Abstract
The CC-chemokine eotaxin is a potent eosinophil chemoattractant that stimulates recruitment of eosinophils from the blood to sites of allergic inflammation. Mobilization from the bone marrow is an important early step in eosinophil trafficking during the allergic inflammatory response. In this paper we examine the potential of eotaxin to mobilize eosinophils and their progenitors from bone marrow. Eotaxin stimulated selective, dose-dependent chemotaxis of guinea pig bone marrow eosinophils in vitro. Intravenous injection of eotaxin (1 nmol/kg) into guinea pigs in vivo stimulated a rapid blood eosinophilia (from 3.9 ± 1.2 to 28 ± 9.9 × 104eosinophils/mL at 30 minutes) and a corresponding decrease in the number of eosinophils retained in the femoral marrow (from 9.0 ± 0.8 to 4.8 ± 0.8 × 106 eosinophils per femur). To show a direct release of eosinophils from the bone marrow an in situ perfusion system of the guinea pig femoral bone marrow was developed. Infusion of eotaxin into the arterial supply of the perfused femoral marrow stimulated a rapid and selective release of eosinophils into the draining vein. In addition, eotaxin stimulated the release of colony-forming progenitor cells. The cytokine interleukin-5 was chemokinetic for bone marrow eosinophils and exhibited a marked synergism with eotaxin with respect to mobilization of mature eosinophils from the femoral marrow. Thus, eotaxin may be involved in both the mobilization of eosinophils and their progenitors from the bone marrow into the blood and in their subsequent recruitment into sites of allergic inflammation.
EOSINOPHILS ACCUMULATE in high numbers during allergic reactions, such as allergic asthma and rhinitis. There is considerable evidence that links the accumulation and activation of these cells with tissue injury and lung dysfunction.1-3
In experiments designed to identify local chemical signals stimulating eosinophil recruitment in allergic inflammatory reactions, we purified and sequenced a CC-chemokine, eotaxin, from bronchoalveolar lavage fluid of allergen-challenged sensitized guinea pigs.4,5 The eotaxin gene has since been cloned from guinea pig,6,7mouse,8,9 and human cells.10,11 Eotaxin is a potent eosinophil chemoattractant which signals via CCR3, a chemokine receptor highly expressed on eosinophils.4,5 8-12
There is evidence that eotaxin has an important local role in stimulating the recruitment of eosinophils from blood microvessels into the tissue at sites of allergic inflammation.4-7,9-11,13,14Because circulating numbers of eosinophils are normally low the extent of recruitment is also dependent on factors that regulate the numbers of eosinophils in the blood. We observed that intravenously administered interleukin-5 (IL-5) mobilized a pool of eosinophils from guinea pig bone marrow, producing a rapid blood eosinophilia.15 This effect correlated with a marked increase in local eosinophil recruitment induced by intradermally injected eotaxin.15 We have suggested that mobilization from the bone marrow is an important early step in eosinophil trafficking during the allergic inflammatory response.16
Although the molecular mechanisms involved in mobilization of leukocytes from the bone marrow are poorly understood, it is clear that migration of leukocytes from the marrow hematopoietic compartment into the sinusoidal lumen is essential for this process.17Therefore, it is possible that factors that stimulate a migratory response in leukocytes may induce their mobilization from the bone marrow.18 Factors that stimulate leukocyte migration do so by inducing either a chemotactic or chemokinetic response. IL-5, which mobilizes bone marrow eosinophils, has been shown to induce eosinophil chemokinesis,19,20 but has relatively weak efficacy as a local eosinophil chemoattractant in vivo15 or as a chemotactic agent in vitro.21-24 Eotaxin, in contrast, is a potent eosinophil chemoattractant in vivo and in vitro.4-6,10-12,14 15 Therefore we have investigated whether eotaxin, in addition to recruiting eosinophils into tissue, can mobilize eosinophils from the bone marrow.
An increase in eosinophil progenitor cells has previously been reported in the blood of atopics,25 in asthmatics during exacerbation,26,27 and in nasal polyp tissue.28Furthermore, it has been shown previously that some chemokines can stimulate the mobilization of hematopoietic progenitor cells from the bone marrow.29-31 However, there have been no reports of factors that selectively release eosinophil progenitors. We have therefore investigated whether eotaxin stimulates the mobilization of eosinophil progenitors from the bone marrow.
In this paper we show that eotaxin has a potent and selective effect in mobilizing bone marrow eosinophils in the guinea pig. Marked synergism between eotaxin and IL-5 was observed. In addition, colony-forming progenitors were mobilized in response to eotaxin, but not IL-5. In contrast, differentiation of the progenitors into eosinophils was stimulated by IL-5, but not by eotaxin.
The eosinophil is a minority cell type in the circulation (normally 1% to 2% of blood leukocytes in humans). Despite this, high numbers of eosinophils are able to accumulate at sites of allergic inflammation, which necessitates mechanisms to increase blood levels of these cells acutely. We suggest that these cells can be mobilized from the large reserve of eosinophils that are known to be present in the human bone marrow.32 The results presented here represent the first demonstration of a chemokine, eotaxin, that stimulates the selective release of eosinophils and their progenitors from the bone marrow.
MATERIALS AND METHODS
Animals.
Male Dunkin-Hartley guinea pigs (250 to 350 g) were obtained from Harlan Olac Ltd (Bicester, UK).
Materials.
Synthetic guinea pig eotaxin was a gift from Drs Glen Andrews and Henry Showell (Pfizer Inc, Groton, CT). Human recombinant IL-5 was a gift from Dr T.N.C. Wells (GlaxoWellcome Ltd, Geneva, Switzerland). Penicillin-streptomycin (pen-strep), Iscove's modified Dulbecco's medium (IMDM), fetal calf serum (FCS), phosphate-buffered saline (PBS), Hank's Balanced Salt Solution (HBSS), and Hepes buffer were purchased from Life Technologies (Paisley, UK). Hypnorm (fentanyl citrate, 0.315 mg/mL; fluanisone, 10 mg/mL) was purchased from Janssen Pharmaceutical Ltd (Oxford, UK). Hypnovel (Midazolam, 5 mg/mL) was purchased from Roche (Welwyn, UK). Expiral (sodium pentobarbitone, 200 mg/mL) was purchased from May and Baker (Dagenham, UK). Methocult GF H4534 medium, a Stem Cell Technologies product (Vancouver, Canada), was purchased from Metachem diagnostics Ltd (Northampton, UK). EasyLyse erythrocyte lysis kits were purchased from Universal Biologicals (London, UK). Transwell inserts with 3-μm pores were purchased from Millipore (Watford, UK). Methylene blue and eosin were purchased from Merck (Dagenham, UK). All other reagents were purchased from Sigma Chemical Co (Poole, UK). Kimura's stain for positive identification of eosinophils was prepared as previously described.33
Modified Krebs-Ringer bicarbonate buffer of the following composition was used in perfusion experiments: D-Glucose, 10 mmol/L; CaCl2, 3.33 mmol/L; MgCl2.6H2O, 0.49 mmol/L; KCl, 4.56 mmol/L; NaCl, 120 mmol/L; Na2HPO4, 0.7 mmol/L; NaH2PO4, 1.5 mmol/L; and NaHCO3, 24 mmol/L. The buffer was supplemented with 4% Ficoll T-70 and 0.1% bovine serum albumin (BSA) and gassed with 95% O2 and 5% CO2.
Preparation of guinea pig femoral marrow leukocytes.
HBSS was prepared containing 30 mmol/L Hepes; 0.25% BSA, pH 7.4; with (assay buffer) or without (cell buffer) Ca2+/Mg2+. Femurs were isolated from guinea pigs immediately after killing and the ends removed. The femoral shaft was flushed with 5 mL of cell buffer (containing 10 U/mL heparin). Displaced cells were gently resuspended using a syringe fitted with a 21G needle. Cells were then centrifuged (200g, 7 minutes, 20°C) and the cell pellet resuspended in 1 mL of cell buffer. Erythrocytes were removed using hypotonic shock lysis (addition of 10 mL of 0.2% NaCl followed by 10 mL of 1.6% NaCl to restore isotonicity). After centrifugation (200g, 7 minutes, 20°C) the leukocyte pellet was resuspended in assay buffer. Total nucleated leukocyte numbers were determined by counting Kimura-stained samples in an improved Neubauer hemacytometer.
Transwell chemotaxis assay.
Guinea pig femoral marrow leukocytes were prepared as described above. For some experiments cells were preincubated with IL-5 for 15 minutes at 37°C. Bone marrow leukocytes (3 × 106 cells in 0.2 mL of assay buffer) were placed in the upper chamber of Transwell filters (3-μm pore diameter) that were in turn placed in individual wells of a 24-well cell culture plate (lower chamber) containing 0.3 mL of assay buffer to which eotaxin (0 to 10 nmol/L) had been added. The chambers were incubated for up to 60 minutes at 37°C. Cells migrating into the bottom chamber were counted using a fluorescence-activated cell sorter (FACS) flow cytometer (FACScan; Becton Dickinson, Mountain View, CA), with relative cell counts obtained by acquiring events for a set time period of 60 seconds. This counting method was highly reproducible and enabled gating on the different leukocyte populations and the exclusion of debris. Counts obtained in this way closely matched those obtained by light microscopy.
Measurement of blood eosinophilia in vivo.
Guinea pigs were sedated with Hypnorm (0.2 mL intramuscularly [i.m.]). Eotaxin (1 nmol kg−1) or PBS/0.1% low endotoxin BSA, was injected intravenously (IV) via the marginal ear vein. Peripheral blood samples (100 μL) were collected from the marginal ear vein into heparin (10 U/mL) before and at 5, 15, 30, 60, and 120 minutes after injection of eotaxin or vehicle. Erythrocytes were lysed using EasyLyse reagents. Leukocytes were washed once with PBS and resuspended in Kimura's stain before counting in an improved Neubauer hemacytometer (greater than 200 leukocytes per sample). Nucleated Kimura-positive leukocytes were recorded as eosinophils. After 120 minutes, the guinea pigs were killed with Expiral (1.5 mL intraperitoneally) and the right femur was removed. Femoral marrow leukocytes were prepared as described above. Total nucleated leukocyte numbers were determined in an improved Neubauer hemacytometer and differential cell counts obtained from cytocentrifuge preparations stained with methylene blue and eosin.
Perfusion of the guinea pig femoral bone marrow in situ.
Guinea pigs were anesthetized with Hypnorm (0.8 mL i.m.) and Hypnovel (0.4 mL i.m.). The right external iliac artery and vein were exposed. The following arteries and corresponding veins were ligated with 5/0 braided silk suture: caudal abdominal artery, superficial iliac circumflex artery, and pudendoepigastric trunk. Heparin was administered IV via the marginal ear vein (1,000 U/kg) and the animal was killed with Expiral (250 mg/kg by cardiac puncture). Cannulae (polyethylene, 0.8-mm outside diameter; Portex, London, UK) were immediately inserted into the external iliac artery and vein and pushed down under the inguinal ligament into the femoral artery and vein. Cannulae were tied in with 5/0 braided silk suture.
Modified Krebs-Ringer bicarbonate buffer (37°C) was perfused (3.4 mL/min) via the arterial cannula and removed from the venous cannula using a Miniplus peristaltic pump (Anachem, Luton, UK). Perfusion pressure was monitored using a transducer located proximal to the arterial cannula. Agents were administered in vehicle (PBS/0.1% BSA) in pulses of 10- or 30-minute durations to gain information on kinetics and conserve reagents. Perfusate fractions were collected over periods of 10 minutes, centrifuged (300g, 10 minutes, 20°C), and the cell pellet resuspended in Kimura's stain. Nucleated leukocytes and Kimura-positive eosinophils were counted in an improved Neubauer hemacytometer. In some experiments, cytocentrifuge (Shandon Cytospin II) preparations of cells were collected and stained with methylene blue and eosin.
Colony-forming unit (CFU) assay.
The femoral bone marrow was perfused for 60 minutes with a 10-minute infusion (t = 0 to 10 minutes) of either 3 nmol/L eotaxin or vehicle (as in Fig 3A). The perfusate was collected and centrifuged (300g, 10 minutes, 20°C), and the cell pellet resuspended in IMDM (containing 100 U/mL pen-strep and 30% FCS). Of the total leukocytes released, 5 × 105 were added to 1.5 mL of Methocult GF H4534 medium of the following composition: 0.9% methylcellulose in IMDM, 30% FCS, 1% BSA, 100 U/mL pen-strep, 0.1 mmol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, and the following recombinant human cytokines: rh Stem cell factor (rhSCF), 50 ng/mL; rh granulocyte-monocyte colony-stimulating factor (rhGM-CSF), 10 ng/mL; and rh IL-3 (rhIL-3), 10 ng/mL. The Methocult GF H 4534 medium was supplemented with either rhIL-5, 3 nmol/L (see Fig 4B); or PBS (A); rhIL-5, 3 nmol/L (B); synthetic guinea pig eotaxin, 3 nmol/L (C) (see Fig 4C). Leukocytes were plated in duplicate 35-mm tissue culture dishes at 5 × 105 leukocytes per dish and maintained in a humidified atmosphere at 37°C, 5% CO2. On day 14, the number of colonies per plate (defined as aggregates of greater than 40 cells) were scored under an inverted microscope. Eosinophil–colony-forming units (Eo-CFU) and granulocyte-monocyte colony-forming units (GM-CFU) were identified from cytospin preparations of individual colonies (10 CFU examined per plate) after staining with methylene blue and eosin. Assessment of progenitor cell release in vivo was not feasible because the small volume blood samples collected for time course studies of eosinophil release did not contain sufficient numbers of leukocytes for the colony-forming assays.
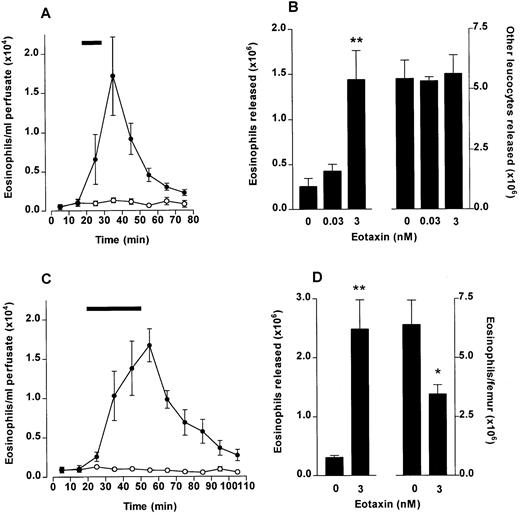
Eosinophil release from bone marrow induced by eotaxin. The femoral bone marrow was perfused in situ with modified Krebs-Ringer bicarbonate buffer via the external iliac artery and 10-minute fractions were collected from the external iliac vein. (A) Kinetics of eosinophil release after a 10-minute infusion (indicated by ▩) of eotaxin (3 nmol/L) or vehicle (PBS/0.1% BSA). Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = five to six perfusions). (•), Eotaxin 3 nmol/L; (○), Vehicle. (B) Total eosinophil release and total release of other leukocytes induced by a 10-minute infusion of eotaxin (0.03 and 3 nmol/L) or vehicle. Results show the total number of eosinophils or other leukocytes released during the 80-minute perfusion period, mean ± SEM (n = five to six perfusions). A significant difference between eotaxin and vehicle injected group is indicated by **(P < .01). (C) Kinetics of eosinophil release after a 30-minute infusion (indicated by ▩) of eotaxin (3 nmol/L) or vehicle. Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = four perfusions). (•) Eotaxin, 3 nmol/L; (○), Vehicle. (D) Eosinophil mobilization after a 30-minute infusion of eotaxin (3 nmol/L) or vehicle. Results (from experiment shown in C) show the total number of eosinophils released during the 110-minute perfusion period (left y-axis) and the number of eosinophils present in the femoral bone marrow after the 110-minute perfusion period (right y-axis), mean ± SEM (n = four perfusions). A significant difference between eotaxin and vehicle infused groups is indicated by *(P < .05) or **(P < .01).
Eosinophil release from bone marrow induced by eotaxin. The femoral bone marrow was perfused in situ with modified Krebs-Ringer bicarbonate buffer via the external iliac artery and 10-minute fractions were collected from the external iliac vein. (A) Kinetics of eosinophil release after a 10-minute infusion (indicated by ▩) of eotaxin (3 nmol/L) or vehicle (PBS/0.1% BSA). Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = five to six perfusions). (•), Eotaxin 3 nmol/L; (○), Vehicle. (B) Total eosinophil release and total release of other leukocytes induced by a 10-minute infusion of eotaxin (0.03 and 3 nmol/L) or vehicle. Results show the total number of eosinophils or other leukocytes released during the 80-minute perfusion period, mean ± SEM (n = five to six perfusions). A significant difference between eotaxin and vehicle injected group is indicated by **(P < .01). (C) Kinetics of eosinophil release after a 30-minute infusion (indicated by ▩) of eotaxin (3 nmol/L) or vehicle. Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = four perfusions). (•) Eotaxin, 3 nmol/L; (○), Vehicle. (D) Eosinophil mobilization after a 30-minute infusion of eotaxin (3 nmol/L) or vehicle. Results (from experiment shown in C) show the total number of eosinophils released during the 110-minute perfusion period (left y-axis) and the number of eosinophils present in the femoral bone marrow after the 110-minute perfusion period (right y-axis), mean ± SEM (n = four perfusions). A significant difference between eotaxin and vehicle infused groups is indicated by *(P < .05) or **(P < .01).
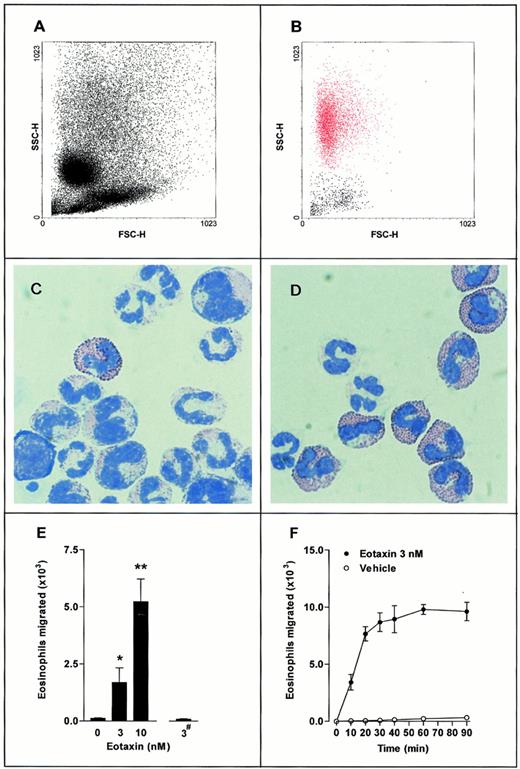
Transwell filter chemotaxis assay of guinea pig bone marrow eosinophils. A suspension of 3 × 106 bone marrow leukocytes was placed in the upper chamber, with eotaxin present in the lower chamber (▪) or upper chamber (#). Leukocytes that accumulated in the lower chamber were quantified using flow cytometry (FACScan; Beckton Dickinson). (A,B) Representative FACS dot-plot light forward scatter/side scatter profile of (A) mixed bone marrow leukocyte population placed into the upper Transwell chamber and (B) leukocytes migrated into the lower chamber in response to eotaxin (3 nmol/L, lower chamber for 30 minutes. Eosinophils shown in red). (C,D) May-Grunwald and Giemsa-stained cytospin preparations of (C) mixed bone marrow leukocyte population placed into the upper Transwell chamber and (D) leukocytes migrated into the lower chamber in response to eotaxin (3 nmol/L, lower chamber for 30 minutes). (E) Total eosinophils migrated in response to eotaxin present in the lower chamber (0 to 10 nmol/L) or upper chamber (3 nmol/L; #). Data represent the number of eosinophils migrated in 1 hour, mean ± SEM, using different cell preparations n = 6 to 12. No significant increase in eosinophil migration was observed when eotaxin (3 nmol/L) was added to the upper chambers. A significant difference between test and control groups is indicated by *(P < .05) or **(P < .01). (F) Time course of eosinophil chemotaxis induced by eotaxin (3 nmol/L, lower chamber). Data represent the number of eosinophils migrated at each time point, mean ± SEM for a single cell preparation performed in triplicate. The results shown are representative of three identical experiments. (•), Eotaxin 3 nmol/L; (○), Vehicle.
Transwell filter chemotaxis assay of guinea pig bone marrow eosinophils. A suspension of 3 × 106 bone marrow leukocytes was placed in the upper chamber, with eotaxin present in the lower chamber (▪) or upper chamber (#). Leukocytes that accumulated in the lower chamber were quantified using flow cytometry (FACScan; Beckton Dickinson). (A,B) Representative FACS dot-plot light forward scatter/side scatter profile of (A) mixed bone marrow leukocyte population placed into the upper Transwell chamber and (B) leukocytes migrated into the lower chamber in response to eotaxin (3 nmol/L, lower chamber for 30 minutes. Eosinophils shown in red). (C,D) May-Grunwald and Giemsa-stained cytospin preparations of (C) mixed bone marrow leukocyte population placed into the upper Transwell chamber and (D) leukocytes migrated into the lower chamber in response to eotaxin (3 nmol/L, lower chamber for 30 minutes). (E) Total eosinophils migrated in response to eotaxin present in the lower chamber (0 to 10 nmol/L) or upper chamber (3 nmol/L; #). Data represent the number of eosinophils migrated in 1 hour, mean ± SEM, using different cell preparations n = 6 to 12. No significant increase in eosinophil migration was observed when eotaxin (3 nmol/L) was added to the upper chambers. A significant difference between test and control groups is indicated by *(P < .05) or **(P < .01). (F) Time course of eosinophil chemotaxis induced by eotaxin (3 nmol/L, lower chamber). Data represent the number of eosinophils migrated at each time point, mean ± SEM for a single cell preparation performed in triplicate. The results shown are representative of three identical experiments. (•), Eotaxin 3 nmol/L; (○), Vehicle.
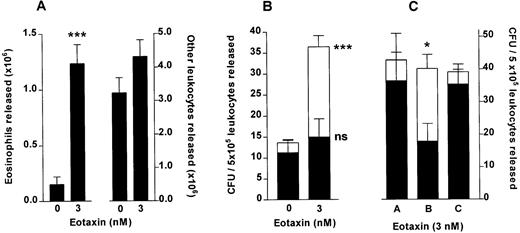
Eotaxin stimulates release of colony-forming units from femoral bone marrow. (A) Total eosinophil and total other leukocyte release from the perfused hind limb stimulated by a 10-minute infusion of eotaxin (3 nmol/L) or vehicle (PBS/0.1% BSA), counted in an improved Neubauer hemacytometer. Results show the total number of eosinophils and other leukocytes released during the 60-minute perfusion period, mean ± SEM (n = seven to eight perfusions). (B) GM-CFU (▪) and Eo-CFU (□) release after a 10-minute infusion of eotaxin (3 nmol/L) or vehicle. The colony-forming assay was performed in Methocult GF H4534 methylcellulose-based medium supplemented with IL-5 (3 nmol/L). Results are expressed as the number of GM-CFU or Eo-CFU present per 5 × 105 total leukocytes released during the 60-minute perfusion period, mean ± SEM of seven to eight perfusions, CFU assay performed in duplicate. A significant difference between Eo-CFU released in eotaxin and in vehicle infused groups is indicated by ***(P < .001). (C) Colony-forming unit assay of leukocytes released by eotaxin (3 nmol/L) performed in Methocult GF H4534 medium supplemented with either PBS (A), IL-5 (3 nmol/L) (B), or eotaxin (3 nmol/L) (C). Results are expressed as the number of GM-CFU (▪) or Eo-CFU (□) present per 5 × 105 total leukocytes released during the 60-minute perfusion period, mean ± SEM of three perfusions, CFU assay performed in duplicate. A significant difference between the number of Eo-CFU formed in the presence of IL-5 compared with either PBS or eotaxin is indicated by *(P < .05).
Eotaxin stimulates release of colony-forming units from femoral bone marrow. (A) Total eosinophil and total other leukocyte release from the perfused hind limb stimulated by a 10-minute infusion of eotaxin (3 nmol/L) or vehicle (PBS/0.1% BSA), counted in an improved Neubauer hemacytometer. Results show the total number of eosinophils and other leukocytes released during the 60-minute perfusion period, mean ± SEM (n = seven to eight perfusions). (B) GM-CFU (▪) and Eo-CFU (□) release after a 10-minute infusion of eotaxin (3 nmol/L) or vehicle. The colony-forming assay was performed in Methocult GF H4534 methylcellulose-based medium supplemented with IL-5 (3 nmol/L). Results are expressed as the number of GM-CFU or Eo-CFU present per 5 × 105 total leukocytes released during the 60-minute perfusion period, mean ± SEM of seven to eight perfusions, CFU assay performed in duplicate. A significant difference between Eo-CFU released in eotaxin and in vehicle infused groups is indicated by ***(P < .001). (C) Colony-forming unit assay of leukocytes released by eotaxin (3 nmol/L) performed in Methocult GF H4534 medium supplemented with either PBS (A), IL-5 (3 nmol/L) (B), or eotaxin (3 nmol/L) (C). Results are expressed as the number of GM-CFU (▪) or Eo-CFU (□) present per 5 × 105 total leukocytes released during the 60-minute perfusion period, mean ± SEM of three perfusions, CFU assay performed in duplicate. A significant difference between the number of Eo-CFU formed in the presence of IL-5 compared with either PBS or eotaxin is indicated by *(P < .05).
Statistical analysis.
Data is presented as mean ± standard error of the mean (SEM). All statistical tests were performed using untransformed data. For analysis of two groups the unpaired two-tail Student's t-test was performed. For analysis of three or more groups one-way analysis of variance was performed, followed by either Bonferroni's multiple comparisons posttest, or Dunnett's posttest for comparison with a control group. A value of P < .05 was considered statistically significant.
RESULTS
Chemotactic effect of eotaxin on bone marrow eosinophils in vitro.
The chemotactic activity of eotaxin on guinea pig bone marrow cell suspensions was investigated in a Transwell filter assay system in vitro. Figure 1A shows the FACS forward scatter/side scatter profile of the mixed bone marrow leukocyte population placed into the upper chamber of the Transwell filter. A cytospin preparation of this cell population is shown in Fig 1C. Figure 1B shows the FACS scatter characteristics of the leukocytes that migrated into the lower chamber in response to eotaxin (3 nmol/L, lower chamber for 30 minutes). The high side-scatter and low forward-scatter profile of the migrated leukocytes (Fig 1B, shown in red) is characteristic of eosinophils. Cytospin preparations of these leukocytes (Fig 1D) showed cells with a bilobed nucleus that stained intensely with May-Grunwald's stain, indicating that these cells were eosinophils.
Figures 1E and F illustrate that basal migration of eosinophils was low, and that a positive gradient of eotaxin stimulated dose-related migration of eosinophils, which reached a maximum at 30 minutes. The effect of eotaxin was selective for eosinophils: there was no increase in the migration of other leukocyte types.
Effect of IV eotaxin.
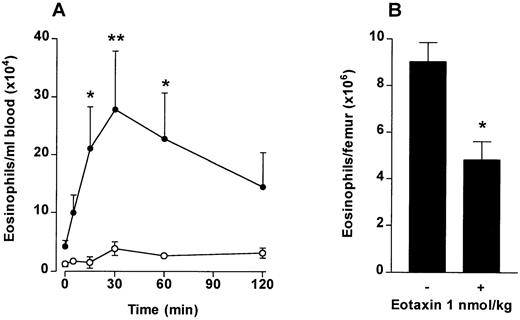
To investigate if chemotaxis across the bone marrow sinus endothelium would induce a blood eosinophilia in vivo, eotaxin (1 nmol/kg) was injected IV into guinea pigs and the numbers of circulating eosinophils monitored over a 2-hour time period. At the end of the experiment the number of eosinophils present in the femoral bone marrow was determined. As shown in Fig 2A, circulating eosinophil levels rose rapidly after the IV eotaxin injection, when compared with PBS-injected controls. Circulating eosinophil numbers were significantly elevated at 15 minutes after eotaxin injection. Maximal levels were attained at 20 to 30 minutes. A significant decrease in eosinophils retained in the femoral bone marrow was observed in the eotaxin-injected group when measured at 2 hours (Fig 2B).
The effect of IV eotaxin on blood and bone marrow eosinophil numbers. After injection of eotaxin, circulating eosinophil numbers were determined at multiple time points over a 2-hour period. Femoral marrow eosinophil numbers were determined after 2 hours. (A) Kinetics of blood eosinophilia induced by eotaxin (1 nmol/kg). Results show the number of eosinophils per milliliter of blood at each time point, mean ± SEM (n = 4 animals). Eotaxin stimulated a significant *(P < .05) or **(P < .01) blood eosinophilia after 15 minutes compared with the vehicle-injected group. (•), Eotaxin 1 nmol/kg; (○), Vehicle. (B) Femoral marrow eosinophil numbers. Results show the total number of morphologically mature eosinophils in femoral bone marrow in one femur 2 hours after injection of eotaxin (1 nmol/kg) or vehicle, mean ± SEM, (n = 4 animals). A significant difference between eotaxin- and vehicle-injected groups is shown by *(P < .05).
The effect of IV eotaxin on blood and bone marrow eosinophil numbers. After injection of eotaxin, circulating eosinophil numbers were determined at multiple time points over a 2-hour period. Femoral marrow eosinophil numbers were determined after 2 hours. (A) Kinetics of blood eosinophilia induced by eotaxin (1 nmol/kg). Results show the number of eosinophils per milliliter of blood at each time point, mean ± SEM (n = 4 animals). Eotaxin stimulated a significant *(P < .05) or **(P < .01) blood eosinophilia after 15 minutes compared with the vehicle-injected group. (•), Eotaxin 1 nmol/kg; (○), Vehicle. (B) Femoral marrow eosinophil numbers. Results show the total number of morphologically mature eosinophils in femoral bone marrow in one femur 2 hours after injection of eotaxin (1 nmol/kg) or vehicle, mean ± SEM, (n = 4 animals). A significant difference between eotaxin- and vehicle-injected groups is shown by *(P < .05).
Effect of eotaxin on eosinophil release from bone marrow perfused in situ.
To show directly that eotaxin stimulates release of eosinophils from the bone marrow, we set up a perfusion system of the femur. The femoral bone marrow was perfused in situ with modified Krebs-Ringer bicarbonate buffer via the external iliac artery and 10-minute fractions were collected from the external iliac vein. Transmission electron microscopy of the perfused bone marrow confirmed that the marrow cytoarchitecture remained intact during this procedure (data not shown). When perfused with buffer alone, approximately 5 × 106 leukocytes were released into the perfusate over an 80-minute period, of which 0.25 × 106 (5% of total) were eosinophils and the remainder were neutrophils (85%) and mononuclear cells (10%). A 10-minute pulse of eotaxin at 3 nmol/L induced a rapid, transient release of eosinophils into the perfusate, which peaked 10 minutes after the start of the infusion (Fig3A) with no effect on other cell types (Fig 3B). Approximately 1.5 × 106 eosinophils were released in total (Fig 3B).
When the infusion of eotaxin was extended from 10 to 30 minutes, the release of eosinophils was considerably prolonged (Fig 3C). As with the 10-minute infusion, release rapidly declined when the infusion was terminated. The release of eosinophils corresponded to a reduction in eosinophils retained in the bone marrow at the end of the perfusion period (30-minute eotaxin infusion; Fig 3D).
Effect of eotaxin on hematopoietic progenitor release from bone marrow perfused in situ.
Perfusion of the femoral bone marrow in situ with eotaxin was performed to determine whether eotaxin stimulated the release of colony-forming cells directly from the bone marrow. A 10-minute pulse of either vehicle or 3 nmol/L eotaxin was infused into the bone marrow. Leukocyte release was similar to that described above: a total of 1.24 ± 0.2 × 106 eosinophils and 4.31 ± 0.5 × 106other leukocytes were released after infusion of eotaxin as compared with 0.2 ± 0.1 × 106 eosinophils and 4.3 ± 0.5 × 106 other leukocytes released when vehicle was infused (Fig4A). This system was suitable for obtaining sufficient numbers of leukocytes for culture, uncontaminated by cells which interfere with the growth of progenitors in semisolid cultures. Leukocytes released were plated in methylcellulose-based medium containing a cytokine profile optimised to detect Eo-CFU and GM-CFU.34 After a 14-day incubation period the number of colonies (aggregates of greater than 40 cells) were counted. There was a significant increase in the total number of colonies formed from leukocytes released in response to eotaxin as compared with vehicle, this increase being accounted for by a significant increase in Eo-CFU with no significant difference in the number of GM-CFU (Fig 4B). Of the total colonies formed, from progenitors mobilized by eotaxin, 59% were identified as Eo-CFU, whereas only 17% of the colonies formed from the vehicle-infused group were Eo-CFU.
In three of these experiments the IL-5 in the methylcellulose medium was replaced by either PBS vehicle or eotaxin (Fig 4C). Leukocytes released by eotaxin infusion were therefore cultured for 14 days in the presence of rhSCF, GM-CSF, and IL-3 together with either PBS (A), IL-5 (B), or eotaxin (C). The results show that the total number of colonies formed was not affected by the presence of either IL-5 or eotaxin in the medium. Of the total colonies, 15% were identified as eosinophil colonies when the medium contained PBS. Addition of eotaxin (3 nmol/L) to the medium had no effect on the percentage of eosinophil colonies formed. In contrast, IL-5 increased the percentage of eosinophil colonies significantly to 55%.
These results show that eotaxin stimulates a direct mobilization of colony-forming progenitors from the bone marrow. Differentiation of these colony-forming cells into eosinophils is dependent on the presence of IL-5. To determine whether IL-5 could mobilize colony-forming progenitors from the bone marrow, IL-5 (0.8 nmol/L) was infused for 60 minutes into the bone marrow. The total number of eosinophils and other leukocytes released (0.8 ± 0.3 × 106 and 4.0 ± 0.5 × 106, respectively) were not significantly different from the release observed with a 10-minute infusion of eotaxin (3 nmol/L; Fig 4A). In colony assays performed exactly as described above, there was no significant increase in the number of colonies formed from leukocytes released in response to IL-5 (Eo-CFU 0.7 ± 0.3 and GM-CFU 7.0 ± 0.5/5 × 105leukocytes plated) as compared with vehicle (Fig 4B). Thus, under these conditions IL-5 did not stimulate the mobilization of eosinophil progenitors from the bone marrow.
To determine whether eosinophil progenitors could be detected in the circulation, blood was collected 30 minutes after IV injection of Guinea pigs with either vehicle or eotaxin 1 nmol/kg. This time point was chosen because it corresponded to the maximal eosinophil release (Fig 2A). The blood was lysed to remove red blood cells and the leukocytes (2 × 105) were plated in methocult medium supplemented with rhSCF, GM-CSF, IL-3, and IL-5. After a 14-day incubation period we did not detect any colonies in either the vehicle- or eotaxin-injected group. This result may reflect the fact that eosinophil progenitors, once released, are rapidly redistributed in vivo, either a result of homing back to the bone marrow or of recruitment into extravascular sites, such as the lung, where we know eotaxin is generated constitutively.16
Potentiating effect of IL-5 on eotaxin-induced chemotaxis of bone marrow eosinophils in vitro.
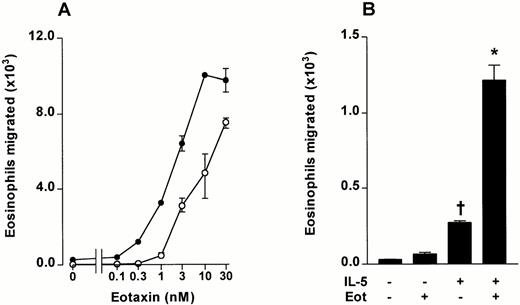
Using the in vitro chemotaxis, assay a detailed dose-response curve of bone marrow eosinophil migration induced by eotaxin was performed. Significant migration was observed at eotaxin concentrations of 1 nmol/L and above (Fig 5A). Preincubation of bone marrow leukocytes with IL-5 (3 nmol/L for 15 minutes at 37°C) induced a small, selective migration of bone marrow eosinophils when subsequently tested in the Transwell system (Fig 5B). The effect was associated with chemokinesis because the same magnitude of migration occurred when IL-5 was added to the upper chamber alone, the lower chamber alone, or both the lower and upper chamber (results not shown). This was in contrast to the chemotactic effect of eotaxin, where migration was not observed with the chemokine in the upper chamber (Fig1E).
The effect of IL-5 on eotaxin-stimulated chemotaxis of guinea pig bone marrow eosinophils. Bone marrow leukocytes were preincubated with IL-5 (3 nmol/L) or vehicle for 15 minutes at 37°C. (A) The effect of IL-5 (3 nmol/L, upper chamber) on bone marrow eosinophil chemotaxis induced by eotaxin (0 to 30 nmol/L, lower chamber). Data represent the number of eosinophils migrated after 1 hour, mean ± SEM of a single cell preparation performed in triplicate. (•), IL-5 3 nmol/L; (○), Vehicle. (B) The effect of IL-5 (3 nmol/L) and eotaxin (0.3 nmol/L) alone or in combination on the chemotaxis of bone marrow eosinophils (results from experiment shown in Fig 5A). Results represent the number of eosinophils migrated after 1 hour, mean ± SEM of a single cell preparation performed in triplicate. The results shown are representative of three identical experiments. Significant difference between IL-5 alone and vehicle indicated by †(P < .05), or between eotaxin together with IL-5 and IL-5 alone by *(P < .05).
The effect of IL-5 on eotaxin-stimulated chemotaxis of guinea pig bone marrow eosinophils. Bone marrow leukocytes were preincubated with IL-5 (3 nmol/L) or vehicle for 15 minutes at 37°C. (A) The effect of IL-5 (3 nmol/L, upper chamber) on bone marrow eosinophil chemotaxis induced by eotaxin (0 to 30 nmol/L, lower chamber). Data represent the number of eosinophils migrated after 1 hour, mean ± SEM of a single cell preparation performed in triplicate. (•), IL-5 3 nmol/L; (○), Vehicle. (B) The effect of IL-5 (3 nmol/L) and eotaxin (0.3 nmol/L) alone or in combination on the chemotaxis of bone marrow eosinophils (results from experiment shown in Fig 5A). Results represent the number of eosinophils migrated after 1 hour, mean ± SEM of a single cell preparation performed in triplicate. The results shown are representative of three identical experiments. Significant difference between IL-5 alone and vehicle indicated by †(P < .05), or between eotaxin together with IL-5 and IL-5 alone by *(P < .05).
IL-5 was able to prime bone marrow eosinophils to enhance migration in response to eotaxin. Preincubation of bone marrow cell suspensions with IL-5 (3 nmol/L for 15 minutes at 37°C) considerably enhanced the migration of eosinophils stimulated by eotaxin in the lower chamber (Fig 5A and B). Synergism was striking at an eotaxin concentration of 0.3 nmol/L, which did not induce significant migration when tested on unprimed cells (Fig 5B).
Effects of IL-5/eotaxin combinations on eosinophil release from perfused bone marrow.
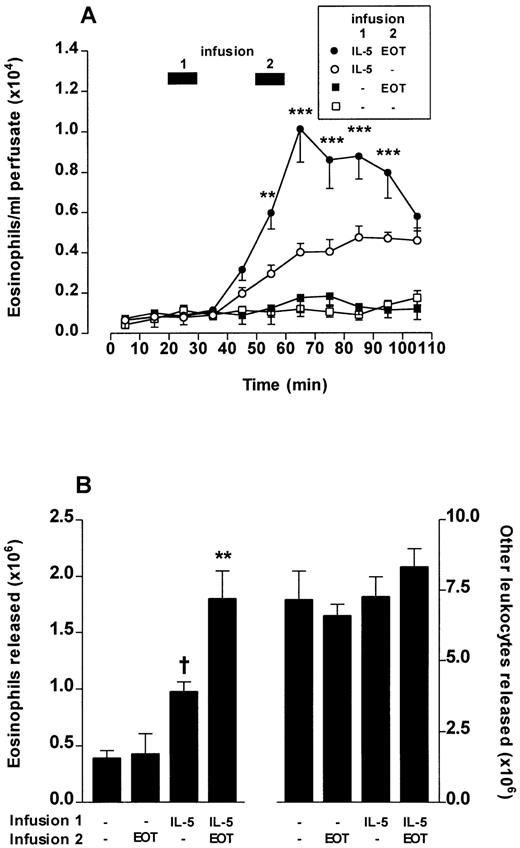
The experiments shown in Fig 5 showed synergism between IL-5 and eotaxin to induce chemotaxis of bone marrow eosinophils in vitro. Experiments were designed to investigate if such synergism was also demonstrable in bone marrow eosinophil release using the in situ perfusion system.
Perfusion of the femoral bone marrow was performed for a total of 110 minutes. After 20 minutes, IL-5 (0.3 nmol/L) or vehicle was infused for 10 minutes. A second infusion of a low dose of eotaxin (0.3 nmol/L) or PBS infusion was performed at 50 to 60 minutes. The results of the first and second infusions are shown in Fig6. Infusions of vehicle/eotaxin induced no significant eosinophil release compared with vehicle/vehicle infusions. IL-5/vehicle infusions induced eosinophil release; however, the kinetics were delayed in onset and of a protracted time course (Fig 6A) when compared with the rapid onset and transient release produced by higher concentrations of eotaxin (Fig 4). A 10-minute pulse of eotaxin (0.3 nmol/L) administered after a first 10-minute pulse of IL-5 produced a rapid and dramatically enhanced eosinophil release (Fig 6A and B). None of these combinations had any effect on the release of other cell types (Fig 6B).
Eotaxin (EOT) and IL-5 act synergistically to release eosinophils from bone marrow. Perfusion of the femoral bone marrow was performed for a total of 110 minutes. (A) Kinetics of eosinophil release induced by a 10-minute infusion (indicated by ▩ 1) of IL-5 (0.3 nmol/L) or vehicle (PBS/0.1% BSA) followed 20 minutes later by a 10-minute infusion (indicated by ▩ 2) of eotaxin (0.3 nmol/L) or vehicle. Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = five to eight perfusions). A significant difference between IL-5 plus eotaxin and IL-5 alone at equivalent time point represented by **(P < .01) or ***(P < .001). (B) Total eosinophil release and total release of other leukocytes induced by a 10-minute infusion of IL-5 (0.3 nmol/L) or vehicle followed 20 minutes later by a 10-minute infusion of eotaxin (0.3 nmol/L) or vehicle. Results show the total number of eosinophils or other leukocytes released during the 110-minute perfusion period, mean ± SEM (n = five to eight perfusions). A significant difference between IL-5 alone and vehicle is indicated by †(P < .05) and a significant difference between IL-5 plus eotaxin and IL-5 alone by **(P < .01).
Eotaxin (EOT) and IL-5 act synergistically to release eosinophils from bone marrow. Perfusion of the femoral bone marrow was performed for a total of 110 minutes. (A) Kinetics of eosinophil release induced by a 10-minute infusion (indicated by ▩ 1) of IL-5 (0.3 nmol/L) or vehicle (PBS/0.1% BSA) followed 20 minutes later by a 10-minute infusion (indicated by ▩ 2) of eotaxin (0.3 nmol/L) or vehicle. Results represent the number of eosinophils per milliliter of perfusate in each 10-minute fraction, mean ± SEM (n = five to eight perfusions). A significant difference between IL-5 plus eotaxin and IL-5 alone at equivalent time point represented by **(P < .01) or ***(P < .001). (B) Total eosinophil release and total release of other leukocytes induced by a 10-minute infusion of IL-5 (0.3 nmol/L) or vehicle followed 20 minutes later by a 10-minute infusion of eotaxin (0.3 nmol/L) or vehicle. Results show the total number of eosinophils or other leukocytes released during the 110-minute perfusion period, mean ± SEM (n = five to eight perfusions). A significant difference between IL-5 alone and vehicle is indicated by †(P < .05) and a significant difference between IL-5 plus eotaxin and IL-5 alone by **(P < .01).
DISCUSSION
The classical experiments of Samter et al35 showed that guinea pig lung produced a factor during anaphylactic shock that induced a blood eosinophilia. These investigators deduced that an “eosinotactic substance” was produced in the lung and that the same substance induced both local eosinophil recruitment in the lung and release of mature eosinophils from the bone marrow into the blood. In contrast, our recent results suggested that these two activities are mediated by separate chemical signals: eotaxin mediating local eosinophil recruitment5 and IL-5 facilitating release of cells from the bone marrow.15,36 Although IL-5 is clearly important in releasing bone marrow eosinophils during an allergic reaction in the lung,16,37 the results presented here show that Samter et al35 were essentially correct in deducing that the same chemoattractant can mediate both local eosinophil recruitment and their release from the bone marrow.
Previous studies have shown that eotaxin is a potent chemoattractant that promotes circulating eosinophils to adhere to and emigrate through a microvessel wall into the extravascular space at sites of inflammation.4,5,11,12,14 15 We evaluated whether this chemoattractant activity could induce migration of eosinophils into the bone marrow sinuses. When tested in a chemotaxis assay in vitro, eotaxin was found to induce a selective chemotaxis of eosinophils from a bone marrow mixed cell suspension (Fig 1). In vivo, IV eotaxin induced a rapid onset and sustained blood eosinophilia, which correlated with a loss of eosinophils from the bone marrow (Fig 2). When infused into the arterial supply of the perfused femoral bone marrow in situ, eotaxin induced a rapid release of eosinophils into the draining vein, correlating with a reduction in retained cells (Fig 3). Release was sustained during the eotaxin infusion, but rapidly declined thereafter. The cells released by eotaxin were not tachyphylactic to the chemokine, as these cells responded to eotaxin when subsequently tested in the chemotaxis assay in vitro (data not shown).
One difference reported in mice with targeted disruption of the eotaxin gene as compared with wild-type mice was a reduction in circulating eosinophil levels despite apparently normal hematopoiesis.38 This finding is consistent with the results presented in this paper. Mould et al39 showed that IV eotaxin stimulated a rapid blood eosinophilia in mice, but in contrast to our results, did not detect a decrease in bone marrow eosinophil numbers. In contrast to the substantial reserve of eosinophils in guinea pig40 and human bone marrow,32 the mouse has only a small number of these cells.41 However, the reserve is increased after sensitization.41
It has previously been reported that the chemokines IL-8 and MIP-1α stimulate the mobilization of hematopoietic progenitor cells from the bone marrow.29-31 Therefore, we have assessed whether eotaxin stimulates the release of hematopoietic progenitors from the guinea pig bone marrow using a methylcellulose-based clonogenic assay. We observed that a 10-minute infusion of 3 nmol/L eotaxin in the femoral bone marrow in situ perfusion system stimulated a significant increase in the release of colony-forming progenitors over basal levels. IL-5 stimulates the mobilization of mature eosinophils from the bone marrow15(Fig 6). However, in this study we did not detect a significant increase in the release of colony-forming progenitor cells in response to IL-5. An increase in eosinophil progenitor cells has been reported in the blood of atopics,25 in asthmatics during exacerbation,26,27 and in nasal polyp tissue.28From the results presented here it seems that colony-forming progenitors express the eotaxin receptor CCR3, and can be mobilized from the bone marrow into the blood by eotaxin. Factors that regulate the subsequent local recruitment of these progenitors into tissues, such as nasal polyp, have not been identified. Eotaxin, which has been detected in human asthmatic lung and nasal polyps10,11,13is a potential candidate for this function. Previously, cytokines have been identified that control colony formation and differentiation.42 In particular, IL-5 is known to be a late differentiation factor for eosinophils.43,44 In this study colony-forming progenitors released in response to eotaxin were cultured in the presence of SCF, IL-3, and GM-CSF together with either PBS, IL-5, or eotaxin. We show that the differentiation of these progenitors into eosinophils was dependent on the presence of IL-5. In contrast, eotaxin, at the same concentration as IL-5, did not induce differentiation of these colonies into eosinophils in these experiments. Therefore, it is possible that IL-545,46together with other cytokines, such as GM-CSF47 and IL-3,48 generated at sites of allergic inflammation, may act locally to support colony formation and differentiation. This hypothesis predicts another example of the cooperative effects of IL-5 and eotaxin.
The detailed mechanisms involved in the mobilization of leukocytes from the bone marrow remain to be established.17 The results presented here suggest that the development of a chemoattractant gradient across the bone marrow sinus endothelium induces eosinophil release. This has parallels with neutrophil release because the IV injection of neutrophil chemoattractants has been shown to induce a transient blood neutropenia followed by a leukocytosis, the latter suggested to involve bone marrow release.18,30,31 In the perfusion system the effect of eotaxin was rapid in onset and transient (Fig 3). This suggests that the migration through the endothelium ceases as the gradient disappears. In contrast, IL-5 induced a sustained release of eosinophils that was delayed in onset (Fig 6). This difference in kinetics may be related to a difference in mechanisms of action. We found in the in vitro Transwell system that eotaxin induces chemotaxis of bone marrow eosinophils, whereas IL-5 induces chemokinesis (Fig 5 and Rankin et al unpublished, 1997). Consistent with these findings, a chemokinetic effect of IL-5 on human peripheral eosinophils has been reported.19 A combination of these effects would seem to be very efficient in mobilizing eosinophils into bone marrow sinuses, ie, eosinophil chemokinesis stimulated by IL-5 and chemotaxis stimulated by a gradient of eotaxin across the sinus endothelium. Accordingly, we found marked synergism between eotaxin and IL-5 in both the Transwell chemotaxis assay system in vitro (Fig 5) and the in situ perfusion system (Fig 6). This was most striking with low doses of eotaxin, which produced insignificant effects on eosinophil migration or release when tested alone.
Neutralization of IL-5 using TRFK5 antibody has been shown to suppress the release of eosinophils from the bone marrow and the resultant blood eosinophilia induced by allergen challenge of guinea pig lung in vivo.16,37 The antibody had no effect on eotaxin generation in these experiments.16 These results could be accounted for by a synergistic interaction between endogenous eotaxin and IL-5 on bone marrow eosinophil release, as we have observed. Furthermore, mice with targeted disruption of the IL-5 gene have a significant, although reduced, level of blood and bone marrow eosinophils.49 Thus, other mediators seem to be able to compensate for the loss of IL-5.
Measurements of eotaxin levels in sensitized guinea pig lung tissue have shown a rapid clearance of the chemokine between 6 and 12 hours after allergen challenge.16 Some of this clearance is likely to be into the local microvasculature. The duffy antigen receptor for chemokines on red blood cells, acting as a chemokine sink, may prevent high levels of circulating, unbound chemokine.50 However, plasma levels of eotaxin, particularly during active phases of inflammation, may be sufficient to exert an important role in regulating blood eosinophil levels, probably in cooperation with IL-5.
These results suggest that the process of bone marrow eosinophil release and tissue eosinophil recruitment share a common feature, fast cell migration across a vascular endothelium, albeit in opposite directions. Furthermore, the data show a dramatic cooperation between chemotaxis and chemokinesis using agents selectively exhibiting these activities.
Supported by the Wellcome Trust and the National Asthma Campaign, London, UK.
Address reprint requests to Sara Rankin, PhD, Leukocyte Biology Section, Biomedical Sciences Division, Imperial College School of Medicine at the National Heart and Lung Institute, London SW3 6LY, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be here-by marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal