RETINOIDS AND IN particular retinoic acid are naturally occurring compounds that regulate the growth and differentiation of a variety of different cell types. The observation that retinoic acid induces the terminal differentiation of human acute promyelocytic leukemia (APL) cells both in vitro1 and in vivo2-4 has had a significant impact in clinical hematology and has altered our approach to therapy for this subtype of acute myelocytic leukemia (AML). Indeed, the addition of retinoic acid to conventional chemotherapy increases the apparent cure rate for patients with APL.5 6 The biologic effects of retinoic acid are generally mediated through specific ligand activated nuclear transcription factors, the retinoic acid receptors (RARs). Interestingly, the great majority of APLs exhibit a characteristic t(15;17) chromosome translocation that generates an aberrant RAR consisting of a chimeric protein fusing a portion of the PML gene on chromosome 15 with RARα on chromosome 17. Indeed, cytogenetic or molecular evidence for the presence of this PML-RARα fusion protein in patient leukemia cells is perhaps the best predictor of initial patient response to retinoic acid.
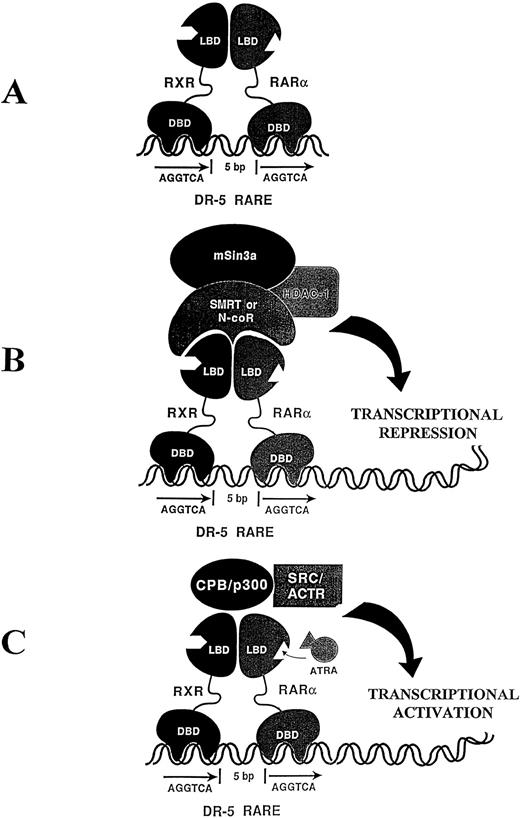
The molecular mechanisms underlying retinoic acid and RAR activity and how this may be disrupted in the PML-RARα APLs has been the subject of considerable investigation. The retinoic acid receptors consist of two distinct families, the RARs and RXRs, both exhibiting modular structures harboring distinct DNA binding and ligand binding domains. These receptors likely mediate their biologic effects by binding as RAR-RXR heterodimers to specific regulatory elements (RAREs), in particular target genes (Fig 1A). The RAR-RXR heterodimer interacts in the absence of ligand with a large ubiquitous nuclear protein (N-CoR), which mediates transcriptional repression through its interaction with other proteins, including mSin3A and histone deacetylase (HDAC)7,8 (Fig 1B). A current model explaining RAR activity suggests that the addition of ligand (RA) results in a distinct conformational change in the RAR-RXR complex, resulting in the release of the repressor protein complex and recruitment of transcriptional coactivators (Fig 1C).9-11Thus, the addition of ligand (RA) normally converts RAR-RXR from a transcriptional repressor (Fig 1B) to a transcriptionalactivator (Fig 1C). Although it is presently unclear exactly how the expression of the aberrant PML-RARα fusion protein leads to leukemia, the PML-RARα product may compete with the normal RARα for binding to RXR and thus interfere with normal RXR-RARα heterodimer formation in a dominant negative manner.12
(A) The retinoic acid receptors, RXR and RARα, harbor distinct DNA-binding domains (DBD) and ligand-binding domains (LBD) and bind as a heterodimer to a specific direct repeat separated by 5 bp making up the retinoic acid response element (RARE). (B) In the absence of ligand, the RXR-RARα heterodimer associates with a transcriptional repressor complex, including N-CoR, mSin3a, and a histone deacetylase (HDAC-1). (C) The addition of all-trans retinoic acid (ATRA) results in a conformational change in the RXR-RAR heterodimer resulting in the release of the repressor complex and recruitment of a transcriptional activator complex exhibiting histone acetyltransferase activity and associated transcriptional activation.
(A) The retinoic acid receptors, RXR and RARα, harbor distinct DNA-binding domains (DBD) and ligand-binding domains (LBD) and bind as a heterodimer to a specific direct repeat separated by 5 bp making up the retinoic acid response element (RARE). (B) In the absence of ligand, the RXR-RARα heterodimer associates with a transcriptional repressor complex, including N-CoR, mSin3a, and a histone deacetylase (HDAC-1). (C) The addition of all-trans retinoic acid (ATRA) results in a conformational change in the RXR-RAR heterodimer resulting in the release of the repressor complex and recruitment of a transcriptional activator complex exhibiting histone acetyltransferase activity and associated transcriptional activation.
An unusual variant of APL is associated with t(11;17), which fuses RARα to a gene on chromosome 11 termed promyelocytic leukemia zinc finger (PLZF). Although the PLZF-RARα leukemias morphologically and histochemically resemble the PML-RARα leukemias, in marked contrast to the PML-RARα leukemias, they are invariably resistant to retinoic acid and exhibit no differentiative/therapeutic response to this compound.13 14
In the present issue of Blood, Guidez et al15 now offer a molecular basis for this marked difference in the clinical response to retinoic acid of the PML-RARα versus the PLZF-RARα promyelocytic leukemias. In a series of convincing experiments, they demonstrate several major differences in the biochemical interactions of the N-CoR transcriptional repressor with the leukemia-specific PLZF-RARα and PML-RARα fusion proteins. First, PLZF, like RARα, but unlike PML, binds the transcriptional corepressor N-CoR. Thus, N-CoR can potentially bind both the RARα and PLZF regions of the chimeric PLZF-RARα fusion protein. In addition, Guidez et al15 demonstrate that this PLZF-RARα binding to N-CoR continues even in the presence of relatively high pharmacological (1 to 10 μmol/L) concentrations of retinoic acid. In contrast, the same concentrations of retinoic acid appear to dissociate N-CoR from the PML-RARα fusion protein. These differences in protein-protein interactions were demonstrated in vitro on glutathione sepharose beads and in vivo in yeast, but the implications for leukemia cells are clear. In PML-RARα leukemias, the addition of retinoic acid dissociates the N-CoR-mSin3-HDAC transcriptional repressor complex from PML-RARα, which relieves transcriptional repression and presumably activates genes involved in terminal differentiation of the promyelocytes. In contrast, in the PLZF-RARα leukemias, retinoic acid, even in relatively high doses, does not dissociate this complex and transcriptional repression is maintained, which presumably maintains the relatively undifferentiated leukemic state of the cells. Thus, these experiments provide a biochemical explanation for the marked difference in the clinical response of these subsets of promyelocytic leukemia to retinoids and emphasize the importance of the N-Cor, mSin3-HDAC transcriptional repression complex in maintaining the leukemic state.
Although these experiments provide persuasive biochemical evidence for the striking difference in the clinical behavior of the PLZF-RARα versus the PML-RARα leukemias, several critical questions remain. Why are such translocations involving RARα confined to the promyelocytic leukemias? The absence of RARα fusion proteins in any other type of leukemia suggests that translocations involving RARα are only leukemogenic in lineage-committed promyelocytes rather than in more immature hematopoietic progenitors, but the molecular basis for this discrepancy remains unclear. What is the normal function of the PML and PLZF proteins and why do these proteins predominate as the fusion partners for RARα in the promyelocytic leukemias? The sequence of these genes suggest they might act as DNA-binding transcription factors, and, under certain experimental conditions, PLZF and PML colocalize to specific nuclear multiprotein structures, the nuclear bodies (NBs).16 However, the specific genes that the PML and PLZF proteins might normally regulate remain unknown. Finally, if transcriptional repression is critical to maintaining the leukemic state as these experiments suggest, then what are the specific target genes that are being repressed in the leukemia cells? To put it another way, what are the genes that are activated by retinoic acid in the PML-RARα leukemias that trigger their terminal differentiation? Such target genes remain elusive, but their identification will be crucial to understanding why APL cells can be induced to undergo terminal granulocytic differentiation by retinoic acid whereas most other acute leukemias cannot.
Address reprint requests to Steven J. Collins, MD, Fred Hutchinson Cancer Research Center, Molecular Medicine Division, 1100 Fairview Ave N, Suite C2-023, Seattle, WA 98109.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal