Abstract
The t(2;5)(p23;q35) translocation, associated with anaplastic large-cell lymphoma (ALCL), results in the expression of a chimeric NPM-ALK protein that can be detected by the ALK1 monoclonal antibody. This report describes the morphologic and phenotypic spectrum of 123 cases of lymphoma that all express ALK protein. The results provide strong evidence that the morphologic patterns of ALCL described in previous reports as representing possible subtypes of ALCL, eg, common type, lymphohistiocytic, or small cell patterns, are morphologic variants of the same disease entity. All of these morphologic patterns could be found within this series, and in some patients different subtypes coexisted in a single biopsy or were found in successive biopsies from a single patient. The link between these morphologic subtypes is further reinforced by the presence in all cases of a highly characteristic large cell, with an eccentric nucleus and an eosinophilic paranuclear region. We suggest that this cell can be considered as a major distinguishing feature of ALK-positive lymphomas. Another characteristic of these tumors was the perivascular pattern of neoplastic cell infiltration seen in a significant number of cases. In addition to ALK protein, all tumors expressed epithelial membrane antigen and lacked CD15, features that may be of value in differentiating ALCL from Hodgkin's disease. In the majority of cases (84%), malignant cells showed both a cytoplasmic and nuclear staining for ALK1 and thus presumably carried the 2;5 translocation, but staining was restricted to the cytoplasm in a few cases, suggesting that translocations other than t(2;5) may induce expression of ALK protein. We conclude from this study that ALK-positive neoplasms represent a distinct entity. Because their morphology is often neither anaplastic nor large cell, we suggest that they should henceforward be referred to as ALK lymphomas.
ANAPLASTIC LARGE-CELL lymphoma (ALCL) was first described in 19851 as a previously unrecognized lymphoid tumor in which the neoplastic cells were labeled by the monoclonal antibody Ki-1 (subsequently shown to recognize the CD30 receptor molecule). This entity was included in the revised Kiel classification2 and in its successor, the REAL scheme,3 and is now widely diagnosed.
Five years after the first description of ALCL, it was noted that tumors carrying the (2;5)(p23;q35) chromosome translocation, a rare cytogenetic abnormality thought initially to be characteristic of malignant histiocytosis, were CD30 (Ki-1)-positive large-cell lymphomas.4-7 Then, in 1994, Morris et al8showed that the (2;5) translocation fuses part of the nucleophosmin (NPM) gene on chromosome 5q35 to a portion of the ALKreceptor tyrosine kinase gene on chromosome 2p23, resulting in expression of a unique chimeric NPM-ALK protein. Antibodies specific for the ALK kinase9,10 have been reported, and the absence of this molecule from normal lymphoid cells means that a positive immunocytochemical reaction for ALK protein is essentially specific for the (2;5) translocation. The major exception is a rare large B-cell lymphoma in which (by an unknown mechanism) full-length ALK protein is expressed.11
Although ALCL is now widely recognized and its association with the (2;5) translocation is beyond doubt, several areas of disagreement and controversy remain. Firstly, estimates of the frequency of the (2;5) translocation (and of the NPM-ALK fusion gene) in this neoplasm vary from less than 15% to more than two thirds.12Secondly, opinions differ as to whether the (2;5) translocation is found in tumors other than ALCL. Some investigators report its presence in a minority of large-cell B-cell lymphomas and pleomorphic T-cell neoplasms,13-15 whereas others argue that it is specific for ALCL.16-18
One reason for these disagreements may lie in the differing criteria used for the diagnosis of ALCL. The range of morphologic features recognized in this tumor is wider than was initially described.1 This is reflected in descriptions in the literature of at least eight putative subtypes.19-24Furthermore, it has been recognized for some years that its original defining marker (the Ki-1/CD30 molecule) can be found on occasion in neoplasms that are clearly not related to ALCL.25 In addition, some investigators consider that occasional cases express B-cell markers, whereas others argue that this phenotype is incompatible with a diagnosis of ALCL.3 16
For these reasons, we have undertaken a review of a large series of lymphomas in which the single criterion for selection was ALK immunoreactivity (and, by implication, the presence of theNPM-ALK fusion gene). It is probable that the NPM-ALKgene is directly involved in the causation of human ALCL because its introduction into murine haemopoietic cells induces a transplantable large-cell lymphoma.26 By selecting a series of lymphomas for review solely on the basis of this genetic anomaly, we aimed to identify a homogeneous entity. In essence, these “ALKomas” are more likely to represent a single clinicopathologic entity than are neoplasms selected on the grounds of morphologic and phenotypic features that are not causally implicated in oncogenic transformation.
This review has enabled us to propose solutions to difficulties that surround the diagnosis and categorization of ALCL. The number of cases reviewed was considerably larger than has been reported previously and included some cases from whom sequential biopsies were obtained. It was culled in part from an extensive input of problem cases sent to one of the investigators (G.D.) over 10 years. In consequence, we also gained insight into morphologic variations that can cause difficulties in the diagnosis of this disease.
MATERIALS AND METHODS
Biopsy Samples
A total of 123 cases of lymphoma expressing ALK protein were identified. These were chosen from a large number of putative cases of ALCL and other lymphomas that had been biopsied at the hospital of one of the investigators (G.D.) or submitted to him for an opinion. They were selected from a total of 145 cases with morphologic features of ALCL that expressed both CD30 and epithelial membrane antigen (EMA) and that did not express B-cell markers. The frequency of ALK-positivity among the cases that satisfied our criteria for ALCL was therefore 85%. The male:female ratio among the 106 patients for which information was available showed a slight excess (1.4:1) of male subjects. Ages ranged from 3 months to 92 years, with a mean age of 21.3 years. In most instances (101 cases), the biopsy was of a lymph node; in a further 18 cases, the sample came from an extranodal area, including skin and bone. In 4 cases, the site of biopsy was unknown.
Special Investigations
Immunohistochemistry.
The anti-ALK monoclonal antibody ALK1 has been described previously.10 Other antibodies were obtained from DAKO A/S (Copenhagen, Denmark; CD3, CD45RO, CD20, anti-LMP1, and anti-EMA), Immunotech (Marseille, France; CD30 and CD15), Biotest (Buc, France; CD43 and MB2), Prof H. Stein (Freie Universität, Berlin; CD30/Ber-H2), and the investigators' laboratories (CD45RA, CD76, CD79a, CBF.78, and BNH.9).27 28
Immunostaining on paraffin sections was performed using the method described by Shi et al,29 with some modifications.16 Briefly, paraffin sections were mounted on glass slides coated with silane (Sigma Chemical Co, St Quentin, France). Sections were deparaffinized, placed in 10 mmol/L Na-citrate buffer (pH 6), and heated in a microwave oven (Whirlpool model; Philips, Eindhowen, Holland) at 900 W for cycles of 20 minutes and 10 minutes. The slides were removed from the oven and allowed to cool for 30 minutes at room temperature. After washing in water, endogeneous peroxidase was blocked with 1% hydrogen peroxide in methanol for 30 minutes. Slides were then rinsed in phosphate-buffered saline before staining using a streptavidin-biotin three-stage technique30 with the DAKO Strept ABC complex/HRP Duet kit (DAKO; code no. K492). Labeling for ALK in 13 cases from which no unstained material was available was performed on destained slides, as previously described.31 Briefly, hematoxylin and eosin-stained slides were immersed in toluene for 12 to 72 hours to remove the coverslip and then placed in successive baths of absolute alcohol, distilled water, and phosphate-buffered saline. This treatment removes eosin staining of the cytoplasm but does not affect nuclear staining. Immunohistochemical staining is then performed as for the unstained sections.
In a few cases, double labeling was carried out by performing first the immunoperoxidase technique for CD31 using nickel enhancement, followed by the alkaline phosphatase–anti-alkaline phosphatase (APAAP) procedure for ALK.
Tissue Specimens
Reverse transcriptase-polymerase chain reaction analysis for NPM-ALK.
This procedure was performed in 20 cases, using the method of Chomczynski and Sacchi as previously described,16 to analyze RNA extracted from 5-μm frozen sections.
In situ hybridization for Epstein-Barr virus (EBV).
This procedure was performed in 12 cases using EBER oligonucleotides, as described previously.32
Histopathologic Evaluation
Morphology.
Each case was reviewed by three pathologists and morphologic features of the neoplastic cells were noted, including the presence of Reed-Sternberg–like forms and giant cells. The degree of tissue involvement, the growth pattern (eg, cohesive, sinusoidal, perivascular, etc), and the abundance of infiltrating cells, including macrophages and plasma cells, was also noted.
The existence of possible morphologic subtypes of ALCL has been proposed in the REAL scheme and elsewhere,3,19-24 and we therefore attempted to assign each case to one of these putative categories. This assessment was made independently by three observers (G.D., Z.M.-B., and D.B.) using conventional hematoxylin and eosin-stained sections. In many instances, sections that had been immunostained (for CD30 and EMA and with antibody BNH9)33were also reviewed. Any controversial cases were reassessed by the three pathologists to achieve a consensus.
Phenotype.
Tumors were considered to be of T-cell origin when the neoplastic cells expressed CD3 or CD43 and/or CD45RO and/or CBF.78 and lacked B-cell–associated markers (CD20, CD79a, and/or CD45RA, CD76, MB2).
RESULTS
Morphologic Features
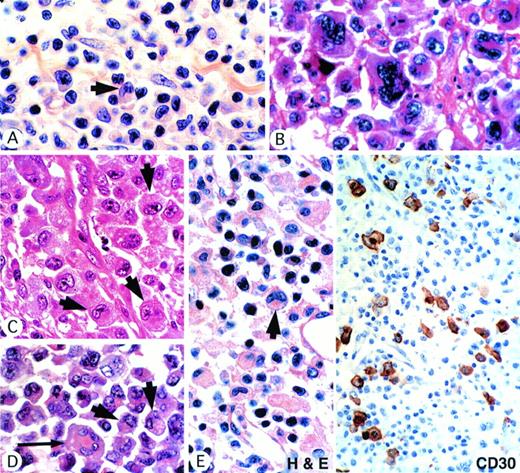
It was evident from the morphologic review that the ALK-positive tumors showed a broad spectrum of features, ranging from small-cell neoplasms that many pathologists might have categorized as pleomorphic T-cell lymphomas (Fig 1A) to cases at the opposite extreme in which very large cells predominated (Fig 1B). However, all cases shared one common feature, notably the presence of a population of large cells with a highly characteristic morphology (Fig1A through E). The nucleus lay eccentrically within these cells and was horseshoe or kidney shaped. In most cases, some crown-like nuclei could also be seen (Fig 1D). Nucleoli were less prominent than in Reed-Sternberg cells and often an eosinophilic region was seen near the nucleus, probably representing a prominent Golgi apparatus (Fig 1A through E).
ALK-positive ALCLs show a wide morphologic spectrum. (A) Small-cell pattern. A predominant population of small cells with irregular nuclei is associated with scattered large hallmark cells (arrow) showing eccentric lobated nuclei. (B) Giant-cell–rich pattern, showing striking cellular pleomorphism. (C) Common-type ALCL showing several hallmark cells (arrows). (D) Common-type ALCL showing several hallmark cells (arrow) and also a cell with crown-like nuclei (long arrow). (E) Lymphohistiocytic variant showing, in the hematoxylin and eosin-stained section, a single hallmark cell (arrow). Note the eosinophilic paranuclear area. Other cells are nonneoplastic, including histiocytes and plasma cells. Immunostaining of this case for CD30 highlights the scattered malignant cell population.
ALK-positive ALCLs show a wide morphologic spectrum. (A) Small-cell pattern. A predominant population of small cells with irregular nuclei is associated with scattered large hallmark cells (arrow) showing eccentric lobated nuclei. (B) Giant-cell–rich pattern, showing striking cellular pleomorphism. (C) Common-type ALCL showing several hallmark cells (arrows). (D) Common-type ALCL showing several hallmark cells (arrow) and also a cell with crown-like nuclei (long arrow). (E) Lymphohistiocytic variant showing, in the hematoxylin and eosin-stained section, a single hallmark cell (arrow). Note the eosinophilic paranuclear area. Other cells are nonneoplastic, including histiocytes and plasma cells. Immunostaining of this case for CD30 highlights the scattered malignant cell population.
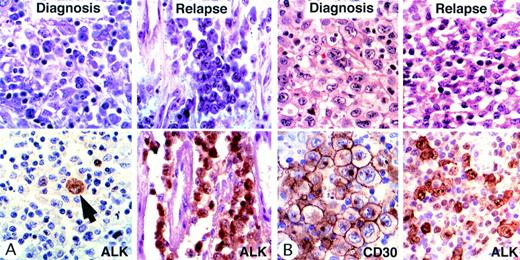
Morphologic transformation in 2 relapsing cases. Giemsa (A) and hematoxylin and eosin (B) staining is shown above and immunostaining is shown below. (Case A) The morphologic appearance at diagnosis was that of a lymphohistiocytic variant with scattered large neoplastic cells. ALK staining confirmed the scarcity of malignant cells (arrow). A few months later, this patient developed lung involvement consisting exclusively of large cells. (Case B) In this case, the morphologic appearance at diagnosis was that of a common-type ALCL with many large cells containing eccentric nuclei. Lymph node biopsy at relapse showed the features of a small-cell variant. Scattered large cells were strongly positive for ALK1, whereas small cells showed only moderate/weak staining.
Morphologic transformation in 2 relapsing cases. Giemsa (A) and hematoxylin and eosin (B) staining is shown above and immunostaining is shown below. (Case A) The morphologic appearance at diagnosis was that of a lymphohistiocytic variant with scattered large neoplastic cells. ALK staining confirmed the scarcity of malignant cells (arrow). A few months later, this patient developed lung involvement consisting exclusively of large cells. (Case B) In this case, the morphologic appearance at diagnosis was that of a common-type ALCL with many large cells containing eccentric nuclei. Lymph node biopsy at relapse showed the features of a small-cell variant. Scattered large cells were strongly positive for ALK1, whereas small cells showed only moderate/weak staining.
Once the characteristic features of these hallmark cells had been recognized, it was easy to identify them in all cases in the series, whatever the overall morphologic appearance (Fig 1A through E). In cases showing lymphohistiocytic features, these cells were less easily identified because of the large numbers of reactive histiocytes, but were nevertheless present (Fig 1E). In small-cell variant cases, these characteristic cells were mainly found around vessels in a perivascular pattern best seen in immunostained sections (see below).
The presence of a neoplastic cell type common to all 123 ALK-positive cases suggested that these cases represent variations on a theme. This view was reinforced by the fact that, when we attempted to assign the cases to one of the proposed subtypes of ALCL (Table 1), almost 15% of cases could not be classified because features of more than one subtype were found in a single biopsy (Table 2).
Histologic Categorization and Age Distribution of ALK-Positive Lymphomas
| Histopathology . | Total . | Children . | Adult . |
|---|---|---|---|
| ALCL common | 78 | 48 | 27 |
| ALCL lymphohistiocytic | 14 | 9 | 5 |
| ALCL small-cell variant | 7 | 4 | 3 |
| ALCL giant cell | 2 | 1 | 1 |
| ALCL mixed | 17 | 10 | 4 |
| Unclassifiable | 5 | 2 | 3 |
| Total | 123 | 74 | 43 |
| Histopathology . | Total . | Children . | Adult . |
|---|---|---|---|
| ALCL common | 78 | 48 | 27 |
| ALCL lymphohistiocytic | 14 | 9 | 5 |
| ALCL small-cell variant | 7 | 4 | 3 |
| ALCL giant cell | 2 | 1 | 1 |
| ALCL mixed | 17 | 10 | 4 |
| Unclassifiable | 5 | 2 | 3 |
| Total | 123 | 74 | 43 |
Morphologic Features of ALK-Positive ALCL in Which More Than One Histologic Pattern Was Found Within a Single Biopsy
| Histopathologic Subtypes . | No. of Cases . |
|---|---|
| Common plus lymphohistiocytic | 5 |
| Common plus small cell | 2 |
| Common plus Hodgkin's-like features | 4 |
| Common plus giant cell | 1 |
| Common plus lymphohistiocytic plus small cell | 2 |
| Lymphohistiocytic plus small cell | 3 |
| Histopathologic Subtypes . | No. of Cases . |
|---|---|
| Common plus lymphohistiocytic | 5 |
| Common plus small cell | 2 |
| Common plus Hodgkin's-like features | 4 |
| Common plus giant cell | 1 |
| Common plus lymphohistiocytic plus small cell | 2 |
| Lymphohistiocytic plus small cell | 3 |
These cases correspond to the “ALCL-mixed” category in Table1.
Furthermore, in 4 of the 6 cases in which a repeat biopsy was performed at the time of relapse, the morphologic features differed from those seen initially, as shown in Table 3 and Fig 2.
Morphologic Transformation in Relapsing ALCL
| Age/Sex . | Relapse After . | Morphology . | |
|---|---|---|---|
| At Diagnosis . | At Relapse . | ||
| 11/M | 7 mo | Lymphohistiocytic | Common* |
| 31/M | 13 yr | Common | Small cell |
| 65/F | 3 yr | Small cell | Common† |
| 12/M | 15 mo | Common | Small cell |
| Age/Sex . | Relapse After . | Morphology . | |
|---|---|---|---|
| At Diagnosis . | At Relapse . | ||
| 11/M | 7 mo | Lymphohistiocytic | Common* |
| 31/M | 13 yr | Common | Small cell |
| 65/F | 3 yr | Small cell | Common† |
| 12/M | 15 mo | Common | Small cell |
*Lung involvement.
Tonsil involvement.
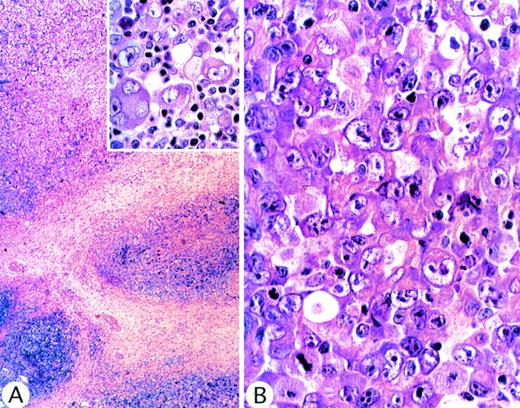
In 4 cases (1 of which showed lung involvement), we recognized features of the putative but controversial Hodgkin's-like form of ALCL, namely vaguely nodular fibrosis associated with capsular thickening and tumor cells resembling Reed-Sternberg cells (Fig3A). However, in all 4 cases, the malignant cells (unlike classical Reed-Sternberg cells) were CD15− and lacked evidence of EBV (ie, EBER RNA). Furthermore, the Hodgkin's-like pattern was seen only in some areas of the lymph node. Elsewhere, the neoplastic infiltrate showed the typical features of ALCL (Fig 3B). We therefore concluded that the resemblance of these cases to Hodgkin's disease was purely superficial and that there was no evidence that they differed from the other cases in this series.
ALCL of common type in which, at low power magnification, (A) sclerotic bands were evident, suggestive of nodular sclerosis Hodgkin's disease. (Inset) Malignant cells, showing Reed-Sternberg–like appearance. These morphologic features are consistent with Hodgkin's disease, but in other areas (B) the tumor showed the typical appearance of common-type ALCL.
ALCL of common type in which, at low power magnification, (A) sclerotic bands were evident, suggestive of nodular sclerosis Hodgkin's disease. (Inset) Malignant cells, showing Reed-Sternberg–like appearance. These morphologic features are consistent with Hodgkin's disease, but in other areas (B) the tumor showed the typical appearance of common-type ALCL.
Immunostaining
ALK protein.
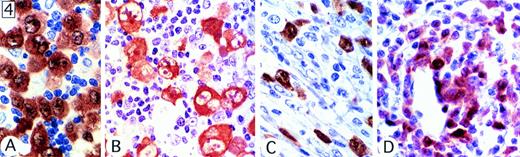
By definition, all cases stained for ALK protein. Eighty-two percent (101 of 123 cases) showed labeling to a similar degree of both the cytoplasm and the nucleus (Fig 4A), but in 16% of cases (20 of 123), staining was limited to the cytoplasm (Fig4B), and in 2 cases, only nuclei were labeled. The cellular distribution of ALK1 immunostaining in cases showing the common, lymphohistiocytic (Fig 4C), and giant cell patterns was comparable. However, in cases of small-cell ALCL only the larger anaplastic cells, often concentrated around vessels, were strongly positive (Fig 4D). In contrast, ALK staining in the small-cell population was variable. In most cases, only a proportion of these cells were positive, and staining was weak and restricted to nuclei.
Patterns of expression of ALK protein. (A) Both cytoplasmic and nuclear expression of the protein was seen in most tumors. (B) ALK staining was confined to the cytoplasm of neoplastic cells in a few cases. This case was of common type, but showed Hodgkin's-like areas. (C) Lymphohistiocytic variant showing scattered positive cells, some of them with a fibroblast-like appearance. (D) Small-cell variant, showing strong staining of large anaplastic cells, associated with only moderate/weak nuclear staining in the small cells.
Patterns of expression of ALK protein. (A) Both cytoplasmic and nuclear expression of the protein was seen in most tumors. (B) ALK staining was confined to the cytoplasm of neoplastic cells in a few cases. This case was of common type, but showed Hodgkin's-like areas. (C) Lymphohistiocytic variant showing scattered positive cells, some of them with a fibroblast-like appearance. (D) Small-cell variant, showing strong staining of large anaplastic cells, associated with only moderate/weak nuclear staining in the small cells.
Lineage markers.
Slightly more than half of the cases appeared to be of the T-cell origin (54%; Table 4), although it was not possible to give a precise figure for the frequency of this phenotype because some cases had been studied in the past with only a limited battery of antibodies. A T-cell phenotype was commoner among cases with lymphohistiocytic, small-cell, or mixed patterns (10 of 14, 7 of 7, and 12 of 17, respectively) than among cases with the common pattern (36 of 78 cases). In no cases was there any evidence for a B-cell derivation.
Reactivity of ALK1-Positive Lymphomas With Antibodies Detecting T-Cell–Associated Antigens
| Histopathologic Subtypes . | Antigens . | Conclusion* . | ||||
|---|---|---|---|---|---|---|
| CD3 (+/tested) . | CD43 (+/tested) . | CD45RO (+/tested) . | CBF.783-151 (+/tested) . | T Phenotype3-152 (+/tested) . | Null Phenotype3-153 (+/tested) . | |
| Common type | 12/44 | 23/55 | 21/39 | 14/26 | 36/78 (46%) | 19/78 (24%) |
| Lymphohistiocytic | 2/5 | 7/9 | 5/9 | 3/4 | 10/14 (71%) | 1/14 (7%) |
| Small cell variant | 3/7 | 5/5 | 3/4 | 7/7 | 7/7 (100%) | 0/7 |
| Giant cell | 0 | 1/1 | 0 | 1/1 | 1/2 | 0/2 |
| Mixed | 5/12 | 6/9 | 6/10 | 7/9 | 12/17 (70%) | 3/17 (17%) |
| Unclassifiable | 1/2 | — | 0 | 0 | 1/5 | 1/5 |
| Total | 23/70 (32%) | 42/79 (53%) | 35/65 (54%) | 31/47 (66%) | 68/123 (54%) | 24/123 (20%) |
| Histopathologic Subtypes . | Antigens . | Conclusion* . | ||||
|---|---|---|---|---|---|---|
| CD3 (+/tested) . | CD43 (+/tested) . | CD45RO (+/tested) . | CBF.783-151 (+/tested) . | T Phenotype3-152 (+/tested) . | Null Phenotype3-153 (+/tested) . | |
| Common type | 12/44 | 23/55 | 21/39 | 14/26 | 36/78 (46%) | 19/78 (24%) |
| Lymphohistiocytic | 2/5 | 7/9 | 5/9 | 3/4 | 10/14 (71%) | 1/14 (7%) |
| Small cell variant | 3/7 | 5/5 | 3/4 | 7/7 | 7/7 (100%) | 0/7 |
| Giant cell | 0 | 1/1 | 0 | 1/1 | 1/2 | 0/2 |
| Mixed | 5/12 | 6/9 | 6/10 | 7/9 | 12/17 (70%) | 3/17 (17%) |
| Unclassifiable | 1/2 | — | 0 | 0 | 1/5 | 1/5 |
| Total | 23/70 (32%) | 42/79 (53%) | 35/65 (54%) | 31/47 (66%) | 68/123 (54%) | 24/123 (20%) |
*In 31 cases, the phenotype of malignant cells could not be determined because these cases had been investigated with a limited panel of antibodies and no material was available for further immunostaining.
CBF.78 is an antibody from the authors' laboratories that detects a T-cell–associated antigen closely related to CD43 antigen.
Tumors were considered to be of T phenotype when CD3 and/or CD43 and/or CBF78 and/or CD45RO were positive in conjunction with negative staining for CD20 and/or CD79a and/or CD45RA and CD76 and/or MB2.
Tumors were considered to be of null phenotype when negative for CD3 and/or CD43 and/or/CBF78 in conjunction with negative staining for CD20 and/or CD79a and/or CD45RA and CD76 and/or MB2. In addition, 20 cases investigated in frozen sections were negative with a large panel of monoclonal antibodies directed against T, B, and macrophage-associated antigens.
CD30 and EMA.
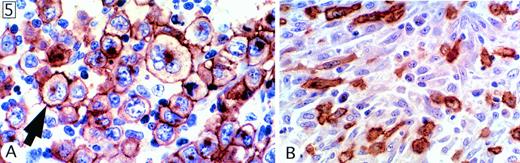
In every case, virtually all malignant cells were strongly reactive for CD30, and the staining pattern was, as previously described, associated mainly with the surface membrane and the Golgi area (Fig 5A). All cases tested (120 of 120) were also positive for EMA. The staining pattern was similar to that of CD30, although, in some cases, only a proportion of malignant cells was positive.
Staining for CD30. (A) Characteristic pattern, as seen in virtually all cases, comprising membrane staining associated with a cytoplasmic dot in the Golgi area. (B) Spindle cells with a fibroblast-like appearance in a lymphohistiocytic case.
Staining for CD30. (A) Characteristic pattern, as seen in virtually all cases, comprising membrane staining associated with a cytoplasmic dot in the Golgi area. (B) Spindle cells with a fibroblast-like appearance in a lymphohistiocytic case.
Other markers.
In a previous study, H and Y blood group-related antigens, detected by antibody BNH.9, were found in a significant proportion of ALCL.28 In the present study, 86 of 108 cases (80%) were reactive with this antibody. However, the number of positive cells varied greatly from case to case. Another carbohydrate antigen, CD15, was not expressed, except for 4 cases (3 showing the common pattern and 1 the features of giant-cell ALCL), in each of which a small proportion of the neoplastic cells was stained.
EBV detection.
No evidence of EBV infection was found in the 64 cases investigated using in situ hybridization and/or LMP-1 staining.
Special Morphologic Features
In addition to the features already described, which were seen in all cases, a number of other histologic characteristics were observed in a minority of biopsies. The two most common features related to the pattern of tumor infiltration, and this was best appreciated in sections immunostained for molecules expressed by the neoplastic cells (ie, CD30, EMA, or ALK).
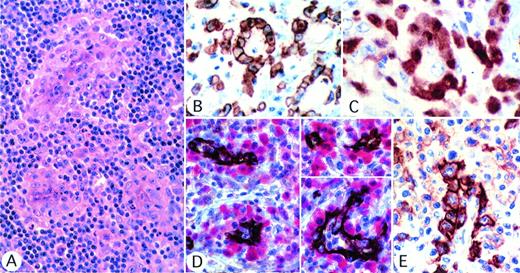
In about three quarters of all cases (84 of 111), the tumor cells showed a tendency to infiltrate lymphatic sinuses, as has been described in earlier publications.19 33 However, an even more distinctive pattern, seen in almost half of the cases (51 of 111), was a perivascular pattern of neoplastic cell infiltration (Fig 6). This appearance was more common when the tumor showed lymphohistiocytic features (9 of 14 cases) than when the common pattern was seen (31 of 70 cases). The perivascular pattern was best seen after single or double immunostaining (Fig 6B through E). Furthermore, all but 1 of the 6 cases with a small-cell appearance showed perivascular distribution, and in these cases large neoplastic cells were clearly associated with vessels (Fig 6E).
Perivascular pattern of tumor cells infiltration. (A) Several tumor cell foci are seen, all localized around vessels (hematoxylin and eosin). (B through D) The perivascular pattern is highlighted by staining for CD30 (B) or ALK (C) or by double labeling for vessels, with anti-CD31 (brown) and for ALK protein (red). (E) In the small-cell variant case, CD30 is strongly expressed by large cells surrounding the vessel, whereas the small cell population is only weakly positive.
Perivascular pattern of tumor cells infiltration. (A) Several tumor cell foci are seen, all localized around vessels (hematoxylin and eosin). (B through D) The perivascular pattern is highlighted by staining for CD30 (B) or ALK (C) or by double labeling for vessels, with anti-CD31 (brown) and for ALK protein (red). (E) In the small-cell variant case, CD30 is strongly expressed by large cells surrounding the vessel, whereas the small cell population is only weakly positive.
In one quarter of all cases, significant fibrosis with collageneous bands was noted (excluding those with only a limited degree of interstitial fibrosis). Spindle cells with a fibroblast-like appearance were found in 20 cases (11%). These cells were evident only in immunostained sections and were commonest (5 of 14 cases) in biopsies showing the lymphohistiocytic pattern (Fig 5B). In 1 case, rare signet ring tumor cells were seen in one area, as recently described in a case of ALCL.24
DISCUSSION
The results of the present study of ALCL expressing ALK protein provide strong evidence that the morphologic patterns described in previous reports as representing possible subtypes of ALCL, eg, common type, lymphohistiocytic, or small-cell patterns,19,20,22 33 are morphologic variants of the same disease entity. All of these morphologic patterns could be found within this series, and in some instances different subtypes coexisted in a single biopsy or were found in successive biopsies from a single patient (Table 3). The link between these morphologic subtypes of ALCL was further reinforced by the presence in all cases of a highly characteristic large cell that can be considered as a major distinguishing hallmark of the disease (Fig 1). One of its distinguishing features is a perinuclear eosinophilic region that we assume represents a prominent Golgi apparatus. We have not shown this directly (by electron microscopy), but it is noteworthy that CD30 staining frequently highlights the same area (Fig 5A).
The proposed Hodgkin's-like form of ALCL is still the subject of controversy.3,19 In 4 cases, typical features of ALCL were associated with areas in which a Hodgkin's-like pattern was seen (Fig5). However, these Reed-Sternberg–like malignant cells were CD15− and lacked EBER RNA (found in 60 % of Hodgkin's cases).34 These results suggest that occasional cases of ALK-positive lymphomas show a superficial resemblance to Hodgkin's disease but can be categorized correctly on careful histologic examination and immunostaining. However, we also believe that many tumors previously diagnosed as Hodgkin's-like ALCL are cases of neoplastic cell-rich Hodgkin's disease (ie, they should be designated ALCL-like Hodgkin's disease rather than Hodgkin's-like ALCL).
In addition to NPM-ALK protein and EMA expression and lack of CD15, a morphologic feature that may be of value in differentiating ALCL from ALCL-like Hodgkin's disease is the perivascular pattern of neoplastic cell infiltration (Fig 6). This has been reported as a frequent feature in a series of cases of the small-cell variant of ALCLs,22but we found it in almost half of our cases. It has not been noted in Hodgkin's disease.35
It may be noted that some putative morphologic variants of ALCL reported previously were not observed in this series of ALK-positive ALCL. Thus, in none of the cases was the number of fibroblast-like spindle cells high enough to give the tumor a sarcomatous appearance, as described by Chan et al,21 and no cases showed features consistent with neutrophil-rich ALCL as described by Mann et al,23 even though infiltrating granulocytes were present in some cases. However, we would argue on the basis of our observation of marked morphologic variability among ALK-positive lymphomas that these are probably not distinct subtypes.
As noted in the description of ALCL in the REAL classification,3 the majority of these ALK-positive ALCL were of T phenotype, and in no case was there evidence for a B-cell origin. T-cell antigen expression by ALCL cases showing the small-cell pattern was of interest because the small-cell population showed stronger staining for T-cell markers than did the larger anaplastic cells. An opposite staining pattern was seen for CD30 and ALK antigens. However, despite the strikingly different morphology of these two cell populations, the expression of NPM-ALK protein by the small cells suggests that they also belong to the malignant cell population and carry the t(2;5).
All tumors expressed EMA, confirming our previous reports on the diagnostic value of this marker in ALCL.33,36 EMA is an epithelial sialomucin,37 encoded by the MUC1 gene on chromosome 1q21-24,38 as is the CD30/Ki-1 molecule (encoded at 1p36).25 This could suggest that the t(2;5) promotes, by an unknown mechanism, the expression of these two antigens by lymphoid cells.
Malignant cells carrying the 2;5 translocation show both cytoplasmic and nuclear staining for NPM-ALK, and this appears to be due to oligomer formation with wild-type NPM and subsequent transport from the cytoplasm to the nucleus directed by nuclear localization signals in the NPM molecule.39 However, in the present study, only about 80% of cases showed both nuclear and cytoplasmic staining pattern. In most of the remaining cases, staining was restricted to the cytoplasm. Because all staining was performed on routinely fixed paraffin-embedded tissue, these differences may be, at least in part, artefactual. However, we have previously reported that, in a case of ALCL carrying a variant t(1;2)(q25;p23) translocation, ALK protein does not enter the nucleus,10 39 and our present findings therefore suggest that, in a significant minority of ALCLs, the ALK gene may fuse to a gene other than NPM. Immunostaining for ALK protein, preferably in cryostat sections, should allow such cases to be identified for more detailed molecular analysis.
In conclusion, our results argue that one can now define, through a combination of morphologic review and immunostaining, a distinct ALK-positive lymphoma entity that tends to occur in young patients. ALK protein expression in these tumors is most commonly due to the creation of the NPM-ALK fusion gene by the 2;5 translocation, although there was at least one exception in this series, a (1;2) translocation, and we suspect that other anomalies involving breakpoints at 2p23 may well be found in this tumor in the future.
These tumors usually express CD30 and EMA and are of T or null phenotype. Their morphology covers a wide morphologic spectrum, but this heterogeneity does not appear to indicate the existence of different disease entities. The hallmark cell we describe may be present only in low numbers but is highly characteristic. It is not seen in other lymphomas and, once recognized, is invaluable, in combination with ALK staining, in defining this lymphoma entity.
Our results inevitably prompt a reevaluation of the term ALCL, which is an inappropriate morphologic description for many of the tumors described in this report (eg, those composed mainly of small cells). There is subjective variation in the ability of hematopathologists to define ALCL (and indeed we suspect that the term anaplastic means different things to different pathologists). Furthermore, there is disagreement as to whether ALCL of B-cell phenotype exists, because it is recognized by the Kiel scheme but not included in the REAL classification. Consequently the term ALCL will continue, in the absence of agreed phenotypic or genotypic criteria, to be applied to a poorly defined group of lymphomas. However, it now seems justifiable to separate out the tumor type that we describe in this report and henceforward to refer to it as ALK lymphoma or even, more colloquially, as “ALKoma”. It must be noted that a quite different, more aggressive ALK-positive lymphoma has been described in adults expressing full-length ALK protein rather than NPM-ALK,11 but these cases are exceedingly rare and the advantages of using the word ALK to rename the tumors we describe in this report should outweigh any potential risk of ambiguity. The initial name for ALCL (Ki-1 lymphoma) was also based on the expression of a marker molecule, but this title was abandoned when it emerged that Ki-1 (CD30) expression was not restricted to one lymphoma type. However, we are confident that ALK expression, given that it appears to be directly implicated in the genesis of these tumors,26 will prove a reliable defining criterion and can be incorporated into their name.
ACKNOWLEDGMENT
The authors thank the IGR and Drs Abd Alsamad, Archambeau, Bertrand, Boccon-Gibod, Bouchind'homme, Bowman-Ferrand, Brousse, Demuet, Deschalotte, Dijoud, Dumont, Fetissof, Galateau, Heymann, Hopfner, Lagacé, Lefèvre-Wuithier, Levillain, Monégier du Sorbier, Morel, Patelli, Patey, Perraudeau, Petrella, Rogez, Rousset, Saint-André, Tapie, Trojani, Validire, and Vancina for their contribution of cases. We also acknowledge the help of Bridget Watson and Beata Ozieblowska in the preparation of, respectively, the text and illustration of this report.
Supported by “Ligue Nationale Contre le Cancer,” the “Délégation à la Recherche Clinique,” the Leukemia Research Fund of Great Britain, National Cancer Institute (NCI) Grants No. CA 01702 and CA 69129 (S.W.M.), the NCI Cancer Center CORE Grant No. CA 21765, the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children's Research Hospital (Memphis, TN), the Chief Scientist Office of the Israeli Ministry of Health (the Yvonne Heymann Trust), and the Tabb Foundation.
Address reprint requests to Georges Delsol, MD, Laboratoire d'Anatomie Pathologique, CHU Purpan, Place du Dr Baylac, 31059, Toulouse Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal