3.67-150 | 64.5 ± 5-150 | 68.3 ± 3.6-150 |
Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. One hundred percent (100%) corresponds to the control value (PBS) (52 CFU-MK/mL, 57 CFU-GM/mL, and 56 BFU-E/mL).
P < .01 as compared with control value determined by the Student's t-test.
Inhibitory Action of PF4 and Its Central Domain on Other Hematopoietic Lineages
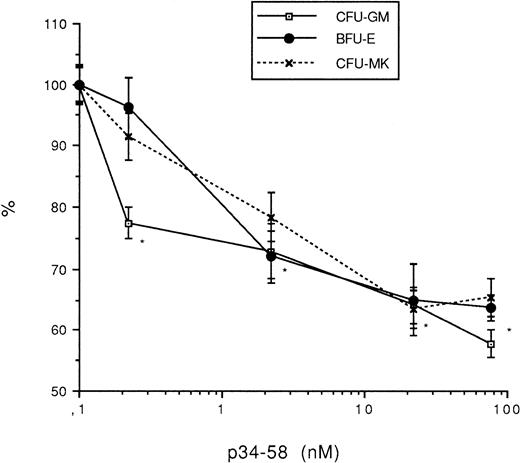
The effects of p34-58 on the growth of other hematopoietic lineages were next analyzed. Murine bone marrow cells were seeded into methylcellulose in the presence of appropriate growth factors and the number of colonies was determined in the presence or the absence of PF4 or p34-58. As reported in Fig 5, the peptide p34-58 impaired to a similar extent the growth of CFU-MK as well as BFU-E and CFU-GM at a concentration of at least 2.2 × 10−9 mol/L, optimally 8.64 × 10−8 mol/L.
Effect of peptide p34-58 (0, 0.2, 2.2, 22, 76.5, 130, and 630 nmol/L) on the formation CFU-MK, CFU-GM, and BFU-E. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of colonies derived from CFU-MK (70 colonies/mL), CFU-GM (84/mL), and BFU-E (64/mL).
Effect of peptide p34-58 (0, 0.2, 2.2, 22, 76.5, 130, and 630 nmol/L) on the formation CFU-MK, CFU-GM, and BFU-E. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to the number of colonies derived from CFU-MK (70 colonies/mL), CFU-GM (84/mL), and BFU-E (64/mL).
Effect of PF4 and p34-58 on Colony Formation From Purified CD34+ Marrow Cells
We next studied the effects of PF4 and p34-58 on the growth of purified CD34+ progenitor population. The murine bone marrow CD34+ population was enriched 17- to 20-fold by flow cytometry sorting. The purity of the isolated CD34+population was 70% to 80%. The enriched CD34+ population was cultured as described above, and its capacity to form colonies in the presence of increasing doses of PF4 or its derived peptides was examined.
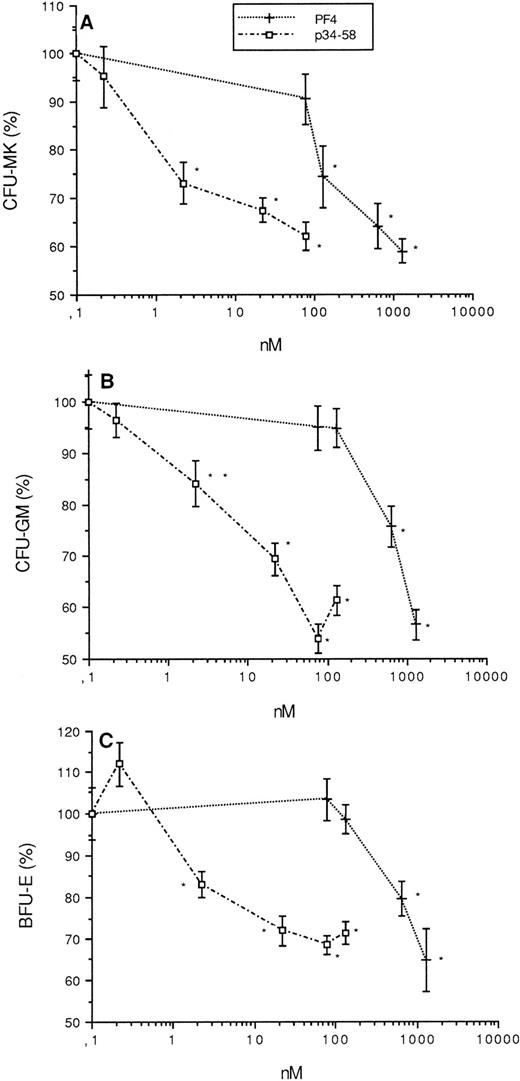
As seen in Fig 6, PF4 and p34-58 retained their ability to inhibit the growth of CFU-MK as well as CFU-GM and BFU-E colonies derived from purified CD34+ cells, similar to their inhibition of colonies derived from total bone marrow cells. Statistically significant inhibition induced by p34-58 was shown at concentrations of 2.2 nmol/L or greater for progenitors of three lineages, whereas that induced by PF4 was seen at 130 nmol/L (1 μg/mL) for CFU-MK and 650 nmol/L (5 μg/mL) for CFU-GM and BFU-E, respectively. These data indicate that p34-58, like PF4, acts directly on hematopoietic progenitors. However, its inhibitory activity is approximately 60-fold for CFU-MK and 300-fold for CFU-GM and BFU-E higher than PF4.
Effect of PF4 (0, 76.5, 130, 630, and 1,300 nmol/L) and p34-58 (0, 0.2, 2.2, 22, 76.5, and 130 nmol/L) on the formation of CFU-MK (A), CFU-GM (B), and BFU-E (C) from purified CD34+of bone marrow. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student'st-test. One hundred percent (100%) corresponds to 60 CFU-MK colonies/mL, 70 CFU-GM colonies/mL, and 60 BFU-E/mL, which were grown from control cultures.
Effect of PF4 (0, 76.5, 130, 630, and 1,300 nmol/L) and p34-58 (0, 0.2, 2.2, 22, 76.5, and 130 nmol/L) on the formation of CFU-MK (A), CFU-GM (B), and BFU-E (C) from purified CD34+of bone marrow. Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. *P < .01 as compared with control value determined by the Student'st-test. One hundred percent (100%) corresponds to 60 CFU-MK colonies/mL, 70 CFU-GM colonies/mL, and 60 BFU-E/mL, which were grown from control cultures.
Effect of Various Anti-PF4 Antibodies on CFU-MK Growth
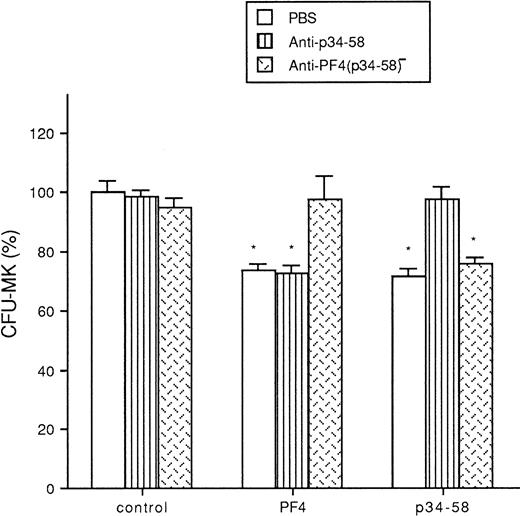
It was found from three experiments that the anti-p34-58, the F(ab′)2 fragments recognizing specifically the peptide p34-58, represents only approximately 1.5% of initial immunopurified anti-PF4 polyclonal antibody, suggesting that in the native PF4 molecule this sequence may be masked. Specificity of the reactivities of the anti-p34-58 and the anti-PF4(p34-58)− [the anti-PF4 F(ab′)2 antibody depleted of the anti-p34-58 fraction] were documented by ELISA (results not shown). The two F(ab′)2 fragment preparations still recognized native PF4 coated in the microassay plate. The anti-p34-58 fraction strongly reacted with insolubilized p34-58 peptide, whereas the anti-PF4(p34-58)− recognized whole PF4 but not p34-58. When added to the cultures, the anti-PF4 (p34-58)− neutralized the inhibitory effect of PF4 on the growth of megakaryocyte progenitor cells but not that of p34-58. In contrast, the antip34-58 F(ab′)2 fragments did not block the effect of PF4 but effectively reversed the inhibition induced by p34-58 (Fig 7).
Effect of anti-p34-58 and anti-PF4 (p34-58)− antibodies in presence of PF4 or p34-58 on the formation of and CFU-MK from total cells of bone marrow. Values are expressed as the mean ± SEM of triplicate determination obtained from three independent experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to 91 CFU-MK colonies/mL of control cultures.
Effect of anti-p34-58 and anti-PF4 (p34-58)− antibodies in presence of PF4 or p34-58 on the formation of and CFU-MK from total cells of bone marrow. Values are expressed as the mean ± SEM of triplicate determination obtained from three independent experiments. *P < .01 as compared with control value determined by the Student's t-test. One hundred percent (100%) corresponds to 91 CFU-MK colonies/mL of control cultures.
Effect of Heparin on the Inhibitory Properties of PF4 and Its Derived Peptides
Although the PF4 C-terminal heparin binding domain is not present in the p34-58 peptide, we tested if heparin could still modulate the inhibitory action of the peptide. As seen in Table 2, the addition of heparin (5 IU/dish) almost completely neutralized the in vitro inhibitory effect of PF4 on the growth of CFU-MK. On the contrary, heparin had no effect on the inhibitory activity of p34-58 (at 2.2 nmol/L).
Effect of PF4 and p34-58 onCFU-MK Growth in the Presence or Absence of Heparin
| . | 0 U/mL Heparin . | 5 U/mL Heparin . |
|---|---|---|
| Control | 100 ± 6.6 | 112 ± 6.6 |
| PF4 | 66.7 ± 2.1* | 99.2 ± 2.9 |
| p34-58 | 65.8 ± 3.7* | 67.9 ± 7.1* |
| . | 0 U/mL Heparin . | 5 U/mL Heparin . |
|---|---|---|
| Control | 100 ± 6.6 | 112 ± 6.6 |
| PF4 | 66.7 ± 2.1* | 99.2 ± 2.9 |
| p34-58 | 65.8 ± 3.7* | 67.9 ± 7.1* |
Values are expressed as the mean ± SEM of triplicate determination obtained from three separate experiments. One hundred percent (100%) corresponds to the control value (PBS) (45 CFU-MK/mL). Formation of CFU-MK from PF4 (630 nmol/L) or p34-58 (22 nmol/L) treated bone marrow cells. Heparin was added after plasma-clot formation.
P < .01 as compared with control value determined by the Student's t-test.
The heparin-binding affinity experiments were performed as another approach to determine the relationship between heparin and PF4 as well as related peptides. The results show that PF4, as previously reported, was effectively retained onto heparin-coated beads and dissociated from heparin by NaCl at a starting concentration of 0.5 mol/L. At 0.75 mol/L, NaCl eluted 50% of bound PF4. In contrast, the peptides p34-58 and p17-58 did not bind to heparin.
DISCUSSION
The present work provides evidence for a new functional domain in the central region of PF4, which is implicated in the hematopoietic inhibitory properties of this chemokine and is active in a heparin-independent manner. This is based on the following observations. (1) The peptide p34-58 possesses a 60- to 300-fold increased inhibitory activity than PF4 on hematopoietic colony formation from either bone marrow or purified CD34+ cells. (2) This peptide similarly inhibits the growth of CFU-MK as well as CFU-GM and BFU-E, whereas PF4 is more active on CFU-MK. (3) Unlike PF4, p34-58 lacks affinity for heparin and its inhibitory activity cannot be abrogated by heparin. (4) The antibody against p34-58 neutralizes the inhibitory activity of p34-58 but not that of native PF4, suggesting that the region 34-58 is poorly or not accessible on the native molecule. (5) Preservation of the DLQ motif in position 54-56 is essential for the activity of p34-58.
PF4 has previously been demonstrated to be an important negative regulator of hematopoiesis, particularly megakaryocytopoiesis.11-13 The exact mechanism of action of PF4 remains to be elucidated. The basic nature of the C-terminus of PF4 confers to the molecule a high affinity for heparin and other sulfated glycans, which was considered to be involved in the mechanism of action of PF4. Accordingly, heparin abrogates the inhibitory effect of PF4 on myeloid colony formation. But other domains of PF4 may be implicated in the functionality of this hematopoietic regulator. Recent studies on structure-function relationships of chemokines have shown that the DLQ motifs of PF4 located in positions 7-9 and 54-56 are necessary for this protein to inhibit myeloid progenitor proliferation.21
Several approaches were therefore made to determine functional domains of PF4 implicated in regulating negatively hematopoiesis. First, the effects of the peptides corresponding to the central and C-terminal domains of PF4 were studied under the same experimental conditions for culturing hematopoietic progenitors. We found in our system that p47-70 and p47-58 retained the inhibitory activity of PF4 but p58-70 was not active. To detect other functional determinants on PF4, we synthesized a longer peptide overlapping the sequence 17-58. This peptide significantly inhibited megakaryocyte colony formation from murine bone marrow at lower concentrations than the native molecule. In an attempt to define the sequence responsible for this strong activity, a shorter peptide including region 34-58 (p34-58) was designed and activity of this peptide was compared with p17-58, p47-58, and PF4. The p34-58 induced a similar inhibition of CFU-MK at comparable molar concentrations as p17-58. Maximum inhibition induced by p17-58 or p34-58 was similar to that caused by PF4, but it occurred at an approximately 60-fold lower molar concentration. The peptide p34-58 contains the motif DLQ at position 54-56. This sequence may be involved in the inhibitory properties of the peptide, because the mutation DLQ → ALA suppresses the inhibitory properties of the peptide. Furthermore, the partial deletion of the DLQ motif in the peptide p47-55 resulted in a complete loss of activity when compared with the peptide p47-58.19 Indeed, PF4 contains two DLQ sequences located at positions 7-9 and 54-56. Mutation of the first DLQ motif has been demonstrated to completely suppress the inhibitory action of PF4 on hematopoietic colony formation.21 In the same work, participation of the DLQ(54-56) in the activity of PF4 was not well established. Thus, our results support for the first time the functional importance of the DLQ motif located in position 54-56 within the region 34-58.
Although all the active peptides tested in our experiments contain the DLQ motif at position 54-56, effective doses of p47-58 were more similar to the native PF4 and lower than the longer peptides. Consequently, differences between p34-58 and p47-58 may be related to other structural features. PF4 contains three large loops that participate in joining the three β-sheet strands and the C-terminal α-helix.25 The p34-58 sequence forms two complete β-sheet strands in the PF4 molecule, whereas p47-58 includes only the sequence of the last β-sheet. The secondary structure of a PF4 peptide overlapping the domain 38-57 has been solved and shows that the native conformation is conserved.26 27 Thus, it is possible that the structures of peptides analyzed in the present study are quite similar to the native. Moreover, we have observed that the Cys 36 and 52, which do not associate together to form a disulfide bridge in the native PF4, remain in a reduced state in the peptide p34-58. These findings can also explain why the native as well as the two mutated peptides p34-58S36 and p34-58S36S52 keep the same enhanced activity.
Members of CXC chemokine family share a significant overall homology up to 25% to 40% and similar three-dimensional conformations. When the sequence 34-58 of PF4 is compared with the equivalent domain of other CXC chemokines, homologies increase to 65% to 70%. IL-8 also manifests hematopoietic inhibitory activity and acts synergistically with PF4 in inhibiting cell proliferation.21,28 Thus, we studied the biological importance of the central region of other chemokines and particularly IL-8. But, contrary to PF4, a peptide corresponding to the region 34-58 of IL-8 did not retain the inhibitory activity of the chemokine (results not shown). This finding suggests that the functionality of this region may be specific for PF4. This is consistent with the lack of activity on myeloid proliferation of a recombinant chimeric PF4 molecule in which the domain 51-60 was replaced by the corresponding IL-8 sequence.21
To examine the role of the domain 34-58 in the PF4 molecule, a second series of experiments was performed using two purified antibodies against PF4 and p34-58, respectively. Purified F(ab′)2 anti-p34-58 completely abrogated the inhibitory action of peptide p34-58, but it could not reverse the negative effect of PF4. This difference can be related to the accessibility of the antibody to its epitope within the whole PF4 molecule. Indeed, the anti-p34-58 was purified from a total F(ab′)2 anti-PF4 polyclonal antibody and was found to be present only in limiting amounts (∼1.5%). Previous observations on three-dimensional structures of PF4 have pointed out that the majority of 34-58 sequence is inside of PF4 tetramer.25Taken together, these data indicate that the p34-58 sequence is usually masked within whole PF4 molecule, probably explaining why the whole PF4 is much less active than its central peptides such as p17-58 and p34-58 observed in the present study. Indeed, it has been reported that the cleavage of PF4 at position between 16 and 17 results in a dramatic increase in endothelial cell proliferation inhibition.15This observation could be related to an exposition of the masked activity of the PF4 34-58 domain.
We have previously shown that PF4 directly impaired MK development from human cord blood CD34+ progenitor cells.25 In this study, we tested the p34-58 on an enriched CD34+population purified from murine bone marrow cells. Basically, no difference in the inhibitory profiles of either PF4 or p34-58 was observed on both enriched CD34+ cells and total bone marrow cells. It seems possible that p34-58 as well as PF4 may act directly on the growth of hematopoietic progenitors. It was interesting to note that the inhibitory effect of p34-58 was not restricted to the megakaryocytic lineage, because it also impaired the growth of BFU-E and CFU-GM from bone marrow cells in vitro. Concerning PF-4 specificity, it has been previously demonstrated by Gewirtz et al11 and our group12 13 and confirmed by the present work that a higher concentration is required to inhibit the development of erythropoietic or macrophage-granulopoietic colonies, compared with its effect on the megakaryocyte lineage. These results suggest that p34-58, in addition to its increased inhibitory activity, may act on hematopoiesis through a mechanism that is not exactly the same as PF4.
PF4 has high affinity for heparin and its activity to modulate cell proliferation as well as its binding to cell surface can be abrogated by heparin.17,18,29 C-terminal peptides including the major heparin domain still retained the inhibitory activity of the native molecule in hematopoietic11 and endothelial cell systems.14 15 These data suggest that PF4 and its related C-terminal peptides act on cell proliferation by heparin-dependent manner. To explore further mechanism of action of PF4 and its related peptides, the present work has studied the relationship between progenitor proliferation inhibitory activity and heparin binding properties of PF4 as well as related peptides. Although the inhibitory activity of PF4 on hematopoiesis is completely heparin-dependent, p34-58, unlike PF4, lacked affinity for heparin and its inhibitory activity on megakaryocytopoiesis could not be abrogated by heparin. Our results indicate that the p34-58 functions as an inhibitor of hematopoiesis in a heparin-independent manner.
The phenomenon that the progenitors of megakaryocytic lineage are more sensitive to the action of PF4 than those of other hematopoietic lineages is an interesting issue worthy of further elucidation. It has been shown that heparin and several other GAGs can significantly stimulate in vitro and in vivo megakaryocytopoiesis.30-32Endogenous GAGs are also implicated in the regulation of megakaryocyte growth, because treatment of cells with heparinase and chondroitinase or by the inhibition of proteoglycan synthesis inhibits the growth of megakaryocytic cells.18,30 However, at the same concentration, the GAGs failed to stimulate granulopoiesis in the presence of GM-CSF or IL-3,30 31 suggesting that hematopoietic progenitors of different lineage could have different sensitivity to GAGs. Concerning its high affinity for GAGs, PF4, unlike p34-58, which lacks affinity for heparin, may inhibit megakaryocytopoiesis more efficiently than granulopoiesis and erythropoiesis by interaction with endogenous GAGs in culture.
All these observations have led to establishment of a hypothetical model of action of PF4. Chemokine PF4 is able to inhibit directly the growth of hematopoietic progenitors through two action pathways. The first pathway is related to its heparin-binding properties. PF4 binds to GAGs present on cellular surface via its C-terminal cationic tail. Such a binding will block the interaction of cells with some growth factors, resulting in an inhibition of cell proliferation. This pathway is similar to the interaction of FGF-2 with cell surface heparin-like molecules, a model pointing out the action of FGF-2 dependent of its heparin binding properties.33 PF4 contains another important functional domain in its central region, which lacks affinity for heparin and acts in a heparin-independent manner. This domain is usually masked in whole PF4 molecule. This first interaction of PF4 with heparin-like molecules on cell surface may cause a change of conformation of this molecule, which facilitates a subsequent contact of the central function domain of PF4 with cells and induces ultimately inhibition of proliferation. Artificial removal of C-terminal and N-terminal regions from PF4 results in a full exposure of this functional domain and thus gives rise to a potent inhibitor of hematopoiesis.
In conclusion, the present study has provided evidence for a new functional domain in the central region of PF4, implicated in the inhibitory properties of this chemokine. Our findings can have important implications for the understanding of the mechanism of action of PF4 in modulating proliferation of hematopoietic progenitors. The future identification of the receptor of PF4 would therefore allow determination of the exact mechanism of action of PF4.
ADDENDUM
The full description of the computerized automatic image analysis system used to quantify megakaryocyte colonies is now in press.34
ACKNOWLEDGMENT
The authors thank Dr Jack Levin for his critical review of the manuscript and Valérie Drouet for her helpful technical assistance.
Supported by a grant from the Association pour la Recherche sur le Cancer to Z.C.H.
Address reprint requests to Jacques P. Caen, MD, IVS-Hôpital Lariboisière, 8 Rue Guy-Patin, 75475 Paris Cedex 10, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal