Abstract
During development, mice with mutations of stem cell factor (SCF) or its receptor c-kit exhibit defects in melanogenesis, as well as hematopoiesis and gonadogenesis. Consequently, accumulating evidence suggests that the c-kit/SCF system plays a crucial role in all of these processes and in tumors which derive from them. Especially in neuroblastoma (infant tumors of neuroectoderm crest derivation such as melanocytes) it would appear that an autocrine loop exists between c-kit and SCF, and that the functional block of the c-kit receptors with monoclonal antibodies (MoAbs) results in a significant decrease in cellular proliferation. We studied the expression and role of c-kit and SCF in cell lines of soft tissue sarcoma of neuroectodermic origin, such as Ewing's sarcoma (ES) and peripheral neuro-ectodermal tumors (PNET). Using flow cytometry with MoAb CD117 PE, c-kit expression was highlighted in all six of the cell lines examined. This receptor was specifically and functionally activated by SCF, as shown by the binding experiments and the intracellular phosphotyrosine and immunoprecipitation studies that were performed. Using reverse transcriptase polymerase chain reaction analysis, five of the six cellular lines expressed the mRNA of SCF. In the medium measured by using an enzyme- linked immunosorbent assay, low concentrations of SCF were found: only the TC32 cellular line produced significantly higher levels (32 pg) than control. In serum-free culture the addition of SCF reduced the percentage of apoptotic cells from 25% to 90% in five out of the six cellular lines. This observation was confirmed by (1) the functional block of c-kit with MoAb: after 7 days of culture more than 30% of the cells were apoptotic (range 31.5% to 100%) in five out of six cell lines and there was also a decrease in the percentage of cells in phase S, and (2) c-kitantisense oligonucleotides: in the cellular lines treated with oligonucleotides (in relation to the untreated lines) there was a notable reduction (P < .001) both in the absolute number of cells and the 3H-thymidine uptake. These results indicate that ES and PNET express c-kit and its ligand SCF and that SCF is capable of protecting the tumor cells against apoptosis. Furthermore, the reverse transcriptase–polymerase chain reaction performed on the biopsies revealed the presence of mRNA both of SCF and c-kit in practically all of the samples studied. Our in vitro data lead us to assume that SCF may also inhibit tumor cell apoptosis in vivo.
THE c-kit PROTO-ONCOGENE, encoding a 145- to 165-kD membrane-bound glycoprotein of the transmembrane tyrosine kinase family, has been identified as a homologue of the sarcoma retrovirus HZ4-FeSV transforming gene.1 The ligand for c-kit is alternatively known as the stem cell factor (SCF), mast cell growth factor, steel factor, or kit ligand.2 3
During development, mice with the c-kit or SCF mutations exhibit defects in melanogenesis, as well as hematopoiesis and gonadogenesis.4 Consequently, accumulating evidence suggests that the c-kit/SCF system plays a crucial role in all of these processes.5-9
This system is also present on the neoplastic counterpart of these cells.10,11 In particular, the mRNA coexpression of the SCF and c-kit has been reported in certain cases of acute myeloid leukemia,12 round cell pulmonary tumors,13 and breast carcinoma.14 This has led to the hypothesis of the existence of an autocrine loop between SCF/c-kit.
Cohen et al15 suggested the presence of this loop in neuroblastoma, neuroectoderm derived infant tumors, and also showed that the c-kit block with the use of a monoclonal antibody (MoAb) is capable of inducing a reduction of the growth of colonies in agar and of the percentage of cells in S phase.15Comparable experiments, conducted on human neuroblastoma lines, with the anti–c-kit blocking antibody, have shown that the SCF could inhibit the apoptosis rather than directly stimulate proliferation.16
No data is available in relation to the c-kit/SCF role in soft tissue sarcoma of neuroectodermic origin, such as Ewing's sarcoma (ES) and peripheral neuroectodermal tumor (PNET).17
In this report we investigated whether c-kit and SCF are expressed in ES and PNET and whether c-kit and SCF play a role in growth regulation of these malignancies.
MATERIALS AND METHODS
Cell lines and tissue samples.
Six cellular lines were studied: three deriving from ES—6647, TC106, and PDE02; and three from PNET—TC32, PDN12, and PDN13. The 6647, TC106, and TC32 was kindly provided by Dr Pagani (Department of Biomedical Science and Human Oncology, University of Turin).18 The PDE-02, PDN12, and PDN13 were obtained in our laboratory from biopsies at diagnosis.19
The human megakaryoblastic leukemia cell line M-07e20 and an Epstein-Barr virus (EBV)-transformed lymphoblastoid cell line were used as controls. All cell lines were maintained in RPMI 1640 media supplemented with 10% fetal calf serum (FCS) and 0.2 mg/mL of penicillin/streptomycin. Thirteen surgical biopsies collected from five ES and eight PNET were obtained from our pathology laboratory after rigorous histological evaluation. In three cases cytogenetic results were not available. The classical cytogenetic translocation t(11;22)(q24;q21) was identified in 9 out of the 10 remaining cases, and in the last case, a PNET, the i(1q), t(2;21)(p21;q22) was shown.
Cytofluorimetric detection of surface c-kit receptor.
With the anti-CD117 phycoerythrin (PE)-conjugated MoAb (Ancell Corp, Bayport, MN), 5 × 105 cells were incubated at 4°C for 30 minutes, then washed twice in phosphate-buffered saline (PBS) and analyzed by flow cytometry (Epics-XL; Coulter, Miami, FL). PE-conjugated isotypic antibody was used as control and M-07e was used as a positive control.
To quantify the average number of c-kit receptors per cell, the Quantum (TM) 27 R-Phycoerythrin-conjugated kit (Flow Cytometry Standard Corporation, San Juan, Puerto Rico) was used. This contains a set of calibrated standards, with four populations of microbeads displaying increasing and predetermined fluorescence intensity (expressed in terms of the number of molecules of equivalent soluble fluorochromes [MESF]) and one reference blank population. Using this method, the relative channel number obtained by flow cytometry analysis of a cell population is directly transformed into the number of MESF. The linear regression equation, correlating the channel number with the specific MESF value, was calculated using specific software (Quickcal V2.0; Flow Cytometry Standard Corporation).
SCF binding.
To block process receptor internalization, 1 × 105 cells were collected by culture and treated with PBS at 4°C with 0.01% NaN3. Cells were then incubated for 60 minutes at 4°C with 10 μL of biotinylated SCF (rh Stem Cell Factor Biotin Conjugate; R&D Systems, Minneapolis, MN) at a concentration of 0.6 μg/mL or with a biotinylated negative control. Ten microliters of avidin-fluorescein isothiocyanate (FITC) were added and incubated for a further 30 minutes at 4°C in the dark. The cells were then washed to remove unreacted avidin fluorescein and resuspended in 0.2 mL of PBS for final flow cytometric analysis. An anti-SCF neutralizing antibody (R&D Systems) was used as a control to show binding specificity.
Staining of intracellular phosphotyrosine.
One hundred microliters of cells suspended at 1 × 106/mL in PBS containing 1% FCS were mixed with an equal volume of PBS containing 2% paraformaldehyde (PFA), 1 mmol/L EDTA, 2 mmol/L NaVO4, and 0.1% saponin. After 10 minutes of incubation at room temperature, the cells were washed twice in PBS and stained with antiphosphotyrosine-PE MoAb (Py-PE kindly provided by F. Lund-Johansen, DNAX Research Institute, Palo Alto, CA).21 Immunostained cells were washed in PBS and resuspended in 0.5% PFA and kept at 4°C until cytofluorimetric analysis.
Immunoprecipitation studies.
Cells were resuspended in serum-free RPMI medium, incubated for 24 hours at 37°C 5% CO2, then untreated and SCF treated cells (10 minutes with 0.5 μg/106 cells) were rapidly pelleted and resuspended in lysis buffer (10 mmol/L TrisHCl, pH 8.6; 1.5 mmol/L MgCl2; 0.14 mol/L NaCl; 1% NP40; 2 mmol/L phenylmethylsulfonylfluoride; 2 μg/mL leupeptin; 2 μg/mL aprotinin; 1 mg/mL pepsin A). Lysates were incubated for a minimum of 30 minutes on ice, frozen, thawed, and centrifuged at 13,000 rpm at 4°C for 20 minutes. Immunoprecipitation of c-kit was performed on the clarified supernatant with a mouse monoclonal anti-human c-kitneutralizing antibody (anti rh SCFR; Boehringer, Mannheim, Germany) coupled to sepharose-proteinA (SIGMA Chemical Co, St Louis, MO).
After rotating for 16 hours at 4°C, immunoprecipitates were washed twice with lysis buffer, once with lysis buffer without NP-40, and once with Tris HCl 50 mmol/L, pH 6.5. Samples then underwent elution from proteinA with sodium dodecyl sulfate (SDS) sample buffer. The resulting protein was subjected to 7% SDS-polyacrylamide gel electrophoresis (PAGE). Protein was transferred electrophoretically to polyvinylidenedifluoride (PDVF). The filter was incubated with a 3% albumin bovine (SIGMA) blocking solution in TBS-Tween (20 mmol/L Tris HCl, pH 7.6; 137 mmol/L NaCl; 0.1% Tween 20) overnight at 4°C. Antiserum anti-phosphotyrosine PY20 (Transduction Laboratories, Lexington, KY) was added to the same solution and incubation was carried out for 2 hours at room temperature. The filters were then washed three times (10 minutes each) with TBS-Tween and reacted for 1 hour at room temperature with rabbit anti-mouse horseradish peroxidase-conjugated (Transduction Laboratories). The filter was washed as above and visualized using ECL western blotting analysis system (Amersham, Buckinghamshire, UK).22
The PDVF filter was stripped of antibody by 200 mmol/L glycine, pH 2.2; SDS 0.1%; and Tween-20 1%, for 2 hours, then reprobed with MoAb anti c-kit (Boehringer).
Reverse transcriptase-polymerase chain reaction (RT-PCR).
The RT-PCR was performed according to the previously reported method.16 The total RNA was extracted by means of the guanidine salts and phenol-chloroform method using Ultraspec RNA (Biotecx Laboratories Inc, Houston, TX). The cDNA products of all samples and a lymphoblastoid cell line, used as a negative control, were obtained by using 2 μg of total RNA and a poliT primer (Reverse Transcription System, PCR-related; Promega, Madison, WI). To verify the quality of the RNA of each sample, RT-PCR amplification of β-actin was performed with appropriate primers (Clontech Lab, Palo Alto, CA) and a single band of the expected size was obtained (data not shown). The same cDNA is used for RT-PCR amplification for SCF and c-kit. The PCR primers for SCF amplification were upstream primer 5′ATTCAAGAGCCCAGAACCCA3′ and downstream primer 5′CTGTTACCAGCCAATGTACG3′. For c-kit amplification the primers were upstream primer 5′GAGTTGGCCCTAGAGTTAGA3′ and downstream primer 5′CCTGGAGTTGGATGCAAGTT3′. The specific primers for the PCR reaction were synthesized with an Oligonucleotide Synthesizer 381A (Applied Biosystems, Foster City, CA). The cycling condition for amplification was as follows: denaturation at 96°C for 3 minutes, then 35 cycles of 94°C for 1 minute, 64°C for 45 seconds, and 72°C for 1 minute; a thermal cycler (Perkin Elmer-Cetus, Norwalk, CT) was used. The PCR products were separated on 2% agarose gel containing 1 μg/mL ethidium bromide.
Detection of soluble SCF by tumor cell lines.
Chemically defined media of cell lines were collected after 72 hours of culture and the SCF protein concentration in the cell supernatants was quantified by using an enzyme-linked immunosorbent assay (R&D Systems) with a sensibility of 4 pg/mL. The medium was used as the control.
Exogenous SCF.
To evaluate the potential role of the SCF, experiments were performed in serum-free conditions. Cells were cultured in RPMI 1640 supplemented with monotioglycerol (SIGMA), 7.5 10−5; Albumine Bovine (SIGMA), 2%; and 0.2 mg/mL of penicillin/streptomycin and Insulin Transferrine Sodium Selenite media supplement (SIGMA) diluted to 1:100, following the manufacturer's instructions.
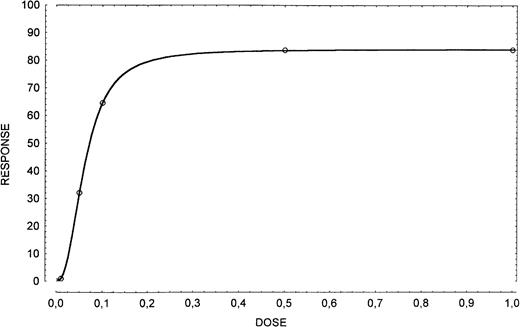
Cells were incubated with 0.5 μg/106 cells of human recombinant SCF (Stem Cell Technologies, Vancouver) and evaluated at 24 hours for apoptosis. Selection of the appropriate working concentration of the SCF was based on a dose-response curve in which the cells were incubated with 0, 0.01, 0.05, 0.1, 0.5, and 1.0 μg/106cells of exogenous SCF and evaluated at 24 hours for apoptosis (Fig1).
Dose-response curve in apoptosis inhibition obtained by using exogenous SCF (0, 0.01, 0.05, 0.1, 0.5, and 1.0 μg/106 cells). The dose of 0.5 μg/106 cells has been considered appropriate.
Dose-response curve in apoptosis inhibition obtained by using exogenous SCF (0, 0.01, 0.05, 0.1, 0.5, and 1.0 μg/106 cells). The dose of 0.5 μg/106 cells has been considered appropriate.
Evaluation of apoptosis.
The cells, cultured in serum-free conditions, were fixed by adding formaldehyde (1% in PBS) for 15 minutes on ice. After resuspension in PBS, cells were stored at 4°C in ethanol (70% in PBS). To perform apoptosis analysis, cells were rehydrated in PBS and cytocentrifuged. The slides were fixed by immersion for 5 minutes in a solution of ethanol:acetic acid (2:1) and then washed twice in PBS and incubated with a cacodylate buffer (0.2 mol/L potassium cacodylate; 2.5 mmol/L Tris HCl; 2.5 mmol/L/cobalt chloride; and 0.25 mg/mL bovine serum albumin, pH 6.6) containing 5 U of TdT (Boehringer) and 0.5 μmol/L of Bio-dUTP (Boehringer) for 1 hour at 37°C. Slides were rinsed in PBS and incubated with 5 μg/mL of fluoresceinated streptavidin (Boehringer) at room temperature for 30 minutes in the dark. Slides were rinsed in PBS, 0.4 μg/mL of propidium iodide was added for 1 minute, and slides were mounted with glycerol for microscopic analysis.23 More than 300 cells were double tested.
Effect of anti-c-kit neutralizing antibody on sarcoma cell growth.
The MoAb anti-human c-kit neutralizing antibody (anti-rhSCFR; Boehringer) at a final concentration of 0.4 μg/104 cells was added to the cells growing in the complete medium. The antibody was added every 72 hours and the cells were assayed at 24 to 72 hours and 7 days for S phase and apoptosis. The experiments were repeated using an isotypic MoAb (mouse anti-human Vβ-8, kindly provided by F. Malavasi, Department of Genetics Biology and Biochemistry, University of Turin) as a negative control. Selection of the appropriate working concentration was based on dilution experiments on the M-07e cell line (data not shown).
Propidium iodide staining.
Cultured in serum-free conditions, 5 × 105 cells were centrifuged and the pellets treated with automated DNA staining kit (DNA-prep; Coulter). Tubes were placed at 4°C in the dark overnight. The PI fluorescence of individual nuclei was measured by flow cytometry and the results obtained analyzed by Multicycle specific software (Phoenix Flow Systems, San Diego, CA).
Treatment of cells with antisense oligonucleotide.
Two cell lines, the ES (6647) and the PNET (TC32) were selected for antisense experiments. The experiments were performed by adopting the specific SCF receptor antisense oligonucleotide kit (Biognostik Antisense kit; Biognostik GmbH, Göttingen, Germany) according to the manufacturer's instructions. The cells were washed four times and resuspended at 2.5 × 104 cells/mL in cell culture media. Aliquots of 100 μL/well were placed in 96-well plates and allowed to adhere for at least 1 hour. Specific c-kit oligo antisense and a control were added directly in the respective wells, in order to make a 2 μmol/L solution. The cells were maintained at 37°C in 5% CO2 and checked daily to examine the different morphology between antisense treated and control cells. The oligos were added daily (2 μL/well). After 7 days, 3 wells were counted in a Neubauer's chamber, the others received 4 μCi/mL of3H-thymidine (Amersham), incubation was continued for 6 hours, and cells were then harvested on glass fiber filter paper. Filter strips were dried and the incorporated radioactivity was measured in a scintillation counter (Canberra Packard International S.A., Zurich, Switzerland). All assays were performed in triplicate.
RESULTS
Direct immunofluorescence analyses.
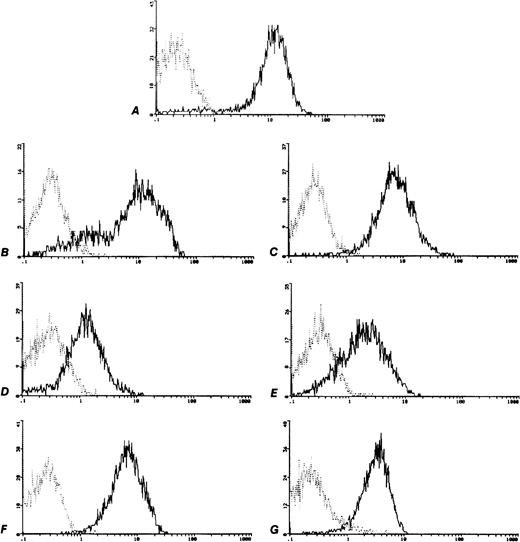
In all of the six cell lines the surface expression of c-kitwas tested in base line culture conditions. All cell lines were positive for c-kit expression, though with a different expression (Fig 2) minimum of 63935 MESF in the TC106 (ES) cell line and a maximum of 330815 MESF in the 6647 (ES) cell line (Table 1). The positive control M07e cell line showed 365960 MESF.
c-kit Expression on human sarcoma cell lines analyzed by flow cytometry. Control MoAb (┈) and anti CD117-PE (—). (A) M07e, (B) 6647 (ES), (C) TC32 (PNET), (D) TC106 (ES), (E) PDN13 (PNET), (F) PDN12 (PNET), (G) PDE02 (ES).
c-kit Expression on human sarcoma cell lines analyzed by flow cytometry. Control MoAb (┈) and anti CD117-PE (—). (A) M07e, (B) 6647 (ES), (C) TC32 (PNET), (D) TC106 (ES), (E) PDN13 (PNET), (F) PDN12 (PNET), (G) PDE02 (ES).
c-kit Expression on Human Sarcoma Cell Lines
| Cell Line . | MESF . |
|---|---|
| M-07e | 365,960 |
| PDN12 | 124,021 |
| TC32 | 129,877 |
| PDN13 | 81,960 |
| 6647 | 330,815 |
| PDE02 | 98,374 |
| TC106 | 63,935 |
| Cell Line . | MESF . |
|---|---|
| M-07e | 365,960 |
| PDN12 | 124,021 |
| TC32 | 129,877 |
| PDN13 | 81,960 |
| 6647 | 330,815 |
| PDE02 | 98,374 |
| TC106 | 63,935 |
c-kit Expression on human Sarcoma cell lines (megakaryoblastic—M-07e; PNET—PDN12, PDN13, and TC32; ES—6647, PDE02, and TC106) obtained by flow cytometry analysis and transformed in terms of number of MESF.
SCF binding.
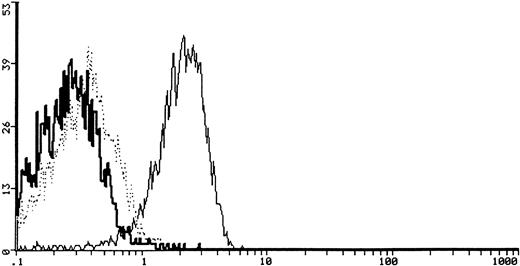
The c-kit binding ability was analyzed by using biotinylated rh-SCF. The cytofluorimetric detection of the SCF-FITC bound to cells was positive in 6 out of 6 (100%) samples tested with specific inhibition. A representative case is shown (6647 ES) in Fig3.
Cytofluorimetric profile of SCF specific binding and SCF binding inhibition after coincubation with 20 μg of goat anti-human SCF neutralizing antibody in a representative case (6647, ES) of sarcoma cells. The heavy solid line represents the background of the negative staining control, the light solid line represents the SCF-FITC staining, and the dotted line represents the SCF-FITC staining after coincubation with anti-human SCF antibody.
Cytofluorimetric profile of SCF specific binding and SCF binding inhibition after coincubation with 20 μg of goat anti-human SCF neutralizing antibody in a representative case (6647, ES) of sarcoma cells. The heavy solid line represents the background of the negative staining control, the light solid line represents the SCF-FITC staining, and the dotted line represents the SCF-FITC staining after coincubation with anti-human SCF antibody.
Protein tyrosine phosphorylation induced by rhSCF.
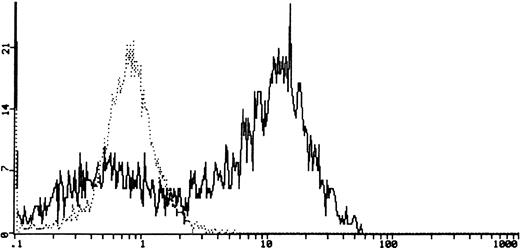
Using flow cytometry, we tested the ability of the SCF to induce protein tyrosine phosphorylation in the six cell lines. We observed a 10-fold increase, compared with untreated cells, of intracellular phosphotyrosine 10 minutes after stimulation with rhSCF (a representative case PDN12 is shown in Fig4). The specific c-kitphosphorylation after SCF treatment was shown by using Western blotting analysis on c-kit immunoprecipitates (Fig5).
Cytofluorimetric detection of intracellular phosphotyrosine in PDN12 (PNET) cell line. The dotted line (┈) represents phosphotyrosine in cells in serum-free condition for 24 hours. The solid line (—) shows the phosphotyrosine 10 minutes after stimulation with rhSCF (0.5 μg/106 cells).
Cytofluorimetric detection of intracellular phosphotyrosine in PDN12 (PNET) cell line. The dotted line (┈) represents phosphotyrosine in cells in serum-free condition for 24 hours. The solid line (—) shows the phosphotyrosine 10 minutes after stimulation with rhSCF (0.5 μg/106 cells).
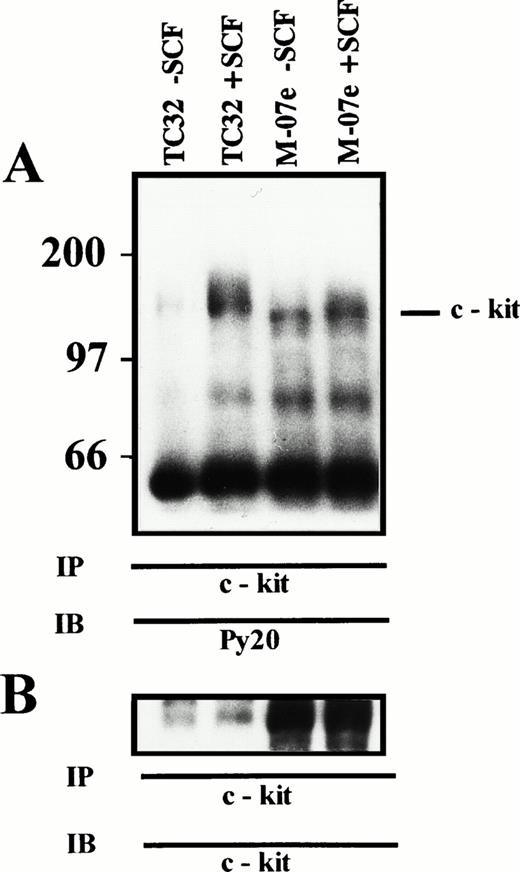
(A) SCF-induced tyrosine phosphorylation of c-kit. TC32 (PNET) and M-07e (control) cells were incubated for 10 minutes with 0.5 μg/106 cells rhSCF, lysed, and immunoprecipitated with c-kit antiserum. Proteins were resolved by 7% SDS-PAGE, transferred to PDVF, and immunoblotted with antiphosphotyrosine antibody. Molecular mass of protein standards are indicated in kD. (TC32 − SCF), TC32 untreated cells; (TC32 + SCF), TC32 SCF treated cells; (M-07e − SCF), M-07e untreated cells; (M-07e + SCF), M-07e treated cells. (B) c-kit Immunoblot. The immunoblot from (A) was stripped and reprobed with an anti–c-kit MoAb.
(A) SCF-induced tyrosine phosphorylation of c-kit. TC32 (PNET) and M-07e (control) cells were incubated for 10 minutes with 0.5 μg/106 cells rhSCF, lysed, and immunoprecipitated with c-kit antiserum. Proteins were resolved by 7% SDS-PAGE, transferred to PDVF, and immunoblotted with antiphosphotyrosine antibody. Molecular mass of protein standards are indicated in kD. (TC32 − SCF), TC32 untreated cells; (TC32 + SCF), TC32 SCF treated cells; (M-07e − SCF), M-07e untreated cells; (M-07e + SCF), M-07e treated cells. (B) c-kit Immunoblot. The immunoblot from (A) was stripped and reprobed with an anti–c-kit MoAb.
SCF and c-kit RNA expression.
All of the cellular lines studied, apart from TC106 (ES), expressed the mRNA of SCF (Fig 6). In addition, 13 sarcoma biopsies were examined for c-kit and SCF mRNA expression; 12 out of the 13 (92%) samples examined seemed to coexpress RNA for SCF and c-kit; whereas one sample only showed SCF mRNA (Fig 7A and B).
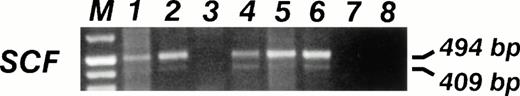
SCF mRNA RT-PCR analysis. The specific 409- and 494-bp PCR fragments are visible in 5 out of 6 (83%) of sarcoma cell lines. Lane 1, 6647 (ES); lane 2, TC32 (PNET); lane 3, TC106 (ES); lane 4, PDN13 (PNET); lane 5, PDN12 (PNET); lane 6, PDE02 (ES); lane 7, B lymphoblastoid EBV-transformed cell line SCF mRNA neg; lane 8, No DNA.
SCF mRNA RT-PCR analysis. The specific 409- and 494-bp PCR fragments are visible in 5 out of 6 (83%) of sarcoma cell lines. Lane 1, 6647 (ES); lane 2, TC32 (PNET); lane 3, TC106 (ES); lane 4, PDN13 (PNET); lane 5, PDN12 (PNET); lane 6, PDE02 (ES); lane 7, B lymphoblastoid EBV-transformed cell line SCF mRNA neg; lane 8, No DNA.
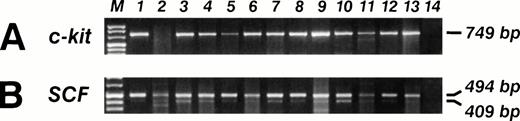
SCF and c-kit mRNA RT-PCR analysis in 13 sarcoma's biopsies (lanes 1 through 13), and B lymphoblastoid EBV-transformed cell line SCF and c-kit mRNA neg (lane 14). (A) The specific c-kit 749-bp PCR fragment is visible in 12 out of 13 (92%) samples. (B) The SCF specific 409- and 494-bp PCR fragments are visible in 13 out of 13 (100%) samples.
SCF and c-kit mRNA RT-PCR analysis in 13 sarcoma's biopsies (lanes 1 through 13), and B lymphoblastoid EBV-transformed cell line SCF and c-kit mRNA neg (lane 14). (A) The specific c-kit 749-bp PCR fragment is visible in 12 out of 13 (92%) samples. (B) The SCF specific 409- and 494-bp PCR fragments are visible in 13 out of 13 (100%) samples.
Detection of SCF production by tumor cell lines.
Detectable levels, higher than medium alone, were found in five of six supernatants. The TC106 (ES) cell line showed no SCF production compared with medium alone. Four cell lines showed a low production level (less than 12 pg), whereas TC32 (PNET) production was 36 pg (Table 2).
Soluble SCF Production by Sarcoma Cell Lines
| Cell Line . | SCF Production (pg/mL) . |
|---|---|
| PDN12 | 12.0 |
| TC32 | 36.0 |
| PDN13 | 8.5 |
| 6647 | 5.5 |
| PDE02 | 9.0 |
| TC106 | 5.0 |
| Medium alone | 5.0 |
| Cell Line . | SCF Production (pg/mL) . |
|---|---|
| PDN12 | 12.0 |
| TC32 | 36.0 |
| PDN13 | 8.5 |
| 6647 | 5.5 |
| PDE02 | 9.0 |
| TC106 | 5.0 |
| Medium alone | 5.0 |
Soluble SCF production by Sarcoma cell lines (PNET—PDN12, PDN13, and TC32; ES—6647, PDE02, and TC106) grown in serum-free medium assessed using enzyme immunoassay.
Effect of the SCF on apoptosis.
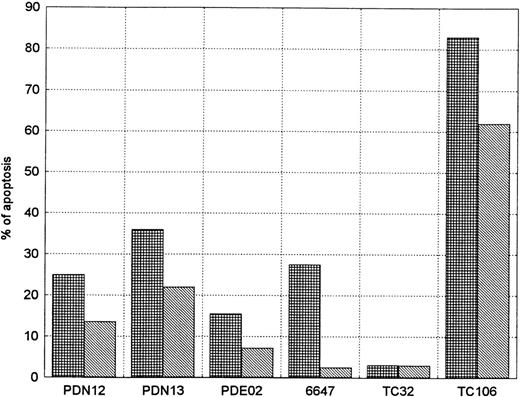
After 24 hours, five out of the six cell lines (without SCF) showed apoptosis ranging from 15.5% in PDE02 (ES) to 83% in TC106 (ES). This phenomenon was not present in TC32 (PNET). SCF addition, in the responders' cell lines, showed a decrease in the percentage of apoptotic cells in relation to nontreated cells, with a more evident decrease in 6647 (ES) (90%) and in PDE02 (ES) (54%) (Fig8).
SCF activity on apoptosis inhibition in sarcoma cell lines. The evaluation was performed in serum-free conditions 24 hours after administration of exogenous SCF (0.5 μg/106 cells). Each experiment was performed at least twice (mean of two reproducible experiments). Five of six (83%) of the cell lines showed an evident reduction in percentage of the apoptotic cells. (▦), SCF−; (▧), SCF+.
SCF activity on apoptosis inhibition in sarcoma cell lines. The evaluation was performed in serum-free conditions 24 hours after administration of exogenous SCF (0.5 μg/106 cells). Each experiment was performed at least twice (mean of two reproducible experiments). Five of six (83%) of the cell lines showed an evident reduction in percentage of the apoptotic cells. (▦), SCF−; (▧), SCF+.
S Phase and apoptosis after exposure to anti–c-kitneutralizing MoAb.
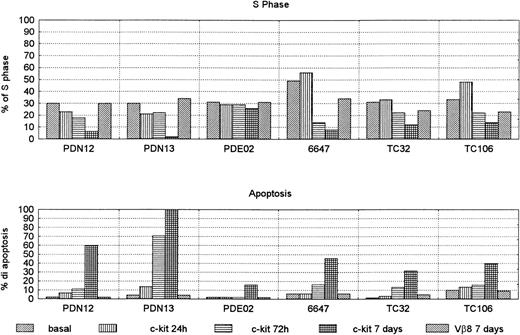
To test whether c-kit expression contributes to Sarcoma cell proliferation or apoptosis, the cell lines were treated in culture with neutralizing c-kit MoAb. After culture with anti–c-kitneutralizing antibody, cell cycle analysis in three cell lines (6647 [ES], TC32 [PNET], and TC106 [ES]) showed an increase of the S phase after 24 hours and a decrease at 72 hours, with a maximum effect after 7 days (Fig 9). In the last 2 PNET cell lines (PDN12 and PDN13) the addition of neutralizing anti–c-kit antibody had a decreasing effect of S phase at 24 hours, reaching 0 value in the PDN13 after 7 days of culture.
S phase and apoptosis in sarcoma cell lines treated with anti c-kit neutralizing antibody 0.4 μg/104 cells for 24 hours, 72 hours, and 7 days. The cells were grown in complete medium. Control cells were treated with Vβ-8 antibody, and the value at 7 days was reported (the results at 24 hours and 72 hours were comparable). Each experiment was performed in duplicate (mean of two reproducible experiments).
S phase and apoptosis in sarcoma cell lines treated with anti c-kit neutralizing antibody 0.4 μg/104 cells for 24 hours, 72 hours, and 7 days. The cells were grown in complete medium. Control cells were treated with Vβ-8 antibody, and the value at 7 days was reported (the results at 24 hours and 72 hours were comparable). Each experiment was performed in duplicate (mean of two reproducible experiments).
All cell lines showed increased apoptotic phenomenon. In fact, on day 7, five out of six (83%) had more than 30% apoptotic cells (range, 31.5% to 100%). By contrast, the control cells treated with Vβ-8 antibody at the same dilution did not modify the apoptosis of S phase. The PDE02 (ES) cell line showed only 16% of apoptosis after 7 days (Fig 10), but a longer incubation time (14 days) increased apoptotic percentage (50%) (data not shown).
Specific action of c-kit neutralizing antibody in two lines. PDE02 (ES) (A,C,E,G) and 6647 (ES) (B,D,F,H) cultured in complete medium. Cytofluorimetric profile of the propidium iodide staining of DNA content in basal condition (A,B) with 0.4 μg/104 cells of Vβ-8 for 7 days (C,D), with 0.4 μg/104 cells of anti–c-kit neutralizing antibody for 72 hours (E,F) and for 7 days (G,H). The reduction of S phase and the increment of the apoptotic phenomenon appear at 72 hours (E,F) and is more evident after 7 days (G,H) (13% in PDE02 and 46% in 6647). These results were confirmed by TdT assay.
Specific action of c-kit neutralizing antibody in two lines. PDE02 (ES) (A,C,E,G) and 6647 (ES) (B,D,F,H) cultured in complete medium. Cytofluorimetric profile of the propidium iodide staining of DNA content in basal condition (A,B) with 0.4 μg/104 cells of Vβ-8 for 7 days (C,D), with 0.4 μg/104 cells of anti–c-kit neutralizing antibody for 72 hours (E,F) and for 7 days (G,H). The reduction of S phase and the increment of the apoptotic phenomenon appear at 72 hours (E,F) and is more evident after 7 days (G,H) (13% in PDE02 and 46% in 6647). These results were confirmed by TdT assay.
Effect of c-kit antisense oligonucleotides on growth of cell lines.
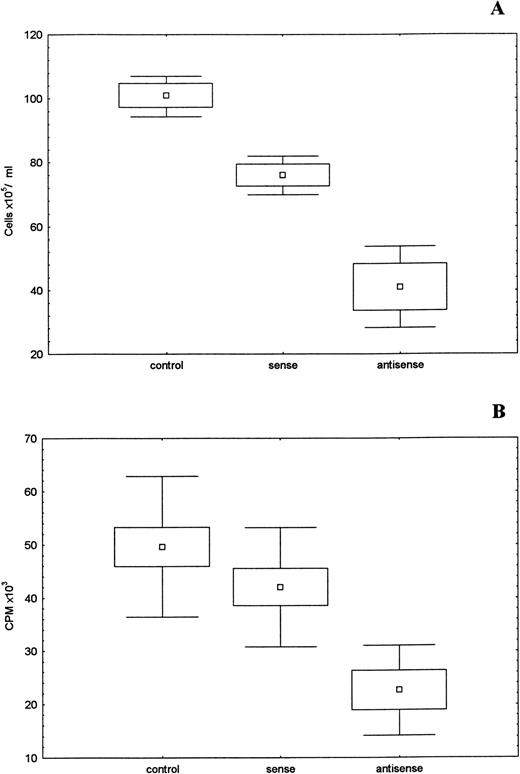
To study the specific role of the SCF receptor, we examined the effect of c-kit sense and antisense oligonucleotides on the growth of two sarcoma cell lines. The treatment of 6647 (PNET) and TC32 (ES) cell lines with c-kit antisense oligonucleotides resulted in an inhibition of cell growth. Treated cells showed a growth depression in comparison with untreated cells or sense-oligos–treated cells. This observation was directly shown by the significant (P < .001) reduction in the absolute number of cells. Even the 3H-thymidine uptake data showed a significant (P < .001) reduction of growth activity in the oligo-treated cells (Fig 11A and B).
Cell growth inhibition by c-kit antisense oligonucleotides. Untreated and treated 6647 cells (sense or antisense oligos 2 μmol/L) incubated 7 days at 37°C 5% CO2. (A) Absolute number of cells, mean of three different experiments. (□), mean; (□), SD; (⊥) SEM. (B) 6647 oligos-treated cells were incubated in triplicate for 6 hours with 4 μCi/mL of3H-thymidine. (□), mean; (□), SD; (⊥), SEM. The differences between treated and untreated cells showed a statistical significance (P < .001).
Cell growth inhibition by c-kit antisense oligonucleotides. Untreated and treated 6647 cells (sense or antisense oligos 2 μmol/L) incubated 7 days at 37°C 5% CO2. (A) Absolute number of cells, mean of three different experiments. (□), mean; (□), SD; (⊥) SEM. (B) 6647 oligos-treated cells were incubated in triplicate for 6 hours with 4 μCi/mL of3H-thymidine. (□), mean; (□), SD; (⊥), SEM. The differences between treated and untreated cells showed a statistical significance (P < .001).
DISCUSSION
This is the first study that underlines the presence of the c-kit/SCF system in ES and PNET and indicates that SCF is involved in cellular proliferation and, above all, is protecting cells from apoptosis. c-kit Was found in all of the cell lines examined, with a consistent level of positivity that was comparable, in certain cases, with that of the M-07e line which was used as a positive control. The binding experiments and the intracellular phosphotyrosine and immunoprecipitation studies showed how this receptor is both specifically and functionally activated by the exogenus SCF. Furthermore, five out of the six cell lines expressed the mRNA of the SCF. Correspondingly, the analysis of the mRNA of c-kit and SCF performed on bioptic samples confirmed and reinforced these observations, suggesting that the hypothesis of a c-kit/SCF autocrine loop.
Significant levels of SCF in the medium were only found in one cellular line (TC32). This may indicate that there was either a failed translation of the message or immediate consumption, by means of interaction with the receptor. The experiments with c-kitblocking antibody and antisense oligonucleotides could suggest that the second hypothesis is more likely. On the other hand, the receptor's activation in TC32 cell line after exposure to exogenous SCF could indicate that endogenus SCF is not able to activate all receptors.
Moreover, the addition of exogenous SCF in the serum-free culture, the c-kit receptor block, and the antisense oligonucleotide experiments (performed only on two cellular lines: 6647 [ES] and TC32 [PNET]), clearly indicated that the SCF is capable of both increasing cellular proliferation and, more importantly, inhibiting the apoptosis in the ES and PNET.
This fact is hardly surprising for a variety of reasons. SCF plays a crucial role in the hematopoiesis, development, and organization of the neural crest and the cells that derive from the crest (eg, melanocytes).4 In the hematopoiesis, in synergy with other growth factors, it stimulates the proliferation of the more immature staminal cells and the commissioned progenitors, protecting them from apoptosis.7,8,24-26 In certain acute myeloid leukemias, the SCF, either by itself or together with other growth factors, is capable of stimulating the self-maintenance and proliferation of leukemia blasts.12 In some human melanoma lines that present mRNA for both SCF and c-kit, the SCF can increase the percentage of S phase cells and the formation of colonies in agar.27Similar results were obtained in neuroblastoma, infant tumors of neuroectoderm derivation, producing the functional block of the c-kit receptors with an MoAb. In this manner Cohen et al15 were the first to assume the function of the autocrine loop between the SCF and its receptor, but a more recent study further indicated that the main role of the SCF in neuroblastomas could be that of protecting the cells from apoptosis.16
Our study underlines the presence of this growth factor and its receptors in ES and PNET, and also explains a functional apoptosis-preventing role. Even if the data indicate that prevention of apoptosis is not complete and decreases if the experiments lasted for 3 days (data not shown), it however suggests that other factors are necessary for the complete cell survival. Whether c-kit and SCF play a significant role in the growth of these soft tissue sarcoma of neuroectodermic origin remains to be determined. The hypothesis that the SCF and c-kit mRNA transcripts from tumor samples were not derived from tumor cells themselves, but from contaminating hematopoietic cells within the sample is not probable fact. All the biopsies were obtained from massively infiltrated tissue. Furthermore, in two cases in which suitable samples were available the c-kit expression was shown by flow cytometry (data not shown). The results obtained on the cellular lines would indicate that SCF/c-kit plays a functional role. Therefore, it can be strongly suggested that this role is also present in tumors in vivo. This data also suggests that among the growth factors used in therapeutic protocol, SCF must be used cautiously with these patients.
ACKNOWLEDGMENT
We thank Fabio Malavasi, MD, for providing the Vβ-8 antibody; F. Lund-Johansen, MD, for providing the Py-PE antibody; and A. Pagani, MD, for providing cell lines. We are indebted to Mary Lo Bianco for reviewing the English language of the manuscript.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (A.I.R.C., Milan) and Consiglio Nazionale delle Ricerche (CNR, Rome) grant no. 97.04176.CT04 to E.M. and G.B., MURST ex 60% University of Turin.
Address reprint requests to Enrico Madon, MD, Dept of Pediatrics, University of Turin, Piazza Polonia, 94, 10126 Torino, Italy
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal