Abstract
We have used ultrathin cryosectioning and immunogold cytochemistry to study the position of α-granules in the endocytic and biosynthetic pathways in megakaryocytes and platelets. Morphologically, we distinguished three types of granules; so-called multivesicular bodies type I (MVB I) with internal vesicles only, granules with internal vesicles and an electron dense matrix (MVB II), and the α-granules with mainly a dense content and often internal membrane vesicles at their periphery. The MVBs were prominent in cultured megakaryocytes and the megakaryoblastic cell line CHRF-288, but were less numerous in bone marrow megakaryocytes and platelets, whereas α-granules were most prominent in mature bone marrow megakaryocytes and in platelets. The internalization kinetics of bovine serum albumin-gold particles and of fibrinogen positioned the MVB subtypes and α-granules sequentially in the endocytic pathway. MVBs contained the secretory proteins von Willebrand factor (vWF) and β-thromboglobulin (β-TG), the platelet-specific membrane protein P-selectin, and the lysosomal membrane protein CD63. Within the MVBs, endocytosed fibrinogen and endogenous β-TG were restricted to the matrix, while vWF was predominantly associated with internal vesicles. CD63 was also observed in association with internal membrane vesicles in the α-granules. These observations, and the gradual morphologic transition from granules containing vesicles to granules containing predominantly dense material, suggest that MVBs represent a developmental stage in α-granule maturation.
AT SITES OF VASCULAR injury, circulating platelets adhere rapidly to the subendothelium and become activated. They subsequently release their granule content and the released proteins in turn contribute to the formation of a platelet aggregate. Most functional proteins are delivered to the major storage compartment in platelets, the α-granules. These proteins may derive from different origins. Von Willebrand factor (vWF) andβ-tromboglobulin (β-TG) are synthesized in the platelet precursor cell, the megakaryocyte, while fibrinogen, also a major component of α-granules, is not synthesized by the megakaryocytes,1-3 but is exclusively recruited from plasma.4-6 The platelet α-granule therefore represents a unique storage compartment that stores both endogenously synthesized proteins, as well as proteins derived from endocytic origin. It has been well documented that uptake and the delivery of fibrinogen to α-granules is mediated by glycoprotein IIb-IIIa (GPIIb-IIIa).7-9 Receptor-mediated endocytosis is a process by which cells internalize surface-bound molecules efficiently via specialized domains on the plasma membrane. In many cell types, clathrin-coated pits are considered to play a key role in the selective uptake of ligands by their appropriate receptor,10 but clathrin-independent mechanisms of internalization have been described as well.11 Endocytosis and incorporation of fibrinogen into the α-granules require surface binding, internalization, and proper intracellular targeting. Little is known about the intracellular trafficking of proteins in megakaryocytes and platelets, especially with regard to the intracellular routing of fibrinogen following internalization and delivery to α-granules. The purpose of the present study was to examine the position of α-granules in the intracellular pathways of endocytosis and biosynthesis. We used ultrathin cryosectioning and immunoelectron microscopy on platelets, bone marrow megakaryocytes (ex vivo and cultured), and on the megakaryoblastic cell line CHRF-288. The formation of α-granules with respect to the endocytic and biosynthetic pathways is discussed and a multivesicular precursor organelle of α-granules is described.
MATERIALS AND METHODS
Tracers and antibodies.
Bovine serum albumin (BSA) was conjugated to 5 nm colloidal gold particles according to standard procedures.12 Before use, the gold probe was dialyzed against culture medium without albumin. Rabbit polyclonal GPIIb-IIIa and rabbit polyclonal P-selectin antibodies were a gift from Dr M.C. Berndt (Melbourne, Australia) and have been previously described.13 Rabbit polyclonal human fibrinogen and human von Willebrand factor (vWF) antibodies were purchased from Dakopatts (Glostrup, Denmark). The rabbit polyclonalβ-tromboglobulin (β-TG) antiserum was exclusively directed to human platelet β-TG.14 Endosomes were distinguished by using a rabbit polyclonal antibody directed against the 300-kD cation-independent mannose-6-phosphate receptor (CI-MPR), which is present in endosomes and absent from lysosomes.15-17 To distinguish lysosomes, a monoclonal antibody RUU-SP 28, that recognizes the lysosomal integral membrane protein CD63,18,19 was used, and rabbit polyclonal antibodies directed to the lysosomal enzymesβ-hexosaminidase, cathepsin-D,17,20 and lysosomal acid phosphatase.21 Secondary antibodies swine antirabbit IgG and rabbit antimouse IgG were purchased from Sigma (St Louis, MO). Protein A-gold particles of 10 and 15 nm were prepared according to Slot and Geuze.12
Platelets and bone marrow megakaryocytes.
Blood was taken from healthy donors and was anticoagulated with 1/10 vol of 0.13 mol/L sodium citrate. Platelet-rich-plasma (PRP) was prepared by centrifugation at 180g for 10 minutes at 22°C. The PRP was mixed in a 1:1 ratio with Krebs Ringer Glucose, pH 5.0 (4 mmol/L KCl, 100 mmol/L NaCl, 20 mmol/L NaHC03, 2 mmol/L Na2SO4, 4.7 mmol/L citric acid, 14.2 mmol/L tri-sodium citrate, and 5 mmol/L glucose) and washed again with the same solution at pH 6.0. Normal bone marrow samples were obtained after informed consent from a patient undergoing heart surgery. The megakaryocyte-rich cell suspension was obtained after centrifugation on Ficoll-Paque (Research Grade, Pharmacia, Uppsala, Sweden).
Megakaryocyte culture.
Megakaryocytes were cultured as previously described.22Briefly, bone marrow cells were obtained after informed consent from donors undergoing bone marrow transplantation. The cells were separated by density centrifugation on Ficoll-Paque (density < 1.077 g/mL) and were cultured in a liquid Iscove's medium (GIBCO, BRL Life Technologies, Paisly, UK) containing 10% aplastic plasma (from thrombocytopenic patients after bone marrow transplantation) and 1% deionized BSA. Nonadherent cells were transferred into culture flasks to enrich the megakaryocyte population. Cells were cultured at 37°C for 13 ± 2 days in a 5% CO2 fully humidified atmosphere.
Megakaryoblastic cell line CHRF-288.
The CHRF-288 cell line, originally derived from a patient with an acute megakaryoblastic leukemia, was a kind gift from Drs M. Liebermann and D. Witte (Cincinnati, OH).23 The cell line was cultured in Fisher's medium (GIBCO, BRL Life Technologies), in the presence of 2 mmol/L L-glutamin, 100 U/mL penicillin, and 100 U/mL streptomycin and 20% heat inactivated horse serum at 37°C in a humidified atmosphere with 5% CO2. An enzyme-linked immunosorbent assay (ELISA) was performed to check the vWF and fibrinogen content in the medium and the immunoreactivity of the antibodies. The rabbit antihuman vWF antibody did not cross-react with vWF in horse serum.
Internalization studies.
Megakaryocytes were used for internalization studies after 11 to 13 days of culture in medium depleted of fibrinogen. The megakaryocytes were incubated for 30 minutes, 60 minutes, and overnight in liquid Iscove's medium, containing 20% normal citrated plasma, to which 150 U/mL desulphatohirudin was added (a kind gift of Dr R.B. Wallis, Ciba-Geigy Pharmaceuticals, Horsham, UK). Control megakaryocytes were directly fixed. Incubation was at 37°C in a 5% CO2, fully humidified atmosphere. For BSA-gold uptake, the cells were rinsed once with albumin-free medium and incubated for 5, 15, 30, 60, and 120 minutes in medium containing BSA-gold (final optical density [OD] 2.0). Immediately after internalization, the cells were put on ice, rinsed once with ice-cold phosphate-buffered saline (PBS) (0.15 mol/L NaCl, 0.1 mol/L phosphate buffer, pH 7.4), and fixed for 1 hour in a mixture of 2% paraformaldehyde and 0.2% glutaraldehyde (final concentration) in 0.1 mol/L phosphate buffer (pH 7.4) at 4°C. Internalization studies in the megakaryoblastic cell line CHRF-288 were performed as described for cultured megakaryocytes.
Electron microscopy.
For standard electron microscopy, cells were fixed at room temperature for 1 hour in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4. They were then washed three times in 0.1 mol/L sodium cacodylate buffer and postfixed for 1 hour in 1% aqueous OsO4. Cells were rinsed three times in distilled water, dehydrated in a graded series of ethanol, and embedded in Epon.
Cryosectioning and immunolabeling.
After fixation, the cells were rinsed three times in 0.1 mol/L phosphate buffer (pH 7.4), and resuspended in 10% gelatin in PBS. The gelatin was solidified at 4°C and cut into small blocks. After infiltration in 2.3 mol/L sucrose at 4°C, the cells were frozen in liquid nitrogen. Ultrathin cryosectioning was performed on a Reichert Ultracut S microtome (Leica Aktiengesellschaft, Reichert Division, Wien, Austria), using a cryo-diamond knife (Diatome, Biel, Switzerland). Sections were picked up with a 2.3 mol/L sucrose drop, or using a recently developed method,24 with a mixture of 2.3 mol/L sucrose and 2% nonbuffered methyl cellulose (25 centipoise; Fluka AG, Buchs, Switzerland), followed by immunolabeling. Alternatively, sections were picked up using a mixture of 1.8% methyl cellulose and 2% uranyl acetate, transferred to formvar carbon-coated copper grids, and immediately embedded in a mixture of 1.8% methyl cellulose and 0.3% uranyl acetate at 4°C (“direct view” method). Immunolabeling was performed at room temperature by floating on drops containing the diluted antibodies, as described previously.25 After incubation, the sections were washed in distilled water and stained for 5 minutes with uranyl-oxalate, pH 7.0, washed again, and embedded in a mixture of 1.8% methyl cellulose and 0.3% uranyl acetate at 4°C.26
Immunogold double labeling was performed as described by Slot et al.27 Briefly, the single-labeled sections were put successively on drops containing PBS, 1% glutaraldehyde in PBS (5 minutes), PBS, PBS/0.02% glycine, and PBS/1% BSA before starting the next incubation. In the internalization experiments where 5 nm BSA-gold was used, we used protein-A gold sizes of 10 nm and 15 nm for the detection of the marker proteins. In studies of fibrinogen uptake, we usually combined the 5-nm gold probe for fibrinogen detection with subsequent 10-nm and 15-nm gold probes for other marker proteins. The specificity of immunolabeling was verified using an irrelevant control antibody. The sections were examined in a Philips CM 10 (Philips, Eindhoven, the Netherlands) or JEOL 1200 EX (JEOL, Tokyo, Japan) electron microscope.
In an attempt to study the kinetics of endocytosis, the number of gold particles associated with each compartment was scored at selected time points. Similarly, kinetic data of endocytosis were obtained in CHRF-288 cells by comparing BSA-gold uptake in MPR- and CD63-positive structures after 10-minute and 60-minute incubations, respectively. The intracellular localization of internalized fibrinogen was determined after overnight incubation with medium containing 20% normal plasma. The subcellular distribution of GPIIb-IIIa, P-selectin, and CD63 antigen, and the biosynthetic proteins vWF and β-TG was determined in the cultured megakaryocytes, as well. For all quantitative data, the megakaryocytes were selected at low magnification and the number of gold particles associated with the various intracellular compartments were counted on micrographs at a nominal magnification of 12,000×.
RESULTS
Structural characteristics of MVBs and α-granules.
The cultured megakaryocytes showed all stages of maturation. Immature megakaryocytes contained only a few α-granules, and generally no demarcating membrane system (DMS), while the large mature megakaryocytes, like those from bone marrow, had a well developed DMS and numerous α-granules. Megakaryoblasts and immature megakaryocytes could also be identified by their differential immunoreactivity for platelet-specific marker proteins such as GPIIb-IIIa, P-selectin, vWF, and β-TG (see below). All maturation stages showed multivesicular bodies (MVBs), and α-granules (Figs 1-3). MVBs were most numerous in the immature megakaryocytes in culture, but were found in bone marrow megakaryocytes (see Fig 6B) and in platelets as well (Fig 4A and C). In the megakaryocytes, MVBs were often located in the trans-Golgi area. The internal membrane vesicles measured 30 to 70 nm and were best visualized using recently developed modifications for the processing of ultrathin cryosections.24 Based on their internal morphology, two classes of MVBs could be distinguished: those showing abundant internal membrane vesicles only (MVB I) (Fig 4A and C), and those containing electron-dense material, together with internal membrane vesicles (MVB II) (Fig 1B). In many type II MVBs, the internal vesicles were situated at the periphery, leaving an electron-dense matrix either in the center or somewhat eccentric (Fig 3B). Internal vesicles were also observed in the periphery of α-granules (Fig 4B and D), but we have not found the tubular elements in the α-granules.28
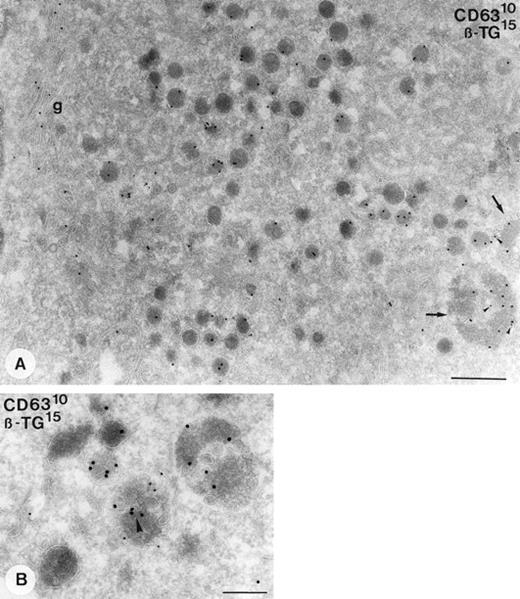
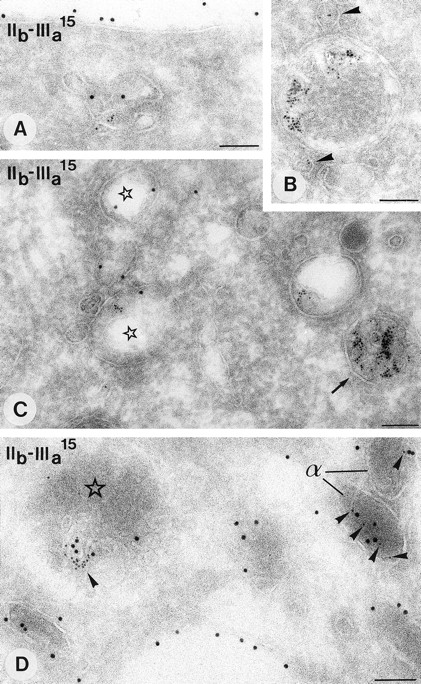
Demonstration of β-TG and CD63 in cultured megakaryocytes. Immunogold double-labeling as indicated on the figure. (A) Specific labeling of β-TG is demonstrated in the Golgi complex (g), α-granules, and MVB II (arrows). Colocalization with CD63 (arrowheads) is restricted to the MVBs. (B) Detailed demonstration of colocalization of β-TG with CD63 in a MVB II. Note that the labeling of CD63 is associated with membranes of the internal vesicles and that of β-TG with the matrix. Bars A, 500 nm; B, 200 nm.
Demonstration of β-TG and CD63 in cultured megakaryocytes. Immunogold double-labeling as indicated on the figure. (A) Specific labeling of β-TG is demonstrated in the Golgi complex (g), α-granules, and MVB II (arrows). Colocalization with CD63 (arrowheads) is restricted to the MVBs. (B) Detailed demonstration of colocalization of β-TG with CD63 in a MVB II. Note that the labeling of CD63 is associated with membranes of the internal vesicles and that of β-TG with the matrix. Bars A, 500 nm; B, 200 nm.
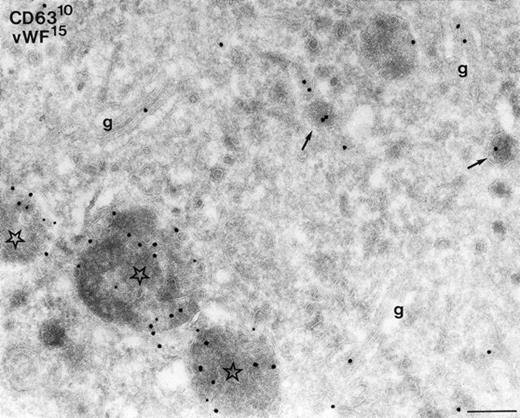
Colocalization of vWF and CD63 in cultured megakaryocytes. Labeling as indicated on the figure. Characteristic labeling of vWF in Golgi-stacks (g), α-granules (arrows), but also in MVBs containing CD63 (asterisks). Note that vWF is often associated with the internal vesicles. Bar, 200 nm.
Colocalization of vWF and CD63 in cultured megakaryocytes. Labeling as indicated on the figure. Characteristic labeling of vWF in Golgi-stacks (g), α-granules (arrows), but also in MVBs containing CD63 (asterisks). Note that vWF is often associated with the internal vesicles. Bar, 200 nm.
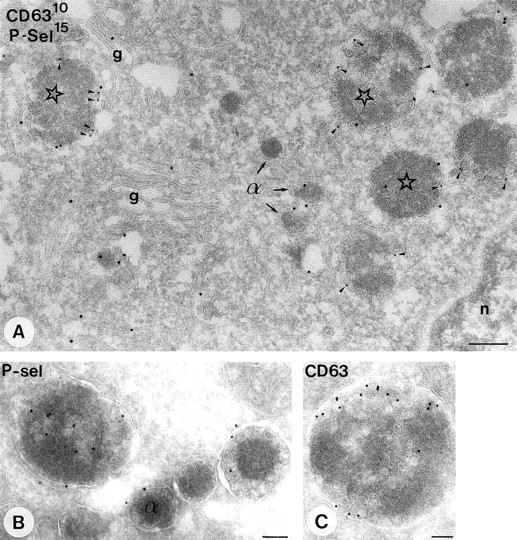
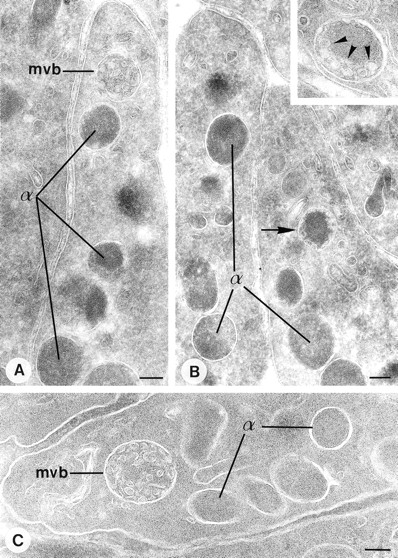
Demonstration of P-selectin and CD63 in cultured megakaryocytes. Labeling as indicated on the figure. P-selectin is present in the Golgi-complex (g), α-granules (α), and MVBs (asterisks). Colocalization with CD63 (arrowheads) is restricted to the MVBs. (B and C) Selected MVBs from megakaryocyte origin. Immunolabeling as indicated on the figure. Bar, 250 nm.
Demonstration of P-selectin and CD63 in cultured megakaryocytes. Labeling as indicated on the figure. P-selectin is present in the Golgi-complex (g), α-granules (α), and MVBs (asterisks). Colocalization with CD63 (arrowheads) is restricted to the MVBs. (B and C) Selected MVBs from megakaryocyte origin. Immunolabeling as indicated on the figure. Bar, 250 nm.
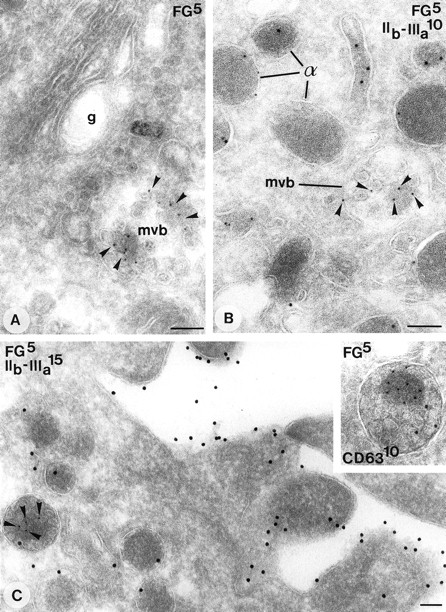
Internalization of fibrinogen. (A and C)Overnight incubation of cultured megakaryocytes with medium containing 20% normal plasma. Labeling as indicated on the figures. (A) Type I MVB in close aposition to the Golgi complex (g) containing fibrinogen. (B) Demonstration of fibrinogen in a type I MVB (arrowheads) in bone marrow megakaryocyte. (C) Typical matrix labeling of internalized fibrinogen in type II MVBs. Inset: Detail of a type II MVB showing fibrinogen associated with the matrix, and CD63 typically with the internal vesicles. Bars, 100 nm.
Internalization of fibrinogen. (A and C)Overnight incubation of cultured megakaryocytes with medium containing 20% normal plasma. Labeling as indicated on the figures. (A) Type I MVB in close aposition to the Golgi complex (g) containing fibrinogen. (B) Demonstration of fibrinogen in a type I MVB (arrowheads) in bone marrow megakaryocyte. (C) Typical matrix labeling of internalized fibrinogen in type II MVBs. Inset: Detail of a type II MVB showing fibrinogen associated with the matrix, and CD63 typically with the internal vesicles. Bars, 100 nm.
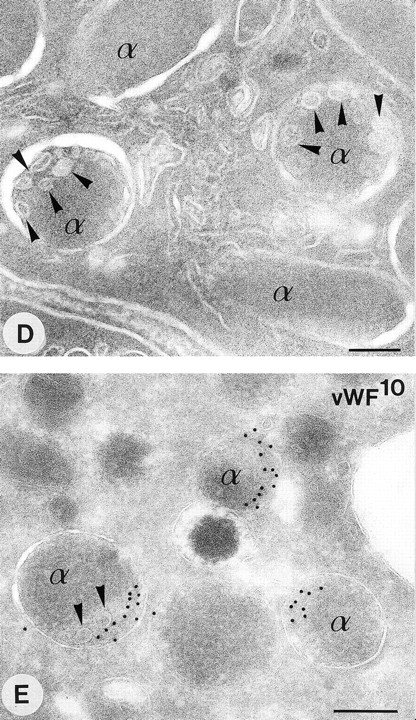
Multivesicular bodies and α-granules in human platelets. Modified pick up approach applied to thin frozen sections as described in Materials and Methods. (A and B) Methyl cellulose sucrose pick up after immuno-gold labeling with a nonspecific control antibody and protein-A gold. (C and D) “direct-view” method. (E and F) Immunolabeling as indicated on the figure. The arrow in (B) indicates an α-granule containing internal vesicles at the periphery. Inset: Higher magnification of an α-granule with eccentrically located internal vesicles. (D) Arrowheads indicate peripheral intragranular vesicles. (E) Immunolabeling of vWF using 10 nm protein-A gold. Gold labeling is located at the eccentric rims of the α-granules. Arrowheads indicate two internal vesicles ≈ 40 nm in diameter. (F) Demonstration of CD63 in platelet α-granules using 5 nm protein-A gold. Arrowheads indicate CD63 associated with internal vesicles, which are often located at extended tips of the α-granules. MVB, multivesicular body, α, α-granules. Bars, 100 nm.
Multivesicular bodies and α-granules in human platelets. Modified pick up approach applied to thin frozen sections as described in Materials and Methods. (A and B) Methyl cellulose sucrose pick up after immuno-gold labeling with a nonspecific control antibody and protein-A gold. (C and D) “direct-view” method. (E and F) Immunolabeling as indicated on the figure. The arrow in (B) indicates an α-granule containing internal vesicles at the periphery. Inset: Higher magnification of an α-granule with eccentrically located internal vesicles. (D) Arrowheads indicate peripheral intragranular vesicles. (E) Immunolabeling of vWF using 10 nm protein-A gold. Gold labeling is located at the eccentric rims of the α-granules. Arrowheads indicate two internal vesicles ≈ 40 nm in diameter. (F) Demonstration of CD63 in platelet α-granules using 5 nm protein-A gold. Arrowheads indicate CD63 associated with internal vesicles, which are often located at extended tips of the α-granules. MVB, multivesicular body, α, α-granules. Bars, 100 nm.
Immunocytochemical characterization of MVBs and α-granules in cultured megakaryocytes.
Immunogold labeling studies were performed on cryosections of cultured megakaryocytes and platelets with antibodies directed against platelet specific membrane proteins (GPIIb-IIIa and P-selectin), secretory proteins (vWF and β-TG), and lysosomal markers (CD63, cathepsin-D and lysosomal acid phosphatase), to further characterize the MVBs and α-granules.
β-TG and vWF.
β-TG (Fig 1) and vWF (Fig 2) were present in the Golgi-complex and trans-Golgi reticulum (TGR), in both types of MVBs and in the α-granules. A quantitative evaluation of the gold labeling patterns is presented in Table 1. Of both vWF and β-TG, the majority of the labeling occurred on the α-granules. There was threefold more labeling of β-TG in the Golgi/TGR than of vWF, while in MVB I and II, β-TG labeling was about half of that of vWF. VWF was often associated with the internal vesicles (Fig 2), whereas β-TG was predominantly associated with the matrix of type II MVBs (Fig 1B). The pattern of labeling for vWF within the α-granules was eccentric, as has been described before,28 and colocalized with the electron-lucent area and occasionally with the small internal vesicles at the eccentric rims (Fig 4E). β-TG was predominantly associated with the matrix of the α-granules (Fig 1A and B).
Relative Label Distribution of Platelet-Specific Markers in Cultured Megakaryocytes
| . | Golgi/TGR . | End . | MVB I . | MVB II . | α . | PM/DMS . |
|---|---|---|---|---|---|---|
| vWF | 8.8 | 3.6 | 28.6 | 24.2 | 34.8 | ND |
| β-TG | 26.7 | 5.1 | 11.1 | 11.8 | 45.3 | ND |
| GPIIb-IIIa | 4.8 | 18.4 | 7.1 | 3.8 | 40.4 | 25.5 |
| P-selectin | 23 | 9.4 | 16.8 | 25.2 | 22.9 | 2.7 |
| CD63 | 1.5 | 14.1 | 43.2 | 39 | 2.2 | ND |
| . | Golgi/TGR . | End . | MVB I . | MVB II . | α . | PM/DMS . |
|---|---|---|---|---|---|---|
| vWF | 8.8 | 3.6 | 28.6 | 24.2 | 34.8 | ND |
| β-TG | 26.7 | 5.1 | 11.1 | 11.8 | 45.3 | ND |
| GPIIb-IIIa | 4.8 | 18.4 | 7.1 | 3.8 | 40.4 | 25.5 |
| P-selectin | 23 | 9.4 | 16.8 | 25.2 | 22.9 | 2.7 |
| CD63 | 1.5 | 14.1 | 43.2 | 39 | 2.2 | ND |
Gold particles were counted over cryosections of cultured megakaryocytes after immunolabeling as indicated. The gold particles were attributed to the selected compartments indicated at the top. The data are expressed as percentages of the total number of gold particles counted over the selected structures.
Abbreviations: PM, plasma membrane; DMS, demarcating membrane system; End, tubulo-vesicular structures reminiscent of endosomes; ND, not determined.
P-selectin and GPIIb-IIIa.
Although P-selectin (Fig 3), as well as GPIIb-IIIa localized to Golgi stacks, TGR, both types of MVBs, and α-granules, there were quantitatively major differences between the two membrane receptors (Table 1). Most of the GPIIb-IIIa was found in α-granules and minor amounts in MVBs, while P-selectin showed a more equal distribution over MVBs I and II and α-granules. In the α-granules, P-selectin was confined to the limiting membrane, and P-selectin was almost absent from the plasma membrane.
CD63 and lysosomal enzymes.
The integral lysosomal membrane protein CD63 was predominantly present in both types of MVBs (Figs 1-3), and specific labeling was observed in a small number of the α-granules, as well, which was best visualized when the immunolabeling was performed with 5 nm protein-A gold (Fig 4F). The lysosomal enzymes β-hexosaminidase, cathepsin-D, and lysosomal acid phosphatase showed only weak immunoreactivity in type I and II MVBs (not shown) and were undetectable in α-granules. The late endosomal marker CI-MPR could not be detected in MVBs and α-granules at all. The labeling of CD63 within the α-granules was observed at the periphery and was consistently associated with the internal vesicles (arrowhead in Fig4F).
Endocytosis of BSA-gold.
Fibrinogen present in the α-granules must be derived from the extracellular environment, as megakaryocytes do not synthesize fibrinogen.1-3 Thus, α-granules are connected to the endocytic pathway of which MVBs are known to be part. To study the relative positions of MVB types and α-granules in the endocytic pathway, we determined the arrival of internalized BSA-gold particles in MVBs and α-granules. Megakaryocytes were incubated with BSA-gold and samples were fixed after 5, 15, 30, and 120 minutes of incubation. BSA-gold was quantitated in lucent endosomes, MVBs, and α-granules. As can be seen in Table 2, at 5 minutes and 15 minutes, BSA-gold could only be detected in endosomes (Fig 5A through C) and MVB I. Starting at about 30 minutes, MVB II and α-granules became labeled. Characteristic examples of compartments that contained internalized BSA-gold are given in Fig 5. Thus, endosomes, MVBs type I and II, and α-granules are sequentially reached by the tracer particles. The sequence of BSA-gold arrival and the gradual morphologic change from a strictly multivesicular compartment toward a compartment with an increased protein content suggest that MVBs represent developmental stages of α-granules.
Appearance of BSA-Gold in MVBs and α-Granules of Cultured Megakaryocytes
| Incubation (min) . | End . | MVB I . | MVB II . | α . |
|---|---|---|---|---|
| 5 | 76 | 24 | 0 | 0 |
| 15 | 57 | 43 | 0 | 0 |
| 30 | 24 | 70 | 5 | 1 |
| 120 | 18 | 64 | 14 | 4 |
| Incubation (min) . | End . | MVB I . | MVB II . | α . |
|---|---|---|---|---|
| 5 | 76 | 24 | 0 | 0 |
| 15 | 57 | 43 | 0 | 0 |
| 30 | 24 | 70 | 5 | 1 |
| 120 | 18 | 64 | 14 | 4 |
Megakaryocytes were incubated for the indicated time intervals with medium containing 5 nm BSA-gold particles and processed for electron microscopy. The number of gold particles associated with each structure was scored at the selected time points. Numbers are expressed as percentages of the total and represent the average of three evaluations (n = 3).
Abbreviations: End, endosomes; MVBs I and II, multivesicular bodies type I and II; α, α-granules.
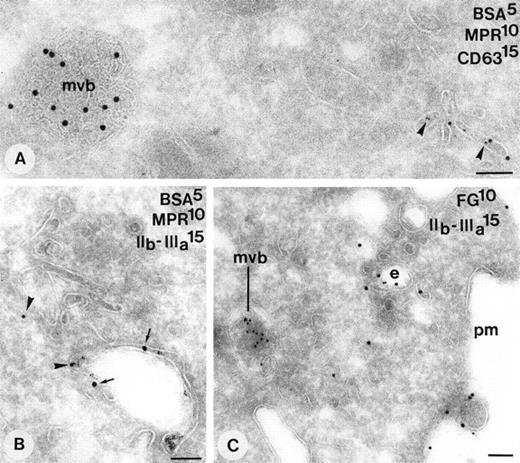
Endocytosis of BSA-gold (5 nm gold) in cultured megakaryocytes. Immunolabeling as indicated on the figure. Compartments that have incorporated the tracer are shown. (A) Characteristic tubulo vesicular endosome close to the plasma membrane containing also GPIIb-IIIa. (B) Endosome with adjacent small vesicles containing the tracer (arrowhead). (C) Tubulo vesicular endosomes (asterisks) and a type I MVB (arrow). (D) MVB type II (asterisk) and two adjacent α-granules (α) both contain the endocytic tracer (arrowheads). Bars, 100 nm.
Endocytosis of BSA-gold (5 nm gold) in cultured megakaryocytes. Immunolabeling as indicated on the figure. Compartments that have incorporated the tracer are shown. (A) Characteristic tubulo vesicular endosome close to the plasma membrane containing also GPIIb-IIIa. (B) Endosome with adjacent small vesicles containing the tracer (arrowhead). (C) Tubulo vesicular endosomes (asterisks) and a type I MVB (arrow). (D) MVB type II (asterisk) and two adjacent α-granules (α) both contain the endocytic tracer (arrowheads). Bars, 100 nm.
Endocytosis of fibrinogen.
After incubation of cultured megakaryocytes with 20% normal plasma for 30, 60, and 120 minutes, fibrinogen was found in the DMS and tubulo-vesicular endosomes adjacent to the plasma membrane. However, the α-granules were still negative at these times. Overnight incubation with plasma resulted in fibrinogen labeling in both types of MVBs (Fig 6A and C), and occasionally in α-granules. In bone marrow megakaryocytes, fibrinogen was more readily detected in the α-granules, and in MVBs as well (Fig 6B). In MVBs type II containing a matrix content, fibrinogen was predominantly associated with this electron-dense matrix (Fig 6C and inset).
Observations in the megakaryoblastic cell line CHRF-288.
The megakaryoblastic cell line CHRF-288 has been described as a useful model to study megakaryocyte function.23 We therefore compared immunocytochemical and endocytic characteristics of these cells with those of megakaryocytes in culture. The absence of a DMS in CHRF-288 cells facilitated the identification of tubulo-vesicular structures as endosomes. CHRF-288 cells possessed a heterogeneous population of granules with a variable number of internal membrane vesicles with or without an electron dense matrix. These MVBs were generally larger than those observed in platelets and in the cultured and bone marrow megakaryocytes. They have previously been suggested to represent α-granules.23 CHRF-288 cells contained only occasionally the classical α-granules, described above.
Distribution of CI-MPR and CD63.
The distribution of GPIIb-IIIa, P-selectin, and vWF in CHRF-288 cells was similar to that in megakaryocytes, but the reactivity was much lower. CI-MPR was detectable in tubulo-vesicular structures close to the plasma membrane (Fig 7A and B) and in an occasional type I MVB with a small number of internal vesicles. Some CI-MPR positive endosomes contained GPIIb-IIIa as well (Fig 7B). The majority of the MVBs in the CHRF-288 cells was positive for CD63 and negative for CI-MPR (Fig 7A).
Demonstration of endosomes in CHRF-288 cells. (A) 10-minute internalization of BSA-gold. Tracer is found in characteristic tubulo vesicular structures that are enriched in CI-MPR (arrowheads). MVBs positive for CD63 are negative for CI-MPR and do not contain the endocytic tracer at these times. (B) Simultaneous demonstration of CI-MPR (arrowheads) and GPIIb-IIIa (small arrows) in an endosome containing tracer. (C) Overnight incubation with medium containing 20% normal plasma. Simultaneous demonstration of fibrinogen and GPIIb-IIIa in an endosome close to the plasma membrane. mvb, multivesicular body; e, endosome; pm, plasma membrane. Bars, 100 nm.
Demonstration of endosomes in CHRF-288 cells. (A) 10-minute internalization of BSA-gold. Tracer is found in characteristic tubulo vesicular structures that are enriched in CI-MPR (arrowheads). MVBs positive for CD63 are negative for CI-MPR and do not contain the endocytic tracer at these times. (B) Simultaneous demonstration of CI-MPR (arrowheads) and GPIIb-IIIa (small arrows) in an endosome containing tracer. (C) Overnight incubation with medium containing 20% normal plasma. Simultaneous demonstration of fibrinogen and GPIIb-IIIa in an endosome close to the plasma membrane. mvb, multivesicular body; e, endosome; pm, plasma membrane. Bars, 100 nm.
Endocytosis of BSA-gold and fibrinogen.
Internalization of BSA-gold was followed in CHRF-288 as described for cultured megakaryocytes. Table 3 shows the slow progression of tracer from endosomes (Fig 7A and B) to MVBs. To further characterize the structures involved in endocytosis, immunodouble labeling was performed for CI-MPR and CD63 after respectively 10 minutes and 60 minutes of BSA-gold internalization. After 10 minutes, the tracer was located for 77% over tubulo-vesicular endosomes showing characteristic staining of CI-MPR (Fig 7A; Table 3), and for 23% over CD63 positive MVBs. Fibrinogen was not detected when CHRF-288 cells were cultured in serum depleted of fibrinogen. After a continuous overnight incubation with 20% normal plasma, fibrinogen was found in endosomes situated near the cell periphery that contained also detectable levels of GPIIb-IIIa (Fig 7C), and in many CD63 positive MVBs (not shown).
Internalization Kinetics of BSA-Gold in CHRF-288 Cells
Tubulo vesicular endosomes (End) and multivesicular bodies (MVB) positive for BSA-gold were scored after 5, 10, 30, and 60-minute intervals. Data are expressed as percentages of the total number of structures evaluated. Immunocytochemical characterization after respectively 10- and 60-minute BSA-gold uptake designated the tubulo vesicular structures as endosomes (MPR positive) and MVBs as (pre)-lysosomal (CD63 positive, MPR negative).
Number of structures evaluated.
DISCUSSION
We have studied the biogenesis of α-granules in platelets, bone marrow megakaryocytes, and in the megakaryoblastic cell line CHRF-288, using immunoelectron microscopy of cells that had internalized exogenous tracer particles or fibrinogen. Several observations indicated the existence of a multivesicular precursor organelle of α-granules, which, like MVBs in most other cells, were endocytic compartments. First, internalized tracer transited MVBs before reaching α-granules, which showed that α-granules represent a post-MVB stage in the endocytic pathway. Next, α-granules contained small membrane vesicles similar in size to the internal membrane vesicles of MVBs. The presence of such vesicles has not been described before and could be detected in our study because of the improved preservation of membranes in the cryosectioning method we used. These 30 to 70 nm vesicles were clearly different from the nonmembranous 20 nm tubules in α-granules described by others and which are probably composed of vWF multimers.29 The vesicles that we found most likely derive from inward vesiculation of the limiting membrane of the MVBs, as in other cell types.30 We have not found the tubular multimers, probably due to the absence of osmium in our preparation procedure.29 Also in support of a formational link between MVBs and α-granules was the identification of a MVB type sharing characteristics of both. This MVB type II contained material similar in density to the content of α-granules and which likewise showed labeling for fibrinogen. Fibrinogen in the α-granules derives from receptor-mediated endocytosis, which is consistent with the presence of the fibrinogen receptor in all MVB types and α-granules. The existence of an intermediate MVB type suggests a maturation of α-granules directly from type II MVBs. However, vesicular trafficking between MVBs and α-granules may also add to the biogenesis of α-granules. Finally, the early MVBs were most abundant in immature megakaryocytes and in the growth-arrested CHRF-288 cells, whereas the α-granules prevailed in the mature bone marrow megakaryocytes and in platelets. In most cell types, lysosomes represent the post-MVB stage in the endocytic route. Dense granules were not preserved in our cryosections, probably because of their extreme dense content. Therefore, the precise position of these organelles in the endocytic pathway and their relation to α-granules remains to be established.
As has been shown in other cells, the endocytic pathway in megakaryocytes probably is connected to the biosynthetic organelles, particularly the Golgi complex and trans Golgi network (TGN) by vesicular transport. Thus, α-granules contain several proteins including P-selectin and CD63 which in resting platelets are absent from the plasma membrane, but on stimulation are translocated to the cell surface by exocytosis of α-granules. Although not yet established, these proteins may be transported from the Golgi/TGN directly to the endocytic compartments, including MVBs and α-granules. Recently, we have shown that the glucose transporter-3 (Glut-3) is mainly localized to the α-granule membrane in resting platelets.31 Glut-3 is a resident plasma membrane protein in other cells and lacks consensus internalization motifs in its cytoplasmic domain.32 After thrombin activation of platelets, α-granules are released and Glut-3 is incorporated into the plasma membrane. Most likely, the Glut-3 in α-granules derives directly from the biosynthetic route in the megakaryocyte without passing through the cell surface. The identity of putative TGN to MVB/α-granule transport intermediates has not been established. However, small granules, emanating from the TGN during megakaryocyte maturation, have been proposed as precursor α-granules.33 These granules did not contain internal membrane vesicles, were smaller than the MVB that we observed, and may mediate transport from the biosynthetic apparatus to the endocytic route in megakaryocytes.
In the MVBs and α-granules, we found differential protein distributions in internal membranes, the limiting membranes, and the electron dense material in the matrices of type II MVBs and α-granules. Thus, the soluble proteins β-TG and fibrinogen were predominantly localized to the matrices, vWF and CD63 were enriched in the internal vesicles, and P-selectin and GPIIb-IIIa were associated both with the internal vesicles and limiting membrane. Differences in intragranular protein distribution have been reported before,14,28,34 35 and may reflect effective sorting and/or retention mechanisms during the formation of MVBs and α-granules.
We have found clathrin-coated buds on the surface of MVBs and in agreement with previous reports on the α-granules, as well (data not shown).36,37 The presence of clathrin coats has been shown on immature secretory granules,38,39endosomes,40 and lysosomes.41 Clathrin-coated buds probably represent stages in the formation of vesicles,42,43 rather than of vesicles that fuse,36,44 and have been implicated in protein sorting and recycling. Specific segregation of the fibrinogen receptor from α-granules45 may be mediated by such vesicles, although we have not found an enrichment for GPIIb-IIIa in the buds.
Finally, during platelet activation, α-granules undergo exocytosis by which their content is released to the exterior. It is envisaged that the internal vesicles of α-granules and MVBs are externalized together with the dense content. Secretion of similar internal vesicles by exocytosis of multivesicular endocytic compartments has been shown in many cell types, in particular in cells of the hematopoietic lineage.46 In B and T lymphocytes, exosomes have been suggested to be involved in antigen presentation47 and target cell killing,48 respectively. Of interest, extracellular microvesicles have also been observed after platelet activation.49 50 Whether exosomes are secreted during platelet activation and play some physiologic role is subject to further investigations.
ACKNOWLEDGMENT
We thank Dr M.A. Liebermann for providing the CHRF-288 cells, Dr Michael C. Berndt for providing the polyclonal anti-GPIIb-IIIa and P-selectin antibodies, and Maurits K. Niekerk and René Scriwaneck for their excellent help in preparing the electron micrographs.
Address reprint requests to Harry F.G. Heijnen, PhD, Department of Hematology, G03.647, University Hospital Utrecht, PO Box 85500, NL-3508 GA Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal