Abstract
Idiotypic structures expressed on the myeloma Ig protein might be regarded as a tumor-specific antigen. Five patients with IgG myeloma were immunized with the purified serum M-component by repeated intradermal injections together with soluble granulocyte-macrophage colony-stimulating factor (GM-CSF). All patients developed an idiotype (Id)-specific T-cell immunity, defined as blood T cells predominantly secreting interferon-γ (IFN-γ) and interleukin-2 (IL-2) (type I cells). Id-specific DNA synthesis was induced in one patient. Delayed-type hypersensitivity against the Id was not evoked. The specific IFN-γ/IL-2 T-cell response was inhibited (46% to 100%) by a major histocompatibility complex (MHC) class I monoclonal antibody (MoAb) in all five patients. A 5% to 37% inhibition by an MHC class II MoAb was seen in four patients. CD4+ as well as CD8+ T cells enriched by magnetic microbeads contained Id-specific cells. The T cells recognized peptides corresponding to the complementarity-determining regions 1, 2, and 3 of the heavy chain of the Id. There was a transient rise of B cells producing IgM anti-idiotypic antibodies in all patients. The results indicate that immunization of myeloma patients using the autologous M-component and soluble GM-CSF may evoke an Id-specific predominantly MHC class I–restricted type I T-cell response.
MULTIPLE MYELOMA (MM) is a lymphoproliferative disorder characterized by a clonal proliferation of B cells producing a monoclonal Ig that can be detected in the serum and/or urine. The unique clonal idiotypic Ig structures are expressed on the cell surface of the myeloma B cells,1 and peptides of the idiotypic Ig may be presented in the groove of major histocompatibility complex (MHC) molecules of the clonal tumor B cells.2,3 The idiotypic structures may serve as tumor-specific antigens.4 The surface-localized, tumor-derived idiotypes (Id) may elicit a humoral and cellular anti-idiotypic response5,6 that may control the growth of the myeloma B-cell clone. Active immunization using the autologous monoclonal Ig as a vaccine may confer resistance to tumor cell challenge in murine myeloma and B-cell lymphomas.4,7 8
Exogenous antigens are processed and presented by antigen-presenting cells (APC). Both the exogenous and endogenous presentation pathways may be used2 resulting in either MHC class II or I presentation.9 The functional activity of APC may be augmented by granulocyte-macrophage colony-stimulating factor (GM-CSF)10,11 In vivo soluble GM-CSF has been shown to enhance the humoral response against hepatitis B vaccine.12In a comparative study, tumor cells engineered to secrete GM-CSF were the most potent stimulators of a specific anti-tumor immunity.13
In the present study, the autologous myeloma IgG protein purified from serum, the Id of which may serve as tumor-specific antigens, was used together with free GM-CSF for immunization of myeloma patients. The specific humoral and cellular anti-Id immunity as well as clinical effects were analyzed.
MATERIALS AND METHODS
Patients.
Five IgG MM patients were included. The median age was 65 years (range, 48 to 74 years). All patients had clinical stage IIA14 with a World Health Organization (WHO) performance status of 0 to 1. Three patients were in a stable partial remission following chemotherapy (nos. 1 and 4) or local radiotherapy (no. 2) 12 to 48 months earlier. Two patients (nos. 3 and 5) were previously untreated. Clinical evaluation of the patients before and after immunization was done according to standard procedures.15 The study was approved by the local Ethic's Committee. Written informed consent was obtained from each patient.
Preparation of monoclonal IgG and F(ab′)2 fragments.
The procedure has been described in detail.16 Briefly, serum was applied to a sterile mabTrapG column (Pharmacia, Uppsala, Sweden). IgG was eluted with 0.1 m glycine-HCl (pH 2.7). Isoelectric focusing (Pharmacia Phast system) estimated that more than 90% of the IgG was monoclonal. The monoclonal IgG was dialysed against sterile NaCl followed by filtration through a Millipore filter (0.20 μm). F(ab′)2 fragments were prepared by pepsin digestion. F(ab′)2 fragments from monoclonal IgG of other myeloma patients were used as controls and were prepared in the same way as the autologous IgG.
Production of complementarity-determining region (CDR) peptides.
Total RNA was extracted from a patient's (no. 4) bone marrow sample.17 First-strand cDNA was synthesized followed by polymerase chain reaction (PCR) using family-specific IgH variable gene framework 1–specific oligonucleotides as 5′ primer and an IgH constant gene–specific 3′ primer.18,19 The PCR products were subjected to fragment analysis20 using an automatic sequencing machine (ALF, Pharmacia). Based on the fragment analysis result (single peak), the relevant PCR product was ligated into the pGEM-T vector (Amersham, Cleveland, OH) and transformed to theEscherichia coli strain JM109. Ten positive colonies, screened by PCR, were isolated and plasmids containing the cloned inserts prepared. They were then sequenced using pGEM-T vector primers (Sp6 and T7). Ten out of 10 clones showed the same sequence, which confirmed the monoclonality of the PCR product as suggested by fragment analysis. This proved that this sequence was carried by the tumor B-cell population.
The amino acid (aa) sequence of the tumor IgH was deduced from the nucleotide sequence, and peptides corresponding to the full CDR 1-3 regions including 2-4 flanking aa were synthesized. The length of the CDR1, 2, and 3 peptides were 15, 20, and 20 aa, respectively. Solid-phase peptide synthesis was performed with 9-fluorenylmethoxycarbonyl methodology.21
Preparation of Id vaccine.
Equal volumes of sterile filtered monoclonal IgG and alum suspension (National Bacteriological Lab, Stockholm, Sweden) were mixed under aseptic conditions and adjusted with sterile 0.9% NaCl to a final IgG concentration of 1 mg/mL. Tests for sterility, pyrogens, and viruses were negative.22
Immunization protocol.
Patients were immunized day 1 intradermally with 0.5 mg of the autologous Id vaccine. Seventy-five μg of GM-CSF (Schering-Plough, Kenilworth, NJ) was injected subcutaneously at the same site daily, days 1 to 4. The complete procedure was repeated after 2, 4, 6, 8, and 14 weeks.
Proliferation assay (DNA synthesis).
The method has been described in detail earlier.23,24Peripheral blood mononuclear cells (PBMC) were stimulated with purified F(ab′)2 fragments of the autologous monoclonal IgG (1 pg/mL to 100 μg/mL) as well as with four allogeneic monoclonal isotype-matched IgG from other myeloma patients purified in the same way and cultured for 6 days. [3H]-thymidine was added during the last 18 hours. ConA (40 μg/mL) and PPD (2.5 μg/mL) were used as positive controls. Tests were run in triplicates, and mean reactivity was calculated for each triplicate. For each testing time, the highest mean value of a triplicate obtained from the five different concentrations of the Id was used. Stimulation index (SI) was calculated by dividing the mean radioactivity for a triplicate of stimulated cells by that of unstimulated cells. The test was performed thrice before the first immunization. The mean + 2 SD stimulation index of the patients using the four control isotypes was 2.68 (range, 0.76 to 3.04) and of five healthy controls using the five different monoclonal IgGs 2.15 (range, 0.55 to 2.84). A cut-off level of less than or equal to 3.20 was used in accordance with previous reports.23 24
Reverse enzyme-linked immunospot (ELISPOT) assay (interferon-γ (IFN-γ), interleukin-2 (IL-2) and IL-4 secreting cells).
The ELISPOT assay for identification of IFN-γ–, IL-2–, or IL-4–secreting cells was used as described.24,25 Briefly, PBMC (1 × 105/well) were incubated with F(ab′)2 fragments of the autologous or isotypic monoclonal IgG (1 pg/mL to 1 μg/mL) or CDR peptides (1 to 10 μg/mL) for 48 hours in humidified air with 5% CO2 at 37°C. As previously reported,25 26 10 pg/mL of F(ab′)2fragments was found to be optimal in the ELISPOT assay in all the patients and used for subsequent experiments. A representative experiment showing the dose-response relationship of Ig-induced cytokine secreting cells is depicted in Fig1. Low concentrations (0.1 to 100 pg/mL) of the Id induced a specific response, whereas at high concentrations (0.1 to 10 μg/mL) the Id-induced T-cell response was less marked as indicated by the increasing numbers of cells also stimulated by the isotypic monoclonal IgG. Cells incubated with medium alone, PPD (2.5 μg/mL) or ConA (10 μg/mL), were used as positive controls. Spots corresponding to cytokine-secreting cells were counted blindly under a dissection microscope. All samples were incubated in duplicate wells. The coefficient of variation between duplicates was 8.5%.
Dose-response relationship of IFN-γ–secreting cells/105 PBMC after incubation with F(ab′)2fragments of autologous (▪) and isotypic control (□) monoclonal IgG.
Dose-response relationship of IFN-γ–secreting cells/105 PBMC after incubation with F(ab′)2fragments of autologous (▪) and isotypic control (□) monoclonal IgG.
The results are expressed as the numbers of stimulated IFN-γ–, IL-2–, or IL-4–secreting cells/105 PBMC. The stimulated number of spots (total number of spots minus the number of spots in cultures with medium only) was determined and is shown. Tests were performed thrice before the first immunization. The mean number of mitogen (ConA)-induced spots was, for IFN-γ, 80 spots (range, 30 to 250); for IL-2, 41 spots (range, 20 to 88), and for IL-4, 32 spots (range, 20 to 80). The mean + 2 SD stimulated number of spots in patients using three isotype-matched (control) IgG F(ab′)2 fragments was, for IFN-γ, 9 spots (range 0 to 14); for IL-2, 7 spots (range, 0 to 14); and for IL-4, 6 spots (range, 0 to 14). As another control for specificity, PBMC from five healthy controls were stimulated with the five different monoclonal IgGs and the mean + 2 SD of stimulated number of spots was, for IFN-γ, 10 spots (range 0 to 12); for IL-2, 4 spots (range, 0 to 9); and for IL-4, 6 spots (range, 0 to 6).
Anti-Id antibody assay.
The ELISPOT assay was also used for detection of B cells secreting IgM antibodies binding to F(ab′)2 fragments of the autologous monoclonal IgG.16 The coefficient of variation between duplicates was 9%. The mean + 2 SD number of spots for five healthy donors using 12 different monoclonal IgGs was 5 (range 0 to 6).
MHC-restricted T-cell response.
To study MHC restriction of the specific T-cell response, mouse MoAbs (IgG2b) against MHC class II molecules (HLA-DR; Immunotec, Marseille, France) or class I molecules (HLA-ABC; Chemicon International Inc, Temecula, CA) were added (1 μg/mL) to the ELISPOT assay for the whole incubation period. One microgram per milliliter of the blocking antibody was optimal as previously shown.27 As a control, a nonrelevant mouse IgG2b MoAb (Immunotec) was used. Results are expressed as percent inhibition compared with cultures without the addition of MHC MoAbs. The unspecific inhibition was 0% to 15%.
Functional analyses of T-cell subsets.
CD4 and CD8 T cells were enriched from freshly prepared PBMC by antibody-coated magnetic microbeads (MiniMACS, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's recommendation. The purity of the respective CD4+ and CD8+ T-cell fractions was greater than 95%. Monocytes were collected by incubation of PBMC in a Petri dish for 30 minutes at 37°C. CD4+ and CD8+ T cells, respectively, were then cultured with 5% autologous adherent cells with or without the addition of F(ab′)2 fragments of the autologous or isotypic control IgG and analyzed for cytokine secretion as described above.
Delayed-type hypersensitivity (DTH).
Statistical methods.
The Mann-Whitney U-test was used to compare differences between data obtained before and during immunization, and between autologous and isotypic (control) IgG.
RESULTS
Id-specific T-cell response (DNA synthesis).
One patient (no. 1) developed an Id-specific T-cell response in the high-dose range (1 to 100 μg/mL of autologous F(ab′)2fragments; Fig 2). The proliferative response gradually increased until week 14 (SI = 43.0 at the highest concentration [100 μg/mL] ) but had disappeared at week 16 and remained then negative. The other patients showed no Id-specific proliferative response (SI ≤ 3.20).
DNA synthesis ([3H]-thymidine incorporation) in PBMC of patient no. 1 (week 14) stimulated with F(ab′)2 fragments of the autologous monoclonal IgG (•), F(ab′)2 fragments of isotypic control monoclonal IgG (□), and pooled human polyclonal IgG (▵) (cpm of PBMC cultured in medium alone was 130). The values for PPD-(2.5 μg/mL) and ConA (40 μg/mL)-stimulated PBMC were 3,933 and 78,865 cpm, respectively.
DNA synthesis ([3H]-thymidine incorporation) in PBMC of patient no. 1 (week 14) stimulated with F(ab′)2 fragments of the autologous monoclonal IgG (•), F(ab′)2 fragments of isotypic control monoclonal IgG (□), and pooled human polyclonal IgG (▵) (cpm of PBMC cultured in medium alone was 130). The values for PPD-(2.5 μg/mL) and ConA (40 μg/mL)-stimulated PBMC were 3,933 and 78,865 cpm, respectively.
Id-specific T-cell response (IFN-γ, IL-2, IL-4 secretion) (ELISPOT).
Before immunization, all patients had low numbers of Id-induced, cytokine-secreting T cells. The median number of spots for IFN-γ was 7 (range 4 to 14); for IL-2 5 (range 3 to 9); and for IL-4, 3 (range 1 to 6). During immunization the numbers of specific IFN-γ–and IL-2–secreting cells increased in all five patients. The differences compared with week 0 were statistically significant (P < .05) at three of four points of time (week 4, 8, and 16) for IFN-γ, and at two of four points (week 8 and 16) for IL-2 (Fig 3A and B). No significant IL-4 response was induced (Fig 3C). The IFN-γ and IL-2 responses, respectively, induced by the autologous monoclonal IgG were statistically significantly higher than those induced by the isotypic control monoclonal IgG (P < .05) at all points (Figs 3A and B). There were no significant changes in the number of cells responding to isotypic control IgG during the immunization period.
Time kinetics of cytokine-secreting cells/105PBMC. PBMC were stimulated in vitro with F(ab′)2fragments of the autologous (•) or isotypic control (○) monoclonal IgG (10 pg/mL). Number of cells secreting IFN-γ. (A), IL-2 (B), and IL-4 (C) was assessed by ELISPOT. Mean ± SEM (n = 5) for each time point are shown. The numbers of IFN-γ– and IL-2–secreting cells induced by the autologous monoclonal IgG was statistically significantly higher compared with week 0 at different points (*P < .05, **P < .01). At all points of time the numbers of IFN-γ and IL-2, but not IL-4–secreting cells, induced by the autologous IgG were significantly different compared with the isotypic control monoclonal IgG (P < .05). Arrows indicate immunization times.
Time kinetics of cytokine-secreting cells/105PBMC. PBMC were stimulated in vitro with F(ab′)2fragments of the autologous (•) or isotypic control (○) monoclonal IgG (10 pg/mL). Number of cells secreting IFN-γ. (A), IL-2 (B), and IL-4 (C) was assessed by ELISPOT. Mean ± SEM (n = 5) for each time point are shown. The numbers of IFN-γ– and IL-2–secreting cells induced by the autologous monoclonal IgG was statistically significantly higher compared with week 0 at different points (*P < .05, **P < .01). At all points of time the numbers of IFN-γ and IL-2, but not IL-4–secreting cells, induced by the autologous IgG were significantly different compared with the isotypic control monoclonal IgG (P < .05). Arrows indicate immunization times.
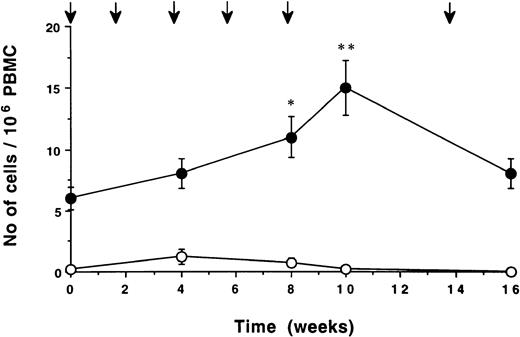
The time kinetics pattern of Id-induced, cytokine-secreting cells is exemplified by patient no. 2 in Fig 4. An increase in the number of IFN-γ– and IL-2–secreting cells, respectively, was seen by repeated immunizations. IL-4–secreting cells remained low, as did the number of IFN-γ–, IL-2–, and IL-4–secreting cells induced by the isotypic control monoclonal IgG. Individual data on IFN-γ– and IL-4–secreting cells of all patients are summarized in Table 1. The number of Id-induced, IFN-γ–producing cells reached a plateau in most patients after 2 to 4 immunizations. Id-induced, IL-4–secreting cells remained low except for patient nos. 4 and 5, who showed positive values at the end of the immunization period/at follow-up. During unmaintained follow-up, idiotype-specific IFN-γ–secreting cells were detectable in all patients 1 to 3 months after the last immunization. Only one patient (no. 2) was followed-up for a long time period, and specific IFN-γ–secreting cells could be detected 1 year after immunization was terminated.
Time kinetics of cytokine secreting cells/105PBMC in patient no. 2. PBMC were stimulated in vitro with F(ab′)2 fragments of the autologous monoclonal IgG (10 pg/mL). Number of cells secreting IFN-γ (▪), IL-2 (▴), and IL-4 (•) was assessed by ELISPOT. Number of cells secreting IFN-γ (□), IL-2 (▵), and IL-4 (○) induced by control monoclonal IgG. Arrows indicate immunization times.
Time kinetics of cytokine secreting cells/105PBMC in patient no. 2. PBMC were stimulated in vitro with F(ab′)2 fragments of the autologous monoclonal IgG (10 pg/mL). Number of cells secreting IFN-γ (▪), IL-2 (▴), and IL-4 (•) was assessed by ELISPOT. Number of cells secreting IFN-γ (□), IL-2 (▵), and IL-4 (○) induced by control monoclonal IgG. Arrows indicate immunization times.
Numbers of Idiotype- and Isotype-Stimulated IFN-γ– and IL-4–Secreting Cells at Different Time Points From Start of Immunization in Myeloma Patients Immunized With the Autologous Idiotype
| Patient No. . | Weeks . | Unmaintained Follow-up . | |||
|---|---|---|---|---|---|
| 0* . | 4 . | 8 . | 16 . | ||
| IFN-γ–secreting cells/105 PBMC | |||||
| 1: Idiotype | 9 | 31 | 22 | 10 | 20 (w20)-151 |
| Isotype | 1 | 2 | 3 | 4 | 4 |
| 2: Idiotype | 4 | 10 | 21 | 36 | 15 (w65) |
| Isotype | 1 | 2 | 2 | 11 | 2 |
| 3: Idiotype | 5 | 27 | 15 | 7 | 23 (w28) |
| Isotype | 2 | 8 | 2 | 2 | ND |
| 4: Idiotype | 14 | 17 | 16 | 31 | 17 (w24) |
| Isotype | 2 | 4 | 2 | 2 | 10 |
| 5: Idiotype | 6 | 53 | 18 | 40 | 25 (w26) |
| Isotype | 4 | 6 | 7 | 9 | 3 |
| IL-4–secreting cells/105 PBMC | |||||
| 1: Idiotype | 4 | 10 | 2 | 9 | 6 (w20) |
| Isotype | 2 | 1 | 0 | 1 | 1 |
| 2: Idiotype | 1 | 1 | 4 | 1 | 1 (w65) |
| Isotype | 0 | 1 | 2 | 0 | 0 |
| 3: Idiotype | 6 | 2 | 3 | 5 | 3 (w28) |
| Isotype | 3 | 6 | 1 | 2 | ND |
| 4: Idiotype | 4 | 4 | 4 | 14 | 19 (w27) |
| Isotype | 1 | 3 | 2 | 9 | 7 |
| 5: Idiotype | 4 | 6 | 5 | 15 | 16 (w26) |
| Isotype | 1 | 3 | 0 | 2 | 1 |
| Patient No. . | Weeks . | Unmaintained Follow-up . | |||
|---|---|---|---|---|---|
| 0* . | 4 . | 8 . | 16 . | ||
| IFN-γ–secreting cells/105 PBMC | |||||
| 1: Idiotype | 9 | 31 | 22 | 10 | 20 (w20)-151 |
| Isotype | 1 | 2 | 3 | 4 | 4 |
| 2: Idiotype | 4 | 10 | 21 | 36 | 15 (w65) |
| Isotype | 1 | 2 | 2 | 11 | 2 |
| 3: Idiotype | 5 | 27 | 15 | 7 | 23 (w28) |
| Isotype | 2 | 8 | 2 | 2 | ND |
| 4: Idiotype | 14 | 17 | 16 | 31 | 17 (w24) |
| Isotype | 2 | 4 | 2 | 2 | 10 |
| 5: Idiotype | 6 | 53 | 18 | 40 | 25 (w26) |
| Isotype | 4 | 6 | 7 | 9 | 3 |
| IL-4–secreting cells/105 PBMC | |||||
| 1: Idiotype | 4 | 10 | 2 | 9 | 6 (w20) |
| Isotype | 2 | 1 | 0 | 1 | 1 |
| 2: Idiotype | 1 | 1 | 4 | 1 | 1 (w65) |
| Isotype | 0 | 1 | 2 | 0 | 0 |
| 3: Idiotype | 6 | 2 | 3 | 5 | 3 (w28) |
| Isotype | 3 | 6 | 1 | 2 | ND |
| 4: Idiotype | 4 | 4 | 4 | 14 | 19 (w27) |
| Isotype | 1 | 3 | 2 | 9 | 7 |
| 5: Idiotype | 4 | 6 | 5 | 15 | 16 (w26) |
| Isotype | 1 | 3 | 0 | 2 | 1 |
*Mean of three analyses done at three different pretreatment time points.
Week from start of immunization.
Abbreviations: ND, not done; w, week.
MHC restriction of the Id-specific T-cell response.
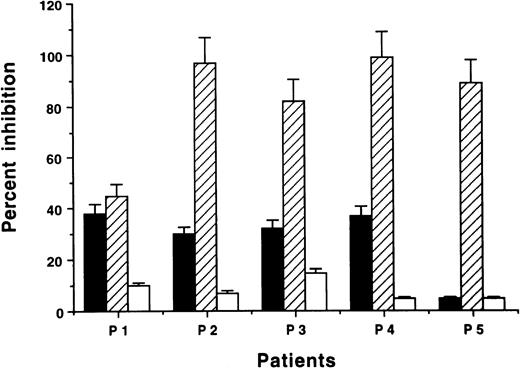
Mouse MoAbs against human MHC class I or II molecules were used to inhibit the Id-induced T-cell response in vitro. MHC class I MoAb markedly suppressed the specific IFN-γ or IL-2 response in four out of five patients (83% to 98% inhibition; Fig.5). In the remaining patient (no. 1), MHC class I MoAb induced an inhibition of 46%. MHC class II MoAb had an inhibitory effect on IFN-γ or IL-2 secretion varying between 5% and 37%.
Inhibition of Id-induced IFN-γ (P1, P2, P4, P5) or IL-2 (P3) cytokine–secreting cells by MHC antibodies (ELISPOT) (mean + SEM). PBMC were stimulated in vitro by F(ab′)2 fragments of the autologous monoclonal IgG (10 pg/mL) in the presence or absence of mouse MoAbs against human MHC class II (▪), MHC class I molecules (▨), or control mouse IgG2b (□) (1 μg/mL). The absolute number of cells/105 PBMC after stimulation with the autologous monoclonal IgG (in the absence of blocking antibodies) for patient nos. 1 through 5 were 18, 30, 22, 16, and 19 cells, respectively. The corresponding numbers obtained with isotypic control IgG were 3, 1, 4, 2, and 3 cells, respectively. Results are expressed as percent inhibition compared with cells incubated without the blocking MoAbs.
Inhibition of Id-induced IFN-γ (P1, P2, P4, P5) or IL-2 (P3) cytokine–secreting cells by MHC antibodies (ELISPOT) (mean + SEM). PBMC were stimulated in vitro by F(ab′)2 fragments of the autologous monoclonal IgG (10 pg/mL) in the presence or absence of mouse MoAbs against human MHC class II (▪), MHC class I molecules (▨), or control mouse IgG2b (□) (1 μg/mL). The absolute number of cells/105 PBMC after stimulation with the autologous monoclonal IgG (in the absence of blocking antibodies) for patient nos. 1 through 5 were 18, 30, 22, 16, and 19 cells, respectively. The corresponding numbers obtained with isotypic control IgG were 3, 1, 4, 2, and 3 cells, respectively. Results are expressed as percent inhibition compared with cells incubated without the blocking MoAbs.
Subset restriction of the Id-specific T-cell response.
Enrichment of T-cell subsets by magnetic microbeads was done in two patients (nos. 1 and 3). The Id-specific IFN-γ response was confined to both CD8+ and CD4+ cells (Fig6).
Stimulated number of Id-induced IFN-γ cytokine–secreting cells/105 cells (ELISPOT) in CD4+ and CD8+ T-cell subpopulations from patients no. 1 (A) and no. 3 (B), enriched from PBMC by magnetic microbeads. CD4+ and CD8+ cells, respectively, were cultured with 5% autologous adherent cells in the presence of F(ab′)2 fragments of the autologous (▨) or isotype-matched allogeneic (▧) monoclonal IgG (10 pg/mL). Standard error of means are shown on top of each column.
Stimulated number of Id-induced IFN-γ cytokine–secreting cells/105 cells (ELISPOT) in CD4+ and CD8+ T-cell subpopulations from patients no. 1 (A) and no. 3 (B), enriched from PBMC by magnetic microbeads. CD4+ and CD8+ cells, respectively, were cultured with 5% autologous adherent cells in the presence of F(ab′)2 fragments of the autologous (▨) or isotype-matched allogeneic (▧) monoclonal IgG (10 pg/mL). Standard error of means are shown on top of each column.
Epitope mapping of Id-specific T-cell response.
Fifteen to 20 aa long peptides of the heavy chain CDR 1, 2, and 3 regions, respectively, of the monoclonal IgG of patient no. 4 were synthesized. Corresponding peptides of a control myeloma monoclonal IgG were also produced. High numbers of IL-2–secreting cells specifically recognizing the CDR 1, 2, and 3 peptides of the autologous but not the allogeneic Ids were noted (Fig 7A). The CDR 1–, CDR3–, and F(ab′)2 fragment–induced specific responses were abrogated by MHC class I MoAb but not by MHC class II MoAb, whereas the CDR2-induced IL-2 response was predominantly class II restricted. No significant IL-4 response was induced by CDR 1-3 peptides (Fig 7B).
CDR 1-3 peptide-reactive T-cells (ELISPOT) secreting IL-2 (A) and IL-4 (B). PBMC of patient no. 4 were stimulated in vitro with autologous (▨) or allogeneic (□) peptides 15-20 aa long corresponding to the CDR1, 2, and 3 regions of the heavy chain and by F(ab′)2 fragments of the autologous monoclonal IgG (▨). The autologous Id-induced response was also assayed in presence of mouse MoAbs against human MHC class II (▧) or MHC class I () (1 μg/mL) molecules. Standard error of means are shown on top of each column.
CDR 1-3 peptide-reactive T-cells (ELISPOT) secreting IL-2 (A) and IL-4 (B). PBMC of patient no. 4 were stimulated in vitro with autologous (▨) or allogeneic (□) peptides 15-20 aa long corresponding to the CDR1, 2, and 3 regions of the heavy chain and by F(ab′)2 fragments of the autologous monoclonal IgG (▨). The autologous Id-induced response was also assayed in presence of mouse MoAbs against human MHC class II (▧) or MHC class I () (1 μg/mL) molecules. Standard error of means are shown on top of each column.
Id-specific T cells in vivo (DTH).
None of the patients developed a DTH reaction against the autologous IgG M-component (data not shown).
Anti-idiotypic (IgM) humoral response.
All five patients developed B cells secreting IgM antibodies binding to F(ab′)2 fragments of the autologous monoclonal IgG (Fig8). The differences between week 0 and weeks 8 and 10, respectively, were statistically significant (P < .05 and P < .01). The anti-idiotypic IgM response induced by the autologous monoclonal IgG was statistically significantly higher than that induced by the isotypic control monoclonal IgG at all points (P < .05). There were no significant changes in the number of cells reactive with the isotypic control IgG during the immunization period. The anti-idiotypic IgM humoral response was transient. In all but one patient (no. 3), the IgM antibody response had diminished by the end of the immunization period.
Anti-idiotypic IgM antibodies. Time kinetics of B cells secreting IgM antibodies binding to F(ab′)2 fragments of the autologous (•) or isotypic control (○) monoclonal IgG were assessed by ELISPOT. Mean ± SEM (n = 5) for each time point are shown. The increase in the numbers of IgM-secreting cells binding to the autologous IgG was statistically significant at week 8 (P < .05) and week 10 (P < .01) compared with baseline. The difference between autologous and isotypic control monoclonal IgG was statistically significant (P < .05) at all points. Arrows indicate immunization times.
Anti-idiotypic IgM antibodies. Time kinetics of B cells secreting IgM antibodies binding to F(ab′)2 fragments of the autologous (•) or isotypic control (○) monoclonal IgG were assessed by ELISPOT. Mean ± SEM (n = 5) for each time point are shown. The increase in the numbers of IgM-secreting cells binding to the autologous IgG was statistically significant at week 8 (P < .05) and week 10 (P < .01) compared with baseline. The difference between autologous and isotypic control monoclonal IgG was statistically significant (P < .05) at all points. Arrows indicate immunization times.
Clinical effects.
All patients had a stable IgG M-component concentration for at least 12 months before study entry and reduced (below the normal range) background Ig (IgM, IgA) levels. One patient (no. 2) was diagnosed with myeloma-related osteolytic lesions of the eighth thoracic vertebra and the eighth left rib. The IgG M-component concentration was 25 g/L. No increased number of plasma cells in a bone marrow sample (obtained from the iliac crest) was found. The patient received local radiotherapy (40 Gy) 55 weeks before immunization, and the M-component concentration then remained stable at 20 g/L until start of immunization. During immunization, the M-component concentration decreased to 7 g/L. At follow-up 1 year after commencement of immunization, the M-component concentration remained at 7 g/L. In parallel, background serum Ig levels were normalized. IgA concentration increased from 1.3 g/L to 1.9 g/L and IgM concentration from 0.2 g/L to 0.6 g/L. There was no urine excretion of the M-component or light chains during the observation period. The M-component concentration as well as the background serum Ig levels of the other four patients remained stable (data not shown).
DISCUSSION
Immunization using the autologous myeloma protein–expressing idiotypic determinants may induce anti-idiotypic antibodies and anti-idiotypic T cells in animals and humans.5,22,28 Protective and therapeutic antitumor immunity were induced in animals with transplantable plasmacytomas.4,7,29 The immune effector functions are, however, not completely understood. Cell-mediated immunity seemed to be of importance,6 but antibodies might also have contributed to the rejection of the plasmacytoma cells.30,31 Effector cells may belong to the CD4Th1, CD4Th2, or the classical CD8 CTL subsets.32,33 Immunization of patients with non-Hodgkin's B-cell lymphoma using the autologous idiotypic Ig coupled to keyhole limpet hemocyanin (KLH)–induced MHC class I–restricted Id-specific CD8+ CTLs, which were associated with a favorable clinical outcome.34
The present pilot study describes the results of Id immunization in five myeloma patients using the myeloma protein (serum M-component) as immunogen and given together with soluble GM-CSF. Id-specific T and B cells were induced in all patients, and a greater than 50% reduction of the M-component concentration was noted in one patient. Based on the cytokine secretion pattern (IFN-γ and IL-2), the majority of the Id-specific T cells could be classified as type I (Th1 or Tc1) cells.35 The main part of the induced T cells appeared to be MHC class I restricted. This may suggest that the immunization led to a predominant Id-specific CD8+ T-cell response. The negative DTH reaction in all patients after challenge with the Id might support such an assumption. Only one patient developed a transient idiotype–specific proliferative T-cell response. These results might be explained by the observation that different T-cell subsets and T cells of varying maturity may have a varying response profile.36 In particular, antigen-specific cell proliferation may not correlate to antigen-specific cytokine production, suggesting that proliferation test only may not be sufficient to reveal an antigen-specific, T-cell population.37 It seems critically important to use several read-out systems to detect and characterize an antigen-specific, T-cell response.
Addition of GM-CSF might be of importance for the enhancement and the quality of the anti-idiotypic immunity.38,39 In a comparative solid tumor model using cytokine gene–transduced melanoma tumor cells for immunization, GM-CSF was shown to be an efficient cytokine for the induction of a protective anti-tumor immunity involving CD8+ as well as CD4+ T cells.13 Immunization with GM-CSF–transduced tumor B cells induced an effective anti-tumor immunity in a mouse lymphoma model. The immunity directed against Id-expressing tumor cells also required CD4+ as well as CD8+ T cells. A humoral response was not detected.38 Immunization of animals with an Id–GM-CSF fusion protein was capable of inducing an Id-specific humoral anti-tumor immunity protecting the animals from a subsequent challenge with the Id-bearing tumor cells.39 The use of soluble GM-CSF as an adjuvant induced a more efficient cellular response against “self” tumor-associated protein antigens including idiotypes than did immunization without the addition of GM-CSF in animal tumor models.40,41 In colorectal carcinoma patients immunized with recombinant carcinoembryonic antigen (rCEA), the rCEA-specific T- and B-cell responses were statistically significantly enhanced by adding soluble GM-CSF compared with patients immunized with rCEA alone.42
GM-CSF most likely acts by increasing the capacity to present tumor antigens.43,44 GM-CSF is a major cytokine for stimulation of dendritic cells (DC). DC pulsed ex vivo with tumor-derived idiotypic protein induced a cellular immune response and tumor regression in patients with B-cell lymphomas.45 DC used as APC could prime a strong CD8 CTL response in lymphocyte populations extensively depleted of CD4+ T cells.46,47 Exogenous antigens may use endogenous MHC class I antigen presentation,9,48-50 and GM-CSF might activate a pathway of antigen processing allowing exogenous soluble proteins to be presented by MHC class I molecules.51 Thus, GM-CSF in addition to augmenting antigen presentation might also contribute to the induction of an MHC class I–restricted response as noted in our study. Moreover, myeloma patients in clinical stages I and II seemed to exhibit an auto T-cell immunity against the Id,23 part of which response was confined to CD8 cells.52 53 Such a cellular immunity might preferentially be boosted by immunization with the Id together with GM-CSF.
In a previous study using the Id alone, 3 out of 5 patients mounted only a weak transient anti-idiotypic T-cell response with no clinical effects.22 In the present study, the induced T-cell immunity appeared to be long lasting, and in one of the patients a reduction of the M-component concentration was noted. The reason(s) for this single clinical effect is not known. A spontaneous regression, occuring simultaneously at the time of immunization, cannot be completely ruled out, although a reduction of the M-component concentration of such a magnitude and unrelated to treatment should be a very rare event in multiple myeloma. Notably, this particular patient developed upon immunization a gradually increasing and marked MHC class I–restricted type I T-cell response, which persisted for 1 year during unmaintained follow-up. Although a clinical effect in one single individual needs to be confirmed, it seems however less likely that the reduction of M-component concentration could be ascribed to GM-CSF. GM-CSF has been reported to act as a growth factor for myeloma cell lines in vitro,54 a notion which may be supported by occasional clinical observations.55
Based on the present results, further studies on the clinical effects of Id immunization in myeloma patients are warranted. It remains to be shown whether human myeloma cells, in accordance to what has been shown in B-cell lymphomas,2,3 can present endogenously derived idiotypic peptides in the context of MHC molecules for recognition by T cells. As immunogen the complete myeloma protein, single chain FV personal molecules,56 or CDR peptides might be used as the Id-specific T cell reactivity might preferentially be located to CDR regions. The prefered clinical situation should be monoclonal gammopathy of undetermined significance at risk, myeloma stage I, or after chemotherapy with the intention to eradicate minimal residual disease.
ACKNOWLEDGMENT
Wen He and Ingrid Eriksson are greatly acknowledged for expert technical assistance and Lena Gahne for excellent typing of the manuscript.
Supported by grants from the Cancer Society in Stockholm, the Swedish Cancer Society, the King Gustav V Jubilee Fund, the Åke Winberg Foundation, the Karolinska Institute Foundation, and the Swedish Society for Cancer and Traffic Injuries.
Address reprint requests to Anders Österborg, MD, PhD, Associate Professor, Department of Oncology (Radiumhemmet), Karolinska Hospital, S-171 76 Stockholm, Sweden.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. DNA synthesis ([3H]-thymidine incorporation) in PBMC of patient no. 1 (week 14) stimulated with F(ab′)2 fragments of the autologous monoclonal IgG (•), F(ab′)2 fragments of isotypic control monoclonal IgG (□), and pooled human polyclonal IgG (▵) (cpm of PBMC cultured in medium alone was 130). The values for PPD-(2.5 μg/mL) and ConA (40 μg/mL)-stimulated PBMC were 3,933 and 78,865 cpm, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/7/10.1182_blood.v91.7.2459/3/m_blod4070702.jpeg?Expires=1765015525&Signature=3ATIZhzxaVqIIM4~N2myW~CQviyCsbyi~OgJcfUBFF9z4RDTfsDkTqR8sOjRAbPmlAMsCJxAychM2FDT3k5Fr8Rx0c9-hhRYhhlgKb6vaXhW5O1BgCEFqhLDUR7GNBkkbqsa26ck25u8AQjgzUe4PbcDuxG5QzE3F1qr0bjQcv1GAlbABZFKhH~vgRh0E0i9j91jC1Rbm9OztUdRZ05nyc83wyHqz-HXT0SI~lRmXcP7EuEsFRjdXBg3q0q20mKZciTx5CmgYS4csbWXmALz0r9ZPTMRnVWes2qyE~Bxr6aTId9oKjed751ukGN3T6~U2vqP~hLiRXPQjnpPxIwtCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal